Abstract
Panax ginseng Meyer has been widely used as a tonic in traditional Korean, Chinese, and Japanese herbal medicines and in Western herbal preparations for thousands of years. In the past, ginseng was very rare and was considered to have mysterious powers. Today, the efficacy of drugs must be tested through well-designed clinical trials or meta-analyses, and ginseng is no exception. In the present review, we discuss the functions of ginseng described in historical documents and describe how these functions are taken into account in herbal prescriptions. We also discuss the findings of experimental pharmacological research on the functions of ginseng in ginseng-containing prescriptions and how these prescriptions have been applied in modern therapeutic interventions. The present review on the functions of ginseng in traditional prescriptions helps to demystify ginseng and, as a result, may contribute to expanding the use of ginseng or ginseng-containing prescriptions.
Keywords: Panax ginseng, Herbal prescription, Historical documents, Modern pharmacological experiments, Translational approaches
INTRODUCTION
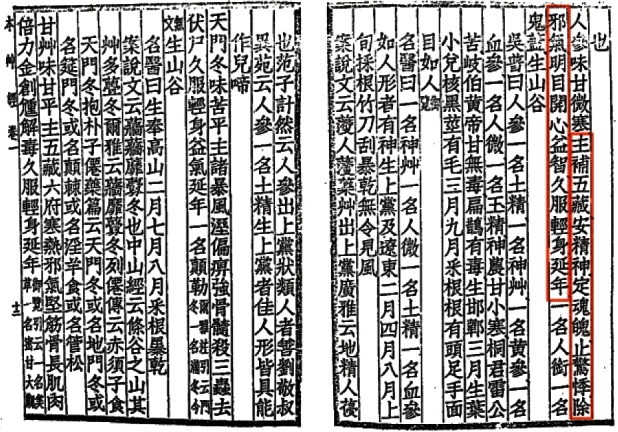
In ancient times, medical practitioners treated patients through shamanism or used materials that were available in their area, such as plants, animals or alcohol. However, these practitioners may not have utilized therapeutic knowledge, and patients may have not received satisfactory medical care. Over time, by trial and error, therapeutic strategies using readily available materials were established, as demonstrated by evidence unearthed from ancient tombs (e.g., the Mawangdui tomb), including the Wushier Bing Fang (五十二病方). Over time, medical information accumulated, and medical practitioners used many suitable medicinal plants or animals for the treatment of diseases, including ginseng. Ginseng is a medicinal plant that has been used in medical practices for more than 2,000 years [1]. The Shennong Bencao Jing (Shennong's Herbal Classic, 神農本草經), one of the first books specializing in herbal medicine, was edited in the 1st century AD. In this text, ginseng was first recognized as a medicinal herb by medical practitioners (Fig. 1). In addition, in the Shang-Han Lun (translated as the treatise on diseases caused by cold factors, 傷寒論), Zhong Jing Zhang (張仲景) described the use of ginseng in herbal prescriptions. However, because ginseng has been cultivated since the 15th century, the type of ginseng that appeared in the Shennong Bencao Jing may have been different from that which is currently in use. In previous centuries, wild ginseng would have been collected from mountainous areas; thus, this plant is commonly referred to as mountain ginseng. Several excellent accounts of mountain ginseng have been presented in the literature. For instance, mountain ginseng has been used to save lives in Korea [1].
Fig. 1. The description of ginseng in the Shennong Bencao Jing (神農本草經), which explains the function of ginseng. The pharmacological functions of ginseng, which was regarded as a high-grade herb, were first described in the Shennong Bencao Jing. The Shennong Bencao Jing was written in the 1st century by an unknown author.
Since standardized ginseng extract G115 was first prepared by Pharmaton Ltd., Lugano-Bioggio, Switzerland, ginseng has been widely used as an active ingredient in modern medicine. For example, the German Commission E approved ginseng as a tonic for invigoration and fortification to treat fatigue, debility or declining sexual capacity. Moreover, ginseng has been approved to enhance concentration and for use during convalescence [2]. These uses were reaffirmed by the World Health Organization in 1999. Ginseng has several beneficial properties, including providing the nourishing effects, anti-fatigue, or immune-enhancing effects, as mentioned in the traditional descriptions of the Shennong Bencao Jing. Therefore, the characteristics of ginseng described in historical documents, such as the Shennong Bencao Jing, the Bencao Gangmu (本草綱目, which was written by Shizhen Li [李時珍] during the Ming Dynasty), and the Hyangyak-jibseongbang (鄕藥集成方) or the Donguibogam (東醫寶鑑, which was published during the Chosun Dynasty), should be examined. The role of ginseng in traditional prescriptions should also be analyzed to expand the use of ginseng in modern medicine. In addition, investigations on the experimental evidence in pharmacological studies of ginseng-based traditional prescriptions would yield information on how traditional knowledge or historical accounts described in the aforementioned documents can be interpreted in contemporary medical terms.
Panax ginseng is a traditional medicinal plant that has been used therapeutically for millennia in East Asia. In Korea, China, and Japan, ginseng is the most valuable of all medicinal herbs. The name Panax means “all healing,” which describes the traditional belief that ginseng can heal all aspects of the body. The most common ginsengs are ginseng (P. ginseng Meyer), Chinese ginseng (P. notoginseng [Burk.] FH Chen), and American ginseng (P. quinquefolium L.).
Among the ginseng varieties, P. ginseng has been extensively researched and has received the most attention. P. ginseng is sensitive to the temperature and soil of the cultivated area. Therefore, P. ginseng is cultivated in limited areas, including Korea, the Manchuria region of China (the region of Dongbei), and the maritime province of Siberia in Russia. Although it is not known whether historical documents described cultivated or wild ginseng, in the present review, we discuss historical accounts concerning P. ginseng, the role of P. ginseng in traditional prescriptions, and experimental evidence from pharmacological and clinical studies of traditional prescriptions containing P. ginseng (Table 1).
Table 1.
Summary of the pharmacological activities of ginseng-containing prescriptions described in the Shang-Han Lun in either animal or human studies
| Name of formula (in Korean) | Name of formula (in Chinese) | Name of formula (in Japanese) | Species | Pharmacological evaluation | References |
|---|---|---|---|---|---|
|
| |||||
| Banhasasim-tang 半夏瀉心湯 | Ban-Xia-Xie-Xin-Tang | Hangeshashin-to | Human | Dyspepsia | [3] |
| Human | Diarrhea | [4,5] | |||
| Mice | Diarrhea | [6] | |||
| Sosiho-tang 小柴胡湯 | Xiao-Chai-hu-Tang | Shosaiko-to | Rat | Anaphylaxis | [7] |
| Human | Hepatic injury | [8-14] | |||
| Rat | Hepatic injury | [15-21] | |||
| Mouse | Hepatic injury | [22] | |||
| Rat | Immune modulation | [23] | |||
| Mouse | Immune modulation | [24-32] | |||
| Insam-tang 人蔘湯 | Ren-Shen-Tang | Ninjin-to | Mouse | Diabetes | [33] |
| Guibi-tang 歸脾湯 | Gu-Pi-Tang | Kihi-to | Rat | Dementia | [34] |
| Mouse | Dementia | [35] | |||
| Rat | Osteopenia | [36] | |||
| Rat | Thrombocytopenic purpura | [37,38] | |||
| Sipjeondaebo-tang 十全大補湯 | Shi-Quan-Da-Bu- Tang | Juzentaiho-to | Mouse | Dementia | [39] |
| Human | Atopic dermatitis | [40] | |||
| Human | Anemia | [41,42] | |||
| Human | Otitis | [43] | |||
| Mouse | Cancer | [44-46] | |||
| Rat | Cancer | [47] | |||
| Mouse | Immune modulation | [48-54] | |||
| Bojungikki-tang 補中益氣湯 | Bu-Zhong-Yi-Qi -Tang | Hochuekki-to | Human | Fatigue | [55,56] |
| Mouse | Fatigue | [57,58] | |||
| Rat | Diabetes | [59] | |||
| Human | Immune modulation | [60-64] | |||
| Mouse | Immune modulation | [65-73] | |||
| Hamster | Cancer | [74] | |||
| Mouse | Lung injury | [75] | |||
| Human | Inflammation | [76,77] | |||
| Mouse | Inflammation | [78] | |||
| Rat | Osteopenia | [79] | |||
| Human | Atopic dermatitis | [80] | |||
| Rat | Liver injury | [81] | |||
| Mouse | Antibacterial | [82] | |||
| Insamyangyoung-tang 人蔘養營湯 | Ren-Shen-Yang- Rong-Tang | Ninjinyouei-to | Mouse | Anemia | [83] |
| Rat | Liver injury | [81] | |||
| Mouse | Amnesia | [84] | |||
| Mouse | Immune modulation | [85-88] | |||
| Rat | Immune modulation | [89] | |||
| Daegeonjung-tang 大建中湯 | Da-Jian-Zhong-Tang | Daikenchu-to | Mouse | Colitis | [90] |
| Rat | Liver injury | [91] | |||
| Dog | Gastrointestinal motility | [92,93] | |||
| Rat | Gastrointestinal motility | [94-96] | |||
| Mouse | Gastrointestinal motility | [97,98] | |||
| Human | Gastrointestinal motility | [99,100] | |||
| Guinea pig | Blood flow | [101,102] | |||
| Human | Blood flow | [103] | |||
| Rat | Blood flow | [104] | |||
| Saengmaek-san 生脈散 | Sheng-Mai-San | Rat | Diabetes | [105] | |
| Mouse | Amnesia | [106] | |||
| Rat | Spinal cord injury | [107] | |||
| Rat | Liver injury | [108] | |||
| Rat | Cerebral ischemia | [109-113] | |||
| Siho-ga-yonggolmoryeu-tang 柴胡加⿓骨牡蠣湯 | Chai-Hu-Jia-Long- Gu-Muli-Tang | Saikokaryukotsuborei-to | Rabbit | Atherosclerosis | [114] |
| Sihokyeji-tang 柴胡桂枝湯 | Chai-Hu-Gui-Zhi -Tang | Saikokeishi-to | Rat | Gastric ulcer | [115] |
| Rat | Liver injury | [116,117] | |||
| Mouse | Hepatic drug-metabolizing enzyme activity | [118] | |||
| Rat | Pancreatitis | [119-122] | |||
| Bekho-ga-Insam -tang 白虎加人蔘湯 | Bai-Hu-Jia-Ren- Shen-Tang | Byakkokaninjin-to | Rat | Water balance | [123] |
| Mouse | Water balance | [124] | |||
| Rat | Salivary secretion | [125] | |||
| Mouse | Salivary secretion | [126] | |||
| Mouse | Atopic dermatitis | [127,128] | |||
| Mouse | Antihyperglycaemia | [129] | |||
| Ome-whan 烏梅丸 | Wu-Mei-Wan | Ubai-gan | Rat | Colitis | [130,131] |
A RETROSPECTIVE ON GINSENG IN HISTORICAL DOCUMENTS
The pharmacological functions of ginseng, which was considered a high-grade herb, were first described in the Shennong Bencao Jing. Ginseng can nourish or tonify 5 vital organs of the body (the spleen, lung, heart, kidney, and liver), has sedative properties, is used for palpitations to restore a normal pulse, dispels pathogenic factors, improves visual acuity and mental activity, and enhances longevity with long-term intake (Fig. 1).
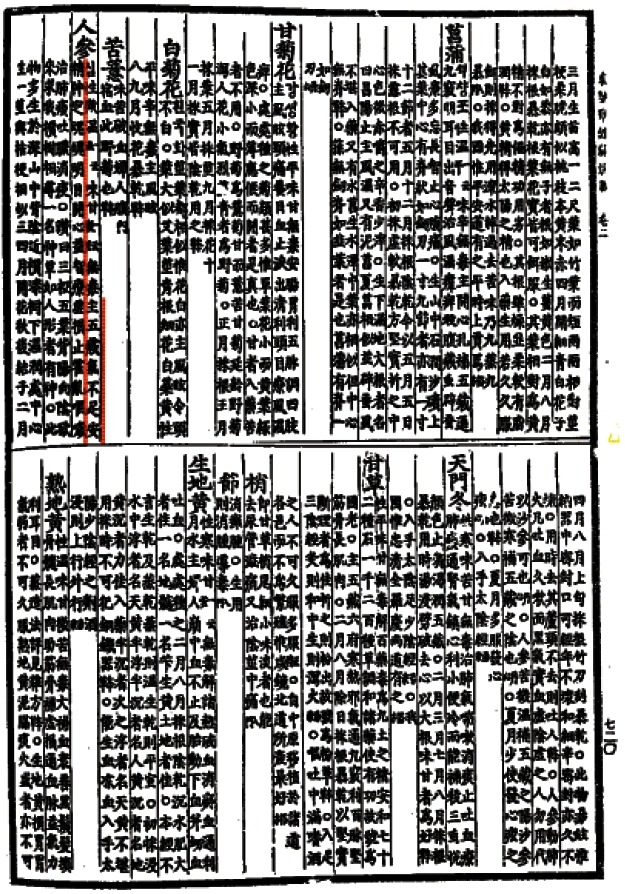
The Shennong Bencao Jing was written in the 1st century by an unknown author, and the Mingyi Bielu (名醫別錄), which described 365 different types of herbs, was written by Hongjing Tao (陶弘景, AD 456-536). Hongjing Tao also published a book titled the Bencaojing Jizhu (本草經集注), in which 730 kinds of herbs were discussed. In the Mingyi Bielu, additional properties of ginseng were described, including curing internal coldness, pain in the chest or abdomen, sensations of fullness in the chest, vomiting, and diarrhea. Ginseng can also be used to relieve thirst and feelings of solidness, to enhance cognitive function, and to improve blood circulation (Fig. 2).
Fig. 2. The description of ginseng in the Mingyi Bielu (名醫別錄), which explains the function of ginseng. The Mingyi Bielu was written by Hongjing Tao.

These pharmacological properties of ginseng were also described in the Bencao Gangmu, which is the most complete and comprehensive pre-modern herbal textbook. In addition, Shizhen Li (李時珍, 1518-1593) discussed several symptoms treatable with ginseng, including general weakness, spontaneous sweating and fever, vertigo and headache, regurgitation and vomiting, alternating fever and chills, chronic diarrhea, increased urination or stranguria, fatigue, externally contracted wind or hot attack, cramps, vomiting blood (hematemesis), bleeding from the rectum, bloody urinary leakage, abnormal uterine bleeding, and discomfort before or after parturition (Fig. 3).
Fig. 3. The description of ginseng in the Bencao Gangmu (本草綱目), which explains the function of ginseng. The Bencao Gangmu is the most complete and comprehensive pre-modern herbal text.

Several published documents have also referred to the descriptions in the Shennong Bencao Jing, Mingyi Bielu, and Bencao Gangmu. The Hyangyak-jibseongbang was published during the Chosun Dynasty and referred to the pharmacological activities of ginseng described in the Mingyi Bielu. The Donguibogam, which was added to the World Heritage List in 2009 by UNESCO, also described the activities of ginseng, including those described in the Shennong Bencao Jing (i.e., the use of ginseng to cure general weakness, acute vomiting with diarrhea [cholera], hiccups and vomiting, atrophic lung disease and phlegm) (Fig. 4).
Fig. 4. The description of ginseng in the Donguibogam (東醫寶鑑), which explains the function of ginseng. The Donguibogam also describes additional activities of ginseng as well as the functions listed in the Bencao Gangmu.
In addition to the aforementioned texts, many published works have described similar activities. Therefore, P. ginseng was likely considered a medicinal herbal for the treatment of general weakness, acute vomiting with diarrhea, anxiety or mental health. It is unclear whether ancient medical practitioners such as Shizhen Li (李時珍), Zhong Jing Zhang (張仲景), or Jun Heo (許浚) knew the functions of ginseng. However, the pharmacological activities listed in historical documents are also supported by recent experimental and clinical studies (vide infra).
Although the pharmacological activities of ginseng were described in the Shennong Bencao Jing, illustrations of the variety of ginseng used at that time were not provided. The Bencao Gangmu described ginseng used in the Baekje Dynasty, and, in this case, an illustration was provided (Fig. 5). The Baekje Dynasty (百濟, 18 BC-AD 660) governed the western part of the Korean Peninsula and actively traded with China, suggesting that the Korean Peninsula might have been suitable for the cultivation of ginseng and that Korean ginseng may have been used in herbal prescriptions prior to the Ming Dynasty. However, the illustration of ginseng in the Bencao Gangmu differs slightly from that of the Bencao Baiyao (本草備要), which was written by Wang Ang (汪昻) in 1694 during the Qing Dynasty. The illustration in the Bencao Baiyao is very similar to P. ginseng (Fig. 6).
Fig. 5. The description of ginseng in the Bencao Gangmu, which explains the origin of ginseng. Baekje (百濟) represents the Baekje Dynasty, which governed the western part of the Korean Peninsula from 18 BC to AD 666.

Fig. 6. Illustrations of ginseng from historical documents. (A) Illustrations of ginseng from the Bencao Gangmu (本草綱目) and (B) the Bencao Baiyao (本草備要) are presented.
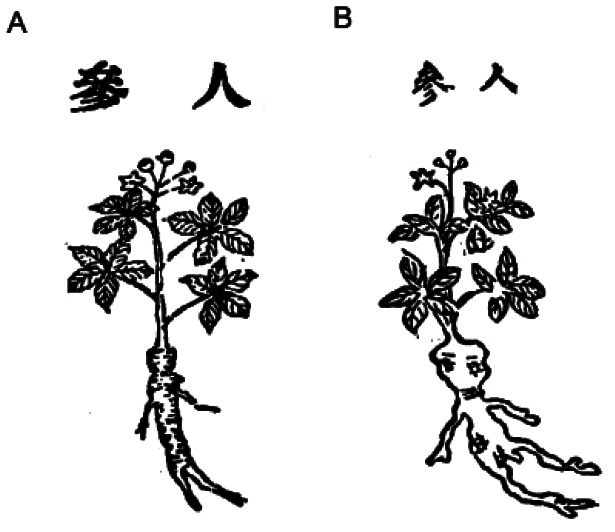
Wang Ang edited the Bencao Baiyao and included herbs from the Shennong Bencao Jing and the Bencao Gangmu. In the Bencao Gangmu, the shape of the ginseng root was described as resembling the human body or the extremities of the human body. Therefore, although the depiction of ginseng differs slightly among historical documents, the description provided in the Bencao Gangmu may have referred to P. ginseng.
THE PHARMACOLOGICAL ACTIVITIES OF PANAX GINSENG IN TRADITIONAL PRESCRIPTIONS
Among the 113 prescriptions (18.6%) described in the Shang-Han Lun, which was written by Zhong Jing Zhang (150-219 AD), 21 prescriptions contained ginseng, including Banhasasim-tang (半夏瀉心湯, Ban-Xia Xie-Xin Tang in Chinese or Hangeshashin-to in Japanese) and Sosiho-tang (小柴胡湯, Xiao-Chai-hu Tang in Chinese or Sho-saiko-to in Japanese). The Shang- Han Lun covers specific symptoms of disorders and their corresponding treatments and is designated as one of the four fundamental texts of traditional Chinese medicine [132]. In addition, among the 3,944 prescriptions (16.6%) in the Donguibogam, which was written by Jun Heo, 653 prescriptions containing ginseng as an ingredient.
Several traditional prescriptions were prepared from ginseng, which was used for its restorative, tonic, nootropic, and anti-aging properties, or for dispelling pathogenic factors, as described in the Shennong Bencao Jing. Among ginseng-containing prescriptions, the classic and basic prescription is Sagunja-tang (四君子湯, Sijunzi Tang in Chinese or Shikunshi-to in Japanese). Sagunjatang was prescribed for conditions such as hypodynamia, lassitude and anorexia. As a main ingredient in Sagunjatang, ginseng exerts various pharmacological activities, such as organ tonification and restorative activities, as described in the Shennong Bencao Jing and Bencao Gangmu. These pharmacological activities were also accomplished by Sipjeondaebo-tang (十全大補湯, Shiquan-da-bu-tang in Chinese or Juzen-taiho-to in Japanese), which was composed of 10 types of herbs, including four herbs (Ginseng Radix, Atractylodis Rhizoma Alba, Poria Sclerotium, Glycyrrhizae Radix et Rhizoma) from Sagunja-tang, four herbs (Angelicae Gigantis Radix, Cnidii Rhizoma, Paeoniae Radix, Rehmanniae Radix Preparata) from Samul-tang (四物湯, Si-Wu-Tang in Chinese, and Shimotsu-to in Japanese) and additional herbal materials, such as Astragali Radix (Astragalus membranaceus) and Cinnamomi Cortex (Cinnamomum cassia). Sipjeondaebo-tang was prescribed to patients who suffered from weakness or spontaneous sweating, which were described as being treatable with ginseng in the Bencao Gangmu. In addition, Doksam-tang (獨蔘湯, Dushen Tang in Chinese or Dokujin-to in Japanese), which was only composed of ginseng, was used for seriously ill patients [133], as depicted in the Shi Yao Shen Shu (十藥神書), which was written by Ke-Jiu Ge (葛 可久) in 1348 during the Yuan Dynasty. In the Shennong Bencao Jing, ginseng was said to dispel pathogenic factors. In the Donguibogam, Talmyung-san (identical to Doksam-tang) was also used for the elimination of pathogenic factors. Recently, An et al. [134] reported the effects of ginseng formulae on stable chronic obstructive pulmonary disease, which represents a pattern of Qi deficiency involving either the lung or the spleen. For seriously ill patients, ginseng can also be used with Aconiti root, which is found in Sambu-tang (蔘附湯, Shen Fu Tang in Chinese), as described in the Fu Ren DA Quan Liang Fang (婦人大全良方), which was written by Zi- Ming Chen (陳自明) during the Song Dynasty. In the aforementioned formulae, ginseng is the main active ingredient and exerts restorative, tonic or nourishing effects on hypodynamia and ameliorates the effects of serious illness, as described in the Shennong Bencao Jing and the Bencao Gangmu.
Although Jeongji-whan prescription (定志丸, Dingzhi Wan in Chinese) is listed in the Yixue Xinwu (醫學心悟), which was written by Goupeng Cheng (程國彭) in 1732, this prescription has not been certified as a herbal prescription in Japan. However, in the mid-1990s, the Nishiyama group in Japan reported the effects of DX-9386, which possesses ingredients that are identical to those of Jeongji-whan, on brain function [135]. The Jeongjiwhan prescription was described as being active against anxiety, palpitation, and amnesia, and ginseng was used as the main ingredient. Kami-guibi-tang (加味歸脾湯, Jia Wei Gu Pi Tang in Chinese or Kami-kihi-to in Japanese), or Guibi-tang (歸脾湯, Gu Pi Tang in Chinese or Kihi-to in Japanese), was described in classical literature as being effective for the treatment of insomnia, anemia, amnesia, depression, and neurosis [35]. Tohda et al. [35] suggested that Guibi-tang is an attractive candidate as an anti-dementia drug. Thus, although the improvement in brain function was not solely dependent on ginseng, these prescriptions made use of the effects of ginseng on anxiety, amnesia, and palpitation, which were described in the Shennong Bencao Jing.
In particular, the Mingyi Bielu stated that ginseng relieves thirst (Sogal in Korean, Xiao Ke in Chinese or Shoukatsu in Japanese, 消渴), and the Bencao Gangmu referred to the description of ginseng in the Mingyi Bielu. As described in historical documents, the symptoms of thirst are similar to those of diabetes. Baekho-ga-Insamtang (白虎加人蔘湯, Byakko-ka-ninjin-to in Japanese or Bai Hu Jia Ren Shen Tang in Chinese), which was a Shang-Han Lun formula, relieves thirst caused by internal fever [126].
In addition to the aforementioned pharmacological properties, ginseng can also be used to alleviate diarrhea and vomiting. Insam-tang (人蔘湯, Ninjin-to in Japanese or Ren Shen Tang in Chinese), which is composed of Ginseng Radix, Glycyrrhizae Radix, Atractyloides Rhizome, and Zingiberis Rhizome, has been used for the treatment of diarrhea or anorexia and tonifies the Qi of the spleen. In Banhasasim-tang, ginseng is used as a coingredient [6]. Even when included as co-ingredient in Banhasasim-tang, ginseng enhances Qi and contributes to the alleviation of diarrhea or intestinal catarrh. Recently, Taiwanese doctors investigated the most common prescriptions from the Shang-Han Lun. Banhasasimtang and Sosiho-tang (Sho-saiko-to in Japanese and Xiao Chai Hu Tang in Chinese) were prescribed at 10.24% and 9.11% in the Shan-Han Lun formulae, respectively.
Additionally, in the Shennong Bencao Jing, ginseng is listed as an anti-aging factor. Kyungok-Ko, a sticky extract composed of Ginseng Radix, Rehmnania juice, Hoelen (Poria Sclerotium), and honey, was described as having anti-aging activities when administered for an extended period of time [136]. Thus, various pharmacological activities of ginseng were described along with those of other active ingredients.
In Doksam-tang, the ginseng content is equal to 37.5 g, and ginseng is the active ingredient in this prescription [137]. According to the German Commission E, crude preparations of 1 to 2 g of dried root powder of ginseng can be taken every day for up to three months. A decoction can be prepared by simmering 3 to 9 g of dried ginseng root in 720 to 960 mL of water [138]. In decoction, ginseng is included at a dosage of 3 to 9 g as the main ingredient in most prescriptions, except for Doksamtang. In some formulations, such as Kami-guibi-tang or Guibi-tang, ginseng is included at a dosage of 15 g [137]. Thus, ginseng has been used at dosages ranging from 3 to 37.5 g. In chemical drugs, the amount of intake rarely exceeds 5 to 10 times the usual dose. Adverse effects or toxicity caused by ginseng overdose should be avoided. However, P. ginseng is well tolerated, and its adverse effects are mild and reversible [139].
THE USE OF GINSENG-CONTAINING PRESCRIPTIONS IN MODERN PHARMACOLOGICAL EXPERIMENTS
Over the last several decades, traditional Chinese medicine has become a significant form of complementary and alternative medicine used by patients in Europe and North America [140]. In addition to traditional Chinese medicine, traditional Japanese medicine (Kampo) and traditional Korean medicine (Hanbang), which are referred to as traditional herbal medicine in this review, have been accepted as a form of complementary and alternative medicine by patients [141,142]. In Western medicine, randomized clinical trials are generally accepted as the most reliable approach to testing the efficacy of medicines and treatments; however, in traditional herbal medicine, clinical evidence was observed and recorded descriptively in the literature [143]. Although the theoretical frameworks of Western medicine and traditional herbal medicine are different, scientific studies on the efficacy of traditional herbal medicine using randomized clinical trial techniques are becoming more popular [143]. Such evidence-based medicine integrates the best evidence from research with clinical expertise and patient values [142]. Using clinical trials or basic scientific research, the efficacy of traditional prescriptions for specific diseases can be determined, which has the potential to expand the use of traditional formulae.
Western research models are used in Japan to study traditional medicine, and the approach is based on conventional Western disease nosology and conventional immunology [142]. To date, most traditional herbal medicine experiments have been performed in vivo on animals or humans by Japanese scientists, and the results have been published in peer-reviewed international journals. Sipjeondaebo-tang is the most commonly investigated herbal medicine by scientists and clinicians. Traditionally, Sipjeondaebo-tang, a decoction with 10 medicinal herbs, has been used to treat patients with anemia, anorexia, or fatigue. The pharmacological activities of Sipjeondaebotang affect the systemic immune functions of T and B cells, macrophages and NK cells and cells of the hematopoietic and intestinal immune system. Nakamoto et al. evaluated the effects of Sipjeondaebo-tang in patients receiving hemodialysis with erythropoietin-resistant anemia [41]. Their results showed that Sipjeondaebotang improved erythropoietin-resistant anemia in endstage renal disease patients, and similar findings were observed in the perioperative period [144]. In addition, Sipjeondaebo-tang exerted a protective influence against intractable and recurrent infections in immature immune systems [43]. Thus, the effects of Sipjeondaebo-tang on the immune system have been confirmed by clinical trials and animal experiments, providing experimental evidence in support of the traditional use of Sipjeondaebo-tang.
Bojungikki-tang (補中益氣湯, Bu-zhong-yi-qi-tang in Chinese or Hochu-ekki-to in Japanese) has been widely used in China, Japan, and Korea for chronic fatigue syndrome and is composed of 10 species of medicinal herbs. In particular, in Bojungikki-tang, Ginseng Radix and Astragali Radix are used in higher proportions. Several clinical trials were conducted to examine whether this formulation alleviates cancer-related fatigue and improves immunological capacity in elderly patients. The results of these trials suggested that Bojungikki-tang could help to improve age- or disease-related impairment in immune function [55,62]. Animal studies also showed that Bojungikki-tang exhibits immunopharmacological activity against microbial infections, and positive results were obtained in a murine model of chronic fatigue syndrome [57,82,145]. Although the main active ingredient in Bojungikki-tang has not been elucidated, Ginseng Radix and Astragali Radix could contribute to the effects of Bojungikki-tang [62]. Insamyangyoung-tang (人蔘養營湯, Ren-shen-yang-rong-tang in Chinese or Ninjin-youeito in Japanese) is another prescription for the modulation of physiological immunity [85].
Several ginseng-containing prescriptions, including Daegeonjung-tang (大建中湯, Da Jian Zhong Tang in Chinese or Dai-kenchu-to in Japanese), Insam-tang (人蔘湯, Ninjin-to in Japanese or Ren Shen Tang in Chinese), and Banhasasim-tang, have been used to treat gastrointestinal problems, such as gastric atony, vomiting and anorexia. Substantial effort has been made to elucidate the pharmacological activities of these formulations. In a human study, Daegeonjung-tang increased gastrointestinal motility and improved ileal function by increasing motilin and vasoactive intestinal peptide levels in the plasma [146,147]. Postoperative ileus is an adverse consequence of abdominal surgery and leads to prolonged periods of hospitalization and increased healthcare costs [148]. Itoh et al. [148] reported the effectiveness of Daegeonjungtang on postoperative ileus, and this prescription has attracted attention for the treatment of postoperative ileus in Japan [149,150]. Insam-tang, which has been used for the treatment of gastroenteritis, gastric atony, gastrectasis, vomiting, and anorexia, is also useful for the treatment of postoperative ileus [151]. Because ginseng is not the main ingredient of either of these prescriptions, the active compound of Insam-tang may be 6-gingerol or 6-shogaol from Zingiberis Rhizome [104,151]. However, ginseng cannot be excluded as a contributor to gastrointestinal motility. Recently, Banhasasim-tang was reported to be effective against irinotecan-induced diarrhea [5]. Kase et al. [6] suggested that Ginseng Radix and Zingiberis Rhizome in Banhasasim-tang accelerate gastrointestinal motility.
Alzheimer’s disease is the most common type of degenerative dementia. Cholinergic hypofunction and inflammatory responses induced by amyloid-β protein are the main causes of this disease. Consequently, acetylcholinesterase inhibitors are clinically used for the treatment of Alzheimer’s disease, and anti-oxidative or anti-inflammatory agents may also be applicable because a redox imbalance is observed in the brains of Alzheimer’s disease sufferers [152]. In addition, degenerative changes in the forebrain cholinergic system are reportedly linked to oxidative stress [153]. Ginseng is a well-known, anti-aging herb, and cognitive functions decrease gradually with age. Several traditional herbal prescriptions containing ginseng have been demonstrated to enhance cognitive activities. Saengmaek-san (生脈散, Shengmaisan in Chinese), which is composed of 3 different kinds of herbs (Ginseng Radix, Ophiopogon Rhizome, and Schisandrae Fructus), has been used to treat symptoms related to cardiovascular diseases, such as heart failure and stroke. This formulation has protective qualities against oxidative damage in vitro and in vivo [154,155]. Recently, Saengmaek-san, which possesses anti-oxidative properties, was reported to ameliorate scopolamine-induced cognitive dysfunctions via acetylcholinesterase inhibition [106]. In addition, DX-9386, which consists of Ginseng Radix, Polygalae Radix, Acorus Radix and Hoelen and is identical to Jeongji-whan, has anti-aging and memory-ameliorating effects [156,157]. Egashira et al. [84] reported that Insamyangyoung-tang may ameliorate memory dysfunction by enhancing the cholinergic system. Guibi-tang was described in historical documents as an effective prescription for psychotic problems such as insomnia, amnesia, depression, and neurosis. Recently, the administration of Guibi-tang for 3 consecutive days was shown to ameliorate spatial and object-recognition memories in amyloid β protein-injected mice [35], suggesting that this formulation is an attractive candidate for anti-dementia drugs. Similar activities were also observed in Kyungok-Ko [158]. In these formulae, ginseng likely exerts its pharmacological effects on the central nervous system.
Diabetes is another serious disease, and its symptoms include severe polydipsia and polyuria. Baekho-ga- Insam-tang is a herbal medicine used for the relief of diuresis, thirst and dermal pruritus, which are associated with diabetes [124]. This result has been confirmed by experiments performed on, KKAy mice, which are genetic animal models of diabetes mellitus [159]. The administration of Baekho-ga-Insam-tang relieves diuresis, thirst, and dermal pruritus by increasing kidney aquaporin 2 and skin aquaporin expression. In addition, Baekhoga- Insam-tang enhances salivary secretion by increasing aquaporin 5 in a rat thirst model [126], suggesting that this formulation could be useful for xerostomia. Thus, the functions and efficacies of the aforementioned ginseng-containing prescriptions have been confirmed by modern experimental pharmacology. We summarized the results of in vivo animal or human studies on ginseng-containing prescriptions in Table 1, which includes the prescriptions mentioned in the present review.
THE VARIETY OF SPECIES IN THE PLANT GENUS PANAX
There are 13 species of ginseng in the Araliaceae Family, including [1] 1) P. ginseng Meyer, 2) P. quinquefolius L., 3) P. japonicus L., 4) P. notoginseng (Burk.) FH Chen, 5) P. pseudoginseng Wallich, 6) P. vietnamensis Ha et Grushv, 7) P. omeiensis J. Wen, 8) P. trifolius L., 9) P. sinensis J. Wen, 10) P. stipuleanatus HT Tsai & KM Feng, 11) P. wangianus Sun, 12) P. zingiberensis CY Wu & KM Feng, and 13) P. major Ting. The most commonly used Panax species include plants 1) to 6) in the list above [160]. P. vietnamensis was found in the mountainous regions of central Vietnam in the 1970s, and P. pseudoginseng has been used as a folk medicine in the Himalayas [161].
Since the pioneering work of Dr. Israel I. Brekhman, a Russian scientist, P. ginseng has been extensively studied [162,163]. For example, in 1964, Wood et al. [164] reported the vasodilatory effect of P. ginseng obtained from Keumsan, Korea, and in 1971, Lee and Huemer [165] reported the antitumor activity of ginseng obtained from Kangwhado, Korea. The Shibata group in Japan has studied the chemistry of the saponins and sapogenins of the white ginseng, P. ginseng, which is cultivated in Japan [161].
Hsu [166] reported the pharmacological activities of P. shinseng Nies, var. notoginseng Burkill and published a picture of P. shinseng var. notoginseng, which has a different morphology than P. ginseng. According to Hsu’s studies, P. shinseng var. notoginseng was first employed as a medicine not much earlier than the time of Shizhen Li’s first report in the Bencao Gangmu. Therefore, this species of ginseng was not mentioned in texts on Chinese medicine produced before this period [166]. Thus, although other species of ginseng are also active, P. ginseng is the most popular and active species.
The therapeutic potency of ginseng is dependent on its geographical locality, dosage, and processing. Red ginseng and sun ginseng refer to processed ginseng [167,168]. Several studies have been conducted to compare the effects of different species of ginseng. For wound healing and hypoglycemic effects, P. ginseng is superior to P. quinquefolius, whereas P. quinquefolius displays better anticancer effects [169]. Similarly, P. ginseng (ginseng in Korea) has the highest therapeutic potency for the treatment of diabetes. P. quinquefolius (American ginseng) is a medium-potency-grade ginseng, and P. japonicus (Japanese ginseng) is considered a lowpotency- grade ginseng [170].
PERSPECTIVES
In the future, a comprehensive and objective evaluation of ginseng should be conducted using evidence-based medicine to further elucidate the intriguing properties of ginseng in terms of treatment of various diseases shown in recent review articles [171,172].
CONCLUSION
In the present review, ethnopharmacological accounts of P. ginseng were discussed. Since the description of ginseng from the Korean Peninsula was first published in the Bencao Gangmu, P. ginseng has become the most commonly used therapeutic ginseng. Modern chemical and biological drugs are suitable for the treatment of diseases with specific causes and pathologies but are not suitable for diseases with multiple factors, such as diabetes, Alzheimer’s disease, and chronic fatigue. Based on historical accounts and recent experimental or clinical studies, ginseng and ginseng-containing herbal prescriptions are useful for the treatment of chronic diseases.
References
- 1.Yun TK. Brief Introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16(Suppl):S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenthal M, Goldberg A, Brinckmann J. Herbal medicine: expanded Commission E monographs. Integrative Medicine Communications; Austin: 2000. [Google Scholar]
- 3.Park JW, Ryu B, Yeo I, Jerng UM, Han G, Oh S, Lee J, Kim J. Banha-sasim-tang as an herbal formula for the treatment of functional dyspepsia: a randomized, double-blind, placebo-controlled, two-center trial. Trials. 2010;11:83. doi: 10.1186/1745-6215-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori K, Kondo T, Kamiyama Y, Kano Y, Tominaga K. Preventive effect of Kampo medicine (Hangeshashinto) against irinotecan-induced diarrhea in advanced nonsmall-cell lung cancer. Cancer Chemother Pharmacol. 2003;51:403–406. doi: 10.1007/s00280-003-0585-0. [DOI] [PubMed] [Google Scholar]
- 5.Naito T, Itoh H, Takeyama M. Comparison of the effects of hange-shashin-to and rikkunshi-to on human plasma calcitonin gene-related peptide and substance P levels. Biol Pharm Bull. 2003;26:1104–1107. doi: 10.1248/bpb.26.1104. [DOI] [PubMed] [Google Scholar]
- 6.Kase Y, Saitoh K, Makino B, Hashimoto K, Ishige A, Komatsu Y. Relationship between the antidiarrhoeal effects of Hange-Shashin-To and its active components. Phytother Res. 1999;13:468–473. doi: 10.1002/(sici)1099-1573(199909)13:6<468::aid-ptr504>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Kim HM, Kim YY, Jang HY, Moon SJ, An NH. Action of Sosiho-Tang on systemic and local anaphylaxis by anal administration. Immunopharmacol Immunotoxicol. 1999;21:635–643. doi: 10.3109/08923979909007131. [DOI] [PubMed] [Google Scholar]
- 8.Deng G, Kurtz RC, Vickers A, Lau N, Yeung KS, Shia J, Cassileth B. A single arm phase II study of a Far-Eastern traditional herbal formulation (sho-sai-ko-to or xiao-chaihu- tang) in chronic hepatitis C patients. J Ethnopharmacol. 2011;136:83–87. doi: 10.1016/j.jep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Lee CH, Wang JD, Chen PC. Risk of liver injury associated with Chinese herbal products containing Radix bupleuri in 639,779 patients with hepatitis B virus infection. PLoS One. 2011;6:e16064. doi: 10.1371/journal.pone.0016064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang JS, Wang KC, Liu HW, Chen MC, Chiang LC, Lin CC. Sho-saiko-to (Xiao-Chai-Hu-Tang) and crude saikosaponins inhibit hepatitis B virus in a stable HBV-producing cell line. Am J Chin Med. 2007;35:341–351. doi: 10.1142/S0192415X07004862. [DOI] [PubMed] [Google Scholar]
- 11.Hsu LM, Huang YS, Tsay SH, Chang FY, Lee SD. Acute hepatitis induced by Chinese hepatoprotective herb, xiaochai- hu-tang. J Chin Med Assoc. 2006;69:86–88. doi: 10.1016/S1726-4901(09)70119-4. [DOI] [PubMed] [Google Scholar]
- 12.Itoh S, Marutani K, Nishijima T, Matsuo S, Itabashi M. Liver injuries induced by herbal medicine, syo-saiko-to (xiao-chai-hu-tang). Dig Dis Sci. 1995;40:1845–1848. doi: 10.1007/BF02212712. [DOI] [PubMed] [Google Scholar]
- 13.Tajiri H, Kozaiwa K, Ozaki Y, Miki K, Shimuzu K, Okada S. Effect of sho-saiko-to(xiao-chai-hu-tang) on HBeAg clearance in children with chronic hepatitis B virus infection and with sustained liver disease. Am J Chin Med. 1991;19:121–129. doi: 10.1142/S0192415X91000193. [DOI] [PubMed] [Google Scholar]
- 14.Hirayama C, Okumura M, Tanikawa K, Yano M, Mizuta M, Ogawa N. A multicenter randomized controlled clinical trial of Shosaiko-to in chronic active hepatitis. Gastroenterol Jpn. 1989;24:715–719. doi: 10.1007/BF02774173. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Furusawa S, Iizuka Y. Preventive effects of a traditional Chinese medicine (Sho-saiko-to) on septic shock symptoms; approached from heme metabolic disorders in endotoxemia. Biol Pharm Bull. 2005;28:165–168. doi: 10.1248/bpb.28.165. [DOI] [PubMed] [Google Scholar]
- 16.Chen MH, Chen JC, Tsai CC, Wang WC, Chang DC, Lin CC, Hsieh HY. Sho-saiko-to prevents liver fibrosis induced by bile duct ligation in rats. Am J Chin Med. 2004;32:195–207. doi: 10.1142/S0192415X04001862. [DOI] [PubMed] [Google Scholar]
- 17.Taira Z, Yabe K, Hamaguchi Y, Hirayama K, Kishimoto M, Ishida S, Ueda Y. Effects of Sho-saiko-to extract and its components, Baicalin, baicalein, glycyrrhizin and glycyrrhetic acid, on pharmacokinetic behavior of salicylamide in carbon tetrachloride intoxicated rats. Food Chem Toxicol. 2004;42:803–807. doi: 10.1016/j.fct.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Kusunose M, Qiu B, Cui T, Hamada A, Yoshioka S, Ono M, Miyamura M, Kyotani S, Nishioka Y. Effect of Shosaiko- to extract on hepatic inflammation and fibrosis in dimethylnitrosamine induced liver injury rats. Biol Pharm Bull. 2002;25:1417–1421. doi: 10.1248/bpb.25.1417. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara K, Mochida S, Nagoshi S, Iijima O, Matsuzaki Y, Takeda S, Aburada M. Regulation of hepatic macrophage function by oral administration of xiao-chai-hu-tang (shosaiko- to, TJ-9) in rats. J Ethnopharmacol. 1995;46:107–114. doi: 10.1016/0378-8741(95)01235-6. [DOI] [PubMed] [Google Scholar]
- 20.Tatsuta M, Iishi H, Baba M, Nakaizumi A, Uehara H. Inhibition by xiao-chai-hu-tang (TJ-9) of development of hepatic foci induced by N-nitrosomorpholine in Sprague- Dawley rats. Jpn J Cancer Res. 1991;82:987–992. doi: 10.1111/j.1349-7006.1991.tb01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaginuma T, Okamura T, Takeuchi T, Nishii O, Fujimori R. Preventive effect of traditional herbal medicine, shosaikoto, on danazol-induced hepatic damage. Int J Gynaecol Obstet. 1989;29:337–341. doi: 10.1016/0020-7292(89)90359-7. [DOI] [PubMed] [Google Scholar]
- 22.Amagaya S, Hayakawa M, Ogihara Y, Ohta Y, Fujiwara K, Oka H, Oshio H, Kishi T. Treatment of chronic liver injury in mice by oral administration of xiao-chai-hu-tang. J Ethnopharmacol. 1989;25:181–187. doi: 10.1016/0378-8741(89)90020-2. [DOI] [PubMed] [Google Scholar]
- 23.Borchers AT, Sakai S, Henderson GL, Harkey MR, Keen CL, Stern JS, Terasawa K, Gershwin ME. Shosaiko-to and other Kampo (Japanese herbal) medicines: a review of their immunomodulatory activities. J Ethnopharmacol. 2000;73:1–13. doi: 10.1016/s0378-8741(00)00334-2. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko M, Kawakita T, Tauchi Y, Saito Y, Suzuki A, Nomoto K. Augmentation of NK activity after oral administration of a traditional Chinese medicine, xiao-chaihu- tang (shosaiko-to). Immunopharmacol Immunotoxicol. 1994;16:41–53. doi: 10.3109/08923979409029899. [DOI] [PubMed] [Google Scholar]
- 25.Tauchi Y, Yamada A, Kawakita T, Saito Y, Suzuki A, Yoshikai Y, Nomoto K. Enhancement of immunoglobulin A production in Peyer’ s patches by oral administration of a traditional Chinese medicine, xiao-chai-hutang (Shosaiko-to). Immunopharmacol Immunotoxicol. 1993;15:251–272. doi: 10.3109/08923979309025998. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura K, Kawakita T, Nakai S, Saito Y, Suzuki A, Nomoto K. Role of B-lymphocytes in the immunopharmacological effects of a traditional Chinese medicine, xiao-chai-hu-tang (shosaiko-to). Int J Immunopharmacol. 1993;15:237–243. doi: 10.1016/0192-0561(93)90100-d. [DOI] [PubMed] [Google Scholar]
- 27.Kawakita T, Nakai S, Kumazawa Y, Miura O, Yumioka E, Nomoto K. Induction of interferon after administration of a traditional Chinese medicine, xiao-chai-hu-tang (shosaiko-to). Int J Immunopharmacol. 1990;12:515–521. doi: 10.1016/0192-0561(90)90115-4. [DOI] [PubMed] [Google Scholar]
- 28.Kawakita T, Mitsuyama M, Kumazawa Y, Miura O, Yumioka E, Nomoto K. Contribution of cytokines to time-dependent augmentation of resistance against Listeria monocytogenes after administration of a traditional Chinese medicine, xiao-chai-hu-tang (Japanese name: shosaiko-to). Immunopharmacol Immunotoxicol. 1989;11:233–255. doi: 10.3109/08923978909005368. [DOI] [PubMed] [Google Scholar]
- 29.Kawakita T, Yamada A, Mitsuyama M, Kumazawa Y, Nomoto K. Protective effect of a traditional Chinese medicine, xiao-chai-hu-tang (Japanese name: shosaiko-to), on Listeria monocytogenes infection in mice. Immunopharmacol Immunotoxicol. 1988;10:345–364. doi: 10.3109/08923978809041426. [DOI] [PubMed] [Google Scholar]
- 30.Kumazawa Y, Takimoto H, Miura S, Nishimura C, Yamada A, Kawakita T, Nomoto K. Activation of murine peritoneal macrophages by intraperitoneal administration of a traditional Chinese herbal medicine, xiao-chai-hutang (Japanese name: shosaiko-to). Int J Immunopharmacol. 1988;10:395–403. doi: 10.1016/0192-0561(88)90126-9. [DOI] [PubMed] [Google Scholar]
- 31.Kawakita T, Yamada A, Kumazawa Y, Nomoto K. Functional maturation of immature B cells accumulated in the periphery by an intraperitoneal administration of a traditional Chinese medicine, xiao-chai-hu-tang (Japanese name: shosaiko-to). Immunopharmacol Immunotoxicol. 1987;9:299–317. doi: 10.3109/08923978709035216. [DOI] [PubMed] [Google Scholar]
- 32.Kawakita T, Yamada A, Mitsuyama M, Kumazawa Y, Nomoto K. Protective effect of a traditional Chinese medicine, xiao-chai-hu-tang (Japanese name: shosaikoto), on Pseudomonas aeruginosa infection in mice. Immunopharmacol Immunotoxicol. 1987;9:523–540. doi: 10.3109/08923978709035230. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Song QH, Hong T, Kitamura H, Cyong JC. Preventive effect of Ninjin-to (Ren-Shen-Tang), a Kampo (Japanese traditional) formulation, on spontaneous autoimmune diabetes in non-obese diabetic (NOD) mice. Microbiol Immunol. 2000;44:299–305. doi: 10.1111/j.1348-0421.2000.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 34.Oh MS, Huh Y, Bae H, Ahn DK, Park SK. The multiherbal formula Guibi-tang enhances memory and increases cell proliferation in the rat hippocampus. Neurosci Lett. 2005;379:205–208. doi: 10.1016/j.neulet.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 35.Tohda C, Naito R, Joyashiki E. Kihi-to, a herbal traditional medicine, improves Abeta(25-35)-induced memory impairment and losses of neurites and synapses. BMC Complement Altern Med. 2008;8:49. doi: 10.1186/1472-6882-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanai S, Taniguchi N, Higashino H. Effect of kami-kihito (jia-wei-gui-pi-tang) for experimental osteopenia. Am J Chin Med. 2005;33:41–48. doi: 10.1142/S0192415X05002643. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzaki T, Nomura S, Yamaoka M, Ozaki Y, Yoshimura C, Xie GL, Katsura K, Kagawa H, Ishida T, Fukuhara S. HLA and HPA typing in idiopathic thrombocytopenic purpura patients treated with Kami-kihi-to. Am J Chin Med. 1998;26:191–198. doi: 10.1142/S0192415X98000245. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi K, Kido H, Kawakatsu T, Fukuroi T, Suzuki M, Yanabu M, Nomura S, Kokawa T, Yasunaga K. Effects of kami-kihi-to (jia-wei-gui-pi-tang) on autoantibodies in patients with chronic immune thrombocytopenic purpura. Am J Chin Med. 1993;21:251–255. doi: 10.1142/S0192415X93000297. [DOI] [PubMed] [Google Scholar]
- 39.Hara H, Kataoka S, Anan M, Ueda A, Mutoh T, Tabira T. The therapeutic effects of the herbal medicine, Juzentaiho- to, on amyloid-beta burden in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;20:427–439. doi: 10.3233/JAD-2010-1381. [DOI] [PubMed] [Google Scholar]
- 40.Chino A, Okamoto H, Hirasaki Y, Terasawa K. A case of atopic dermatitis successfully treated with juzentaihoto (Kampo). Altern Ther Health Med. 2010;16:62–64. [PubMed] [Google Scholar]
- 41.Nakamoto H, Mimura T, Honda N. Orally administrated Juzen-taiho-to/TJ-48 ameliorates erythropoietin (rHuEPO)-resistant anemia in patients on hemodialysis. Hemodial Int. 2008;12(Suppl 2):S9–S14. doi: 10.1111/j.1542-4758.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- 42.Sho Y, Fujisaki K, Sakashita H, Yamaguchi K, Tahara K, Kubozono O, Ido A, Tsubouchi H. Orally administered Kampo medicine, Juzen-taiho-to, ameliorates anemia during interferon plus ribavirin therapy in patients with chronic hepatitis C. J Gastroenterol. 2004;39:1202–1204. doi: 10.1007/s00535-004-1472-0. [DOI] [PubMed] [Google Scholar]
- 43.Maruyama Y, Hoshida S, Furukawa M, Ito M. Effects of Japanese herbal medicine, Juzen-taiho-to, in otitisprone children: a preliminary study. Acta Otolaryngol. 2009;129:14–18. doi: 10.1080/00016480801998838. [DOI] [PubMed] [Google Scholar]
- 44.Tagami K, Niwa K, Lian Z, Gao J, Mori H, Tamaya T. Preventive effect of Juzen-taiho-to on endometrial carcinogenesis in mice is based on Shimotsu-to constituent. Biol Pharm Bull. 2004;27:156–161. doi: 10.1248/bpb.27.156. [DOI] [PubMed] [Google Scholar]
- 45.Lian Z, Niwa K, Gao J, Tagami K, Hashimoto M, Yokoyama Y, Mori H, Tamaya T. Shimotsu-to is the agent in Juzen-taiho-to responsible for the prevention of endometrial carcinogenesis in mice. Cancer Lett. 2002;182:19–26. doi: 10.1016/s0304-3835(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 46.Niwa K, Hashimoto M, Morishita S, Lian Z, Tagami K, Mori H, Tamaya T. Preventive effects of Juzen-taiho-to on N-methyl-N-nitrosourea and estradiol-17beta-induced endometrial carcinogenesis in mice. Carcinogenesis. 2001;22:587–591. doi: 10.1093/carcin/22.4.587. [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto S, Kudo H, Kuwa K, Suzuki S, Kato T, Kawasaki T, Nakayama T, Kasahara N, Okamoto R. Anticancer effects of a Chinese herbal medicine, juzen-taiho-to, in combination with or without 5-fluorouracil derivative on DNA-synthesizing enzymes in 1,2-dimethylhydrazine induced colonic cancer in rats. Am J Chin Med. 1991;19:233–241. doi: 10.1142/S0192415X91000314. [DOI] [PubMed] [Google Scholar]
- 48.Kiyohara H, Matsumoto T, Yamada H. Intestinal immune system modulating polysaccharides in a Japanese herbal (Kampo) medicine, Juzen-Taiho-To. Phytomedicine. 2002;9:614–624. doi: 10.1078/094471102321616427. [DOI] [PubMed] [Google Scholar]
- 49.Dai Y, Kato M, Takeda K, Kawamoto Y, Akhand AA, Hossain K, Suzuki H, Nakashima I. T-cell-immunity-based inhibitory effects of orally administered herbal medicine juzen-taiho-to on the growth of primarily developed melanocytic tumors in RET-transgenic mice. J Invest Dermatol. 2001;117:694–701. doi: 10.1046/j.0022-202x.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto T, Yamada H. Orally administered Kampo (Japanese herbal) medicine, “ Juzen-Taiho-To” modulates cytokine secretion in gut associated lymphoreticular tissues in mice. Phytomedicine. 2000;6:425–430. doi: 10.1016/S0944-7113(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 51.Kiyohara H, Matsumoto T, Yamada H. Lignin-carbohydrate complexes: intestinal immune system modulating ingredients in kampo (Japanese herbal) medicine, juzentaiho-to. Planta Med. 2000;66:20–24. doi: 10.1055/s-2000-11116. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto T, Sakurai MH, Kiyohara H, Yamada H. Orally administered decoction of Kampo (Japanese herbal) medicine, “Juzen-Taiho-To” modulates cytokine secretion and induces NKT cells in mouse liver. Immunopharmacology. 2000;46:149–161. doi: 10.1016/s0162-3109(99)00166-6. [DOI] [PubMed] [Google Scholar]
- 53.Iijima K, Sun S, Cyong JC, Jyonouchi H. Juzen-taiho-to, a Japanese herbal medicine, modulates type 1 and type 2 T cell responses in old BALB/ c mice. Am J Chin Med. 1999;27:191–203. doi: 10.1142/S0192415X99000239. [DOI] [PubMed] [Google Scholar]
- 54.Abe S, Tansho S, Ishibashi H, Inagaki N, Komatsu Y, Yamaguchi H. Protective effect of oral administration of a traditional medicine, Juzen-Taiho-To, and its components on lethal Candida albicans infection in immunosuppressed mice. Immunopharmacol Immunotoxicol. 1998;20:421–431. doi: 10.3109/08923979809034824. [DOI] [PubMed] [Google Scholar]
- 55.Jeong JS, Ryu BH, Kim JS, Park JW, Choi WC, Yoon SW. Bojungikki-tang for cancer-related fatigue: a pilot randomized clinical trial. Integr Cancer Ther. 2010;9:331–338. doi: 10.1177/1534735410383170. [DOI] [PubMed] [Google Scholar]
- 56.Shin HY, Shin CH, Shin TY, Lee EJ, Kim HM. Effect of bojungikki-tang on lipopolysaccharide-induced cytokine production from peripheral blood mononuclear cells of chronic fatigue syndrome patients. Immunopharmacol Immunotoxicol. 2003;25:491–501. doi: 10.1081/iph-120026435. [DOI] [PubMed] [Google Scholar]
- 57.Chen R, Moriya J, Luo X, Yamakawa J, Takahashi T, Sasaki K, Yoshizaki F. Hochu-ekki-to combined with interferon-gamma moderately enhances daily activity of chronic fatigue syndrome mice by increasing NK cell activity, but not neuroprotection. Immunopharmacol Immunotoxicol. 2009;31:238–245. doi: 10.1080/08923970802391525. [DOI] [PubMed] [Google Scholar]
- 58.Chen R, Moriya J, Yamakawa J, Takahashi T, Li Q, Morimoto S, Iwai K, Sumino H, Yamaguchi N, Kanda T. Brain atrophy in a murine model of chronic fatigue syndrome and beneficial effect of Hochu-ekki-to (TJ-41). Neurochem Res. 2008;33:1759–1767. doi: 10.1007/s11064-008-9620-1. [DOI] [PubMed] [Google Scholar]
- 59.Liu XQ, Wu L, Guo XJ. Effect of Bu-Zhong-Yi-Qi-Tang on deficiency of N-glycan/nitric oxide and islet damage induced by streptozotocin in diabetic rats. World J Gastroenterol. 2009;15:1730–1737. doi: 10.3748/wjg.15.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tatsumi K, Shinozuka N, Nakayama K, Sekiya N, Kuriyama T, Fukuchi Y. Hochuekkito improves systemic inflammation and nutritional status in elderly patients with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2009;57:169–170. doi: 10.1111/j.1532-5415.2009.02034.x. [DOI] [PubMed] [Google Scholar]
- 61.Kimura M, Sasada T, Kanai M, Kawai Y, Yoshida Y, Hayashi E, Iwata S, Takabayashi A. Preventive effect of a traditional herbal medicine, Hochu-ekki-to, on immunosuppression induced by surgical stress. Surg Today. 2008;38:316–322. doi: 10.1007/s00595-007-3631-4. [DOI] [PubMed] [Google Scholar]
- 62.Kuroiwa A, Liou S, Yan H, Eshita A, Naitoh S, Nagayama A. Effect of a traditional Japanese herbal medicine, hochu-ekki-to (Bu-Zhong-Yi-Qi Tang), on immunity in elderly persons. Int Immunopharmacol. 2004;4:317–324. doi: 10.1016/j.intimp.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Nabeshima S, Murata M, Hamada M, Chong Y, Yamaji K, Hayashi J. Maturation of monocyte-derived dendritic cells by Hochu-ekki-to, a traditional Japanese herbal medicine. Int Immunopharmacol. 2004;4:37–45. doi: 10.1016/j.intimp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Furuya Y, Akashi T, Fuse H. Effect of Bu-zhong-yi-qitang on seminal plasma cytokine levels in patients with idiopathic male infertility. Arch Androl. 2004;50:11–14. doi: 10.1080/01485010490250515. [DOI] [PubMed] [Google Scholar]
- 65.Matsumoto T, Noguchi M, Hayashi O, Makino K, Yamada H. Hochuekkito, a Kampo (traditional Japanese herbal) medicine, enhances mucosal IgA antibody response in mice immunized with antigen-entrapped biodegradable microparticles. Evid Based Complement Alternat Med. 2010;7:69–77. doi: 10.1093/ecam/nem166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiyohara H, Nagai T, Munakata K, Nonaka K, Hanawa T, Kim SJ, Yamada H. Stimulating effect of Japanese herbal (kampo) medicine, hochuekkito on upper respiratory mucosal immune system. Evid Based Complement Alternat Med. 2006;3:459–467. doi: 10.1093/ecam/nel030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi H, Mizuno N, Kutsuna H, Teramae H, Ueoku S, Onoyama J, Yamanaka K, Fujita N, Ishii M. Hochu-ekkito suppresses development of dermatitis and elevation of serum IgE level in NC/Nga mice. Drugs Exp Clin Res. 2003;29:81–84. [PubMed] [Google Scholar]
- 68.Nakada T, Watanabe K, Matsumoto T, Santa K, Triizuka K, Hanawa T. Effect of orally administered Hochu-ekki-to, a Japanese herbal medicine, on contact hypersensitivity caused by repeated application of antigen. Int Immunopharmacol. 2002;2:901–911. doi: 10.1016/s1567-5769(02)00027-9. [DOI] [PubMed] [Google Scholar]
- 69.Yamaoka Y, Kawakita T, Nomoto K. Protective effect of a traditional Japanese medicine Hochu-ekki-to (Chinese name: Bu-zhong-yi-qi-tang), on the susceptibility against Listeria monocytogenes in infant mice. Int Immunopharmacol. 2001;1:1669–1677. doi: 10.1016/s1567-5769(01)00076-5. [DOI] [PubMed] [Google Scholar]
- 70.Ishimitsu R, Nishimura H, Kawauchi H, Kawakita T, Yoshikai Y. Dichotomous effect of a traditional Japanese medicine, bu-zhong-yi-qi-tang on allergic asthma in mice. Int Immunopharmacol. 2001;1:857–865. doi: 10.1016/s1567-5769(01)00022-4. [DOI] [PubMed] [Google Scholar]
- 71.Kido T, Mori K, Daikuhara H, Tsuchiya H, Ishige A, Sasaki H. The protective effect of hochu-ekki-to (TJ-41), a Japanese herbal medicine, against HSV-1 infection in mitomycin C-treated mice. Anticancer Res. 2000;20:4109–4113. [PubMed] [Google Scholar]
- 72.Harada M, Seta K, Ito O, Tamada K, Li T, Terao H, Takenoyama M, Kimura G, Nomoto K. Concomitant immunity against tumor development is enhanced by the oral administration of a kampo medicine, Hochu-ekki-to (TJ- 41: Bu-Zhong-Yi-Qi-Tang). Immunopharmacol Immunotoxicol. 1995;17:687–703. doi: 10.3109/08923979509037189. [DOI] [PubMed] [Google Scholar]
- 73.Li XY, Takimoto H, Miura S, Yoshikai Y, Matsuzaki G, Nomoto K. Effect of a traditional Chinese medicine, buzhong-yi-qi-tang (Japanese name: Hochu-ekki-to) on the protection against Listeria monocytogenes infection in mice. Immunopharmacol Immunotoxicol. 1992;14:383–402. doi: 10.3109/08923979209005400. [DOI] [PubMed] [Google Scholar]
- 74.Tsuneoka N, Tajima Y, Kitasato A, Fukuda K, Kitajima T, Adachi T, Mishima T, Kuroki T, Onizuka S, Kanematsu T. Chemopreventative effect of hochu-ekki-to (TJ-41) on chemically induced biliary carcinogenesis in hamsters. J Surg Res. 2009;151:22–27. doi: 10.1016/j.jss.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 75.Tajima S, Bando M, Yamasawa H, Ohno S, Moriyama H, Terada M, Takada T, Suzuki E, Gejyo F, Sugiyama Y. Preventive effect of hochu-ekki-to, a Japanese herbal medicine, on bleomycin-induced lung injury in mice. Respirology. 2007;12:814–822. doi: 10.1111/j.1440-1843.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- 76.Yang SH, Yu CL. Antiinflammatory effects of Bu-zhongyi-qi-tang in patients with perennial allergic rhinitis. J Ethnopharmacol. 2008;115:104–109. doi: 10.1016/j.jep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Shinozuka N, Tatsumi K, Nakamura A, Terada J, Kuriyama T. The traditional herbal medicine Hochuekkito improves systemic inflammation in patients with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2007;55:313–314. doi: 10.1111/j.1532-5415.2007.01057.x. [DOI] [PubMed] [Google Scholar]
- 78.Tajima S, Bando M, Yamasawa H, Ohno S, Moriyama H, Takada T, Suzuki E, Gejyo F, Sugiyama Y. Preventive effect of Hochu-ekki-to on lipopolysaccharide-induced acute lung injury in BALB/c mice. Lung. 2006;184:318–323. doi: 10.1007/s00408-006-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanehara M, Ogirima T, Tano K, Maenaka T, Ishida T, Zhang B, Li G, Wang X, Guo Y. Effects of Chinese herbal medicine based on hachimi-jio-gan on osteopenia in rats. J Tradit Chin Med. 2006;26:72–77. [PubMed] [Google Scholar]
- 80.Kobayashi H, Mizuno N, Teramae H, Kutsuna H, Ueoku S, Onoyama J, Yamanaka K, Fujita N, Ishii M. The effects of Hochu-ekki-to in patients with atopic dermatitis resistant to conventional treatment. Int J Tissue React. 2004;26:113–117. [PubMed] [Google Scholar]
- 81.Ochi T, Kawakita T, Nomoto K. Effects of Hochu-ekkito and Ninjin-youei-to, traditional Japanese medicines, on porcine serum-induced liver fibrosis in rats. Immunopharmacol Immunotoxicol. 2004;26:285–298. doi: 10.1081/iph-120037726. [DOI] [PubMed] [Google Scholar]
- 82.Yan X, Kita M, Minami M, Yamamoto T, Kuriyama H, Ohno T, Iwakura Y, Imanishi J. Antibacterial effect of Kampo herbal formulation Hochu-ekki-to (Bu-Zhong-Yi-Qi-Tang) on Helicobacter pylori infection in mice. Microbiol Immunol. 2002;46:475–482. doi: 10.1111/j.1348-0421.2002.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 83.Takano F, Ohta Y, Tanaka T, Sasaki K, Kobayashi K, Takahashi T, Yahagi N, Yoshizaki F, Fushiya S, Ohta T. Oral administration of Ren-shen-yang-rong-tang ‘Ninjin’ yoeito’ protects against hematotoxicity and induces immature erythroid progenitor cells in 5-fluorouracilinduced anemia. Evid Based Complement Alternat Med. 2009;6:247–256. doi: 10.1093/ecam/nem080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Egashira N, Yuzurihara M, Hattori N, Sakakibara I, Ishige A. Ninjin-yoei-to (Ren-Shen-Yang-Rong-Tang) and Polygalae radix improves scopolamine-induced impairment of passive avoidance response in mice. Phytomedicine. 2003;10:467–473. doi: 10.1078/094471103322331403. [DOI] [PubMed] [Google Scholar]
- 85.Nakada T, Watanabe K, Jin GB, Triizuk K, Hanawa T. Effect of ninjin-youei-to on Th1/ Th2 type cytokine production in different mouse strains. Am J Chin Med. 2002;30:215–223. doi: 10.1142/S0192415X0200034X. [DOI] [PubMed] [Google Scholar]
- 86.Nakai S, Kawakita T, Himeno K, Nomoto K. Combined treatments with Ninjin-youei-to (Ren-shen-yang-rongtang) plus a suboptimal dose of prednisolone on autoimmune nephritis in MRL/ lpr mice. Int J Immunopharmacol. 1998;20:275–284. doi: 10.1016/s0192-0561(98)00032-0. [DOI] [PubMed] [Google Scholar]
- 87.Nakai S, Kawakita T, Nagasawa H, Himeno K, Nomoto K. Thymus-dependent effects of a traditional Chinese medicine, ren-shen-yang-rong-tang (Japanese name; Ninjinyouei- to), in autoimmune MRI/ MP-lpr/ lpr mice. Int J Immunopharmacol. 1996;18:271–279. doi: 10.1016/0192-0561(96)84507-3. [DOI] [PubMed] [Google Scholar]
- 88.Nakai S, Kawakita T, Zhou NN, Matsuura K, Oka M, Nagasawa H, Saito Y, Suzuki A, Himeno K, Nomoto K. Treatment effect of a traditional Chinese medicine, renshen- yang-rong-tang (Japanese name: ninjin-youei-to), on autoimmune MRL/ MP-lpr/ lpr mice. Int J Immunopharmacol. 1993;15:589–596. doi: 10.1016/0192-0561(93)90076-b. [DOI] [PubMed] [Google Scholar]
- 89.Harigai E, Nakai S, Kawakita T, Nomoto K. Combined treatment with ren-shen-yang-rong-tang (Japanese name: ninjin-youei-to) plus prednisolone on adjuvant-induced arthritis in Lewis rat. Int J Immunopharmacol. 1995;17:411–418. doi: 10.1016/0192-0561(95)00018-w. [DOI] [PubMed] [Google Scholar]
- 90.Kono T, Kaneko A, Hira Y, Suzuki T, Chisato N, Ohtake N, Miura N, Watanabe T. Anti-colitis and -adhesion effects of daikenchuto via endogenous adrenomedullin enhancement in Crohn’s disease mouse model. J Crohns Colitis. 2010;4:161–170. doi: 10.1016/j.crohns.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 91.Narita M, Hatano E, Tamaki N, Yamanaka K, Yanagida A, Nagata H, Asechi H, Takada Y, Ikai I, Uemoto S. Daikenchu-to attenuates rat sinusoidal obstruction syndrome by inhibiting the accumulation of neutrophils in the liver. J Gastroenterol Hepatol. 2009;24:1051–1057. doi: 10.1111/j.1440-1746.2009.05795.x. [DOI] [PubMed] [Google Scholar]
- 92.Kawasaki N, Nakada K, Suzuki Y, Furukawa Y, Hanyu N, Kashiwagi H. Effect of Dai-kenchu-to on gastrointestinal motility and gastric emptying. Int J Surg. 2009;7:218–222. doi: 10.1016/j.ijsu.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 93.Kawasaki N, Nakada K, Nakayoshi T, Furukawa Y, Suzuki Y, Hanyu N, Yanaga K. Effect of Dai-kenchu-to on gastrointestinal motility based on differences in the site and timing of administration. Dig Dis Sci. 2007;52:2684–2694. doi: 10.1007/s10620-006-9391-y. [DOI] [PubMed] [Google Scholar]
- 94.Tokita Y, Yuzurihara M, Sakaguchi M, Satoh K, Kase Y. The pharmacological effects of Daikenchuto, a traditional herbal medicine, on delayed gastrointestinal transit in rat postoperative ileus. J Pharmacol Sci. 2007;104:303–310. doi: 10.1254/jphs.fp0070831. [DOI] [PubMed] [Google Scholar]
- 95.Tokita Y, Satoh K, Sakaguchi M, Endoh Y, Mori I, Yuzurihara M, Sakakibara I, Kase Y, Takeda S, Sasaki H. The preventive effect of Daikenchuto on postoperative adhesion-induced intestinal obstruction in rats. Inflammopharmacology. 2007;15:65–66. doi: 10.1007/s10787-006-1552-2. [DOI] [PubMed] [Google Scholar]
- 96.Fukuda H, Chen C, Mantyh C, Ludwig K, Pappas TN, Takahashi T. The herbal medicine, Dai-Kenchu-to, accelerates delayed gastrointestinal transit after the operation in rats. J Surg Res. 2006;131:290–295. doi: 10.1016/j.jss.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 97.Kito Y, Suzuki H. Effects of Dai-kenchu-to on spontaneous activity in the mouse small intestine. Effects of Dai-kenchu-to on spontaneous. 2006;42:489–201. doi: 10.1540/jsmr.42.189. [DOI] [PubMed] [Google Scholar]
- 98.Satoh K, Kase Y, Hayakawa T, Murata P, Ishige A, Sasaki H. Dai-kenchu-to enhances accelerated small intestinal movement. Biol Pharm Bull. 2001;24:1122–1126. doi: 10.1248/bpb.24.1122. [DOI] [PubMed] [Google Scholar]
- 99.Iwai N, Kume Y, Kimura O, Ono S, Aoi S, Tsuda T. Effects of herbal medicine Dai-Kenchu-to on anorectal function in children with severe constipation. Eur J Pediatr Surg. 2007;17:115–118. doi: 10.1055/s-2007-965016. [DOI] [PubMed] [Google Scholar]
- 100.Sakakibara R, Odaka T, Lui Z, Uchiyama T, Yamaguchi K, Yamaguchi T, Asahina M, Yamamoto T, Ito T, Hattori T. Dietary herb extract dai-kenchu-to ameliorates constipation in parkinsonian patients (Parkinson’s disease and multiple system atrophy). Mov Disord. 2005;20:261–261. doi: 10.1002/mds.20352. [DOI] [PubMed] [Google Scholar]
- 101.Satoh K, Hashimoto K, Hayakawa T, Ishige A, Kaneko M, Ogihara S, Kurosawa S, Yakabi K, Nakamura T. Mechanism of atropine-resistant contraction induced by Dai-kenchu-to in guinea pig ileum. Jpn J Pharmacol. 2001;86:32–37. doi: 10.1254/jjp.86.32. [DOI] [PubMed] [Google Scholar]
- 102.Satoh K, Hayakawa T, Kase Y, Ishige A, Sasaki H, Nishikawa S, Kurosawa S, Yakabi K, Nakamura T. Mechanisms for contractile effect of Dai-kenchu-to in isolated guinea pig ileum. Dig Dis Sci. 2001;46:250–256. doi: 10.1023/a:1005636412287. [DOI] [PubMed] [Google Scholar]
- 103.Ogasawara T, Morine Y, Ikemoto T, Imura S, Fujii M, Soejima Y, Shimada M. Influence of Dai-kenchu-to (DKT) on human portal blood flow. Hepatogastroenterology. 2008;55:574–577. [PubMed] [Google Scholar]
- 104.Murata P, Kase Y, Ishige A, Sasaki H, Kurosawa S, Nakamura T. The herbal medicine Dai-kenchu-to and one of its active components [6]-shogaol increase intestinal blood flow in rats. Life Sci. 2002;70:2061–2070. doi: 10.1016/s0024-3205(01)01552-1. [DOI] [PubMed] [Google Scholar]
- 105.Ni Q, Wang J, Li EQ, Zhao AB, Yu B, Wang M, Huang CR. Study on the protective effect of shengmai san (see text) on the myocardium in the type 2 diabetic cardiomyopathy model rat. J Tradit Chin Med. 2011;31:209–219. doi: 10.1016/s0254-6272(11)60044-7. [DOI] [PubMed] [Google Scholar]
- 106.Giridharan VV, Thandavarayan RA, Konishi T. Effect of Shengmai-san on cognitive performance and cerebral oxidative damage in BALB/ c mice. J Med Food. 2011;14:601–609. doi: 10.1089/jmf.2010.1362. [DOI] [PubMed] [Google Scholar]
- 107.Seo TB, Baek K, Kwon KB, Lee SI, Lim JS, Seol IC, Kim YS, Seo YB, Namgung U. Shengmai-san-mediated enhancement of regenerative responses of spinal cord axons after injury in rats. J Pharmacol Sci. 2009;110:483–492. doi: 10.1254/jphs.09044fp. [DOI] [PubMed] [Google Scholar]
- 108.Yao HT, Chang YW, Chen CT, Chiang MT, Chang L, Yeh TK. Shengmai San reduces hepatic lipids and lipid peroxidation in rats fed on a high-cholesterol diet. J Ethnopharmacol. 2008;116:49–57. doi: 10.1016/j.jep.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 109.Ichikawa H, Wang L, Konishi T. Prevention of cerebral oxidative injury by post-ischemic intravenous administration of Shengmai San. Am J Chin Med. 2006;34:591–600. doi: 10.1142/S0192415X06004120. [DOI] [PubMed] [Google Scholar]
- 110.Wang NL, Liou YL, Lin MT, Lin CL, Chang CK. Chinese herbal medicine, Shengmai San, is effective for improving circulatory shock and oxidative damage in the brain during heatstroke. J Pharmacol Sci. 2005;97:253–265. doi: 10.1254/jphs.fp0040793. [DOI] [PubMed] [Google Scholar]
- 111.Ichikawa H, Wang X, Konishi T. Role of component herbs in antioxidant activity of shengmai san: a traditional Chinese medicine formula preventing cerebral oxidative damage in rat. Am J Chin Med. 2003;31:509–521. doi: 10.1142/S0192415X03001193. [DOI] [PubMed] [Google Scholar]
- 112.Ichikawa H, Konishi T. In vitro antioxidant potentials of traditional Chinese medicine, Shengmai San and their relation to in vivo protective effect on cerebral oxidative damage in rats. Biol Pharm Bull. 2002;25:898–903. doi: 10.1248/bpb.25.898. [DOI] [PubMed] [Google Scholar]
- 113.Xuejiang W, Magara T, Konishi T. Prevention and repair of cerebral ischemia-reperfusion injury by Chinese herbal medicine, shengmai san, in rats. Free Radic Res. 1999;31:449–455. doi: 10.1080/10715769900301011. [DOI] [PubMed] [Google Scholar]
- 114.Yoshie F, Iizuka A, Kubo M, Komatsu Y, Matsumoto A, Itakura H, Takeda H, Matsumiya T, Kondo K. Protective effects of Saiko-ka-ryukotsu-borei-to (Chai-Hu-Jia-Long-Gu-Mu-Li-Tang) against atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic (KHC) rabbits. Pharmacol Res. 2001;43:481–488. doi: 10.1006/phrs.2001.0804. [DOI] [PubMed] [Google Scholar]
- 115.Chen CY, Kuo TL, Sheu SY, Kuo TF. Preventive effects of Chinese herb chai-hu-gui-zhi-tang extract on water immersion restraint stress-induced acute gastric ulceration in rats. J Vet Med Sci. 2010;72:679–685. doi: 10.1292/jvms.09-0284. [DOI] [PubMed] [Google Scholar]
- 116.Horie Y, Kajihara M, Mori S, Yamagishi Y, Kimura H, Tamai H, Kato S, Ishii H. Japanese herbal medicine, Saiko-keishi-to, prevents gut ischemia/reperfusion-induced liver injury in rats via nitric oxide. World J Gastroenterol. 2004;10:2241–2244. doi: 10.3748/wjg.v10.i15.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tatsuta M, Iishi H, Baba M, Narahara H, Yano H, Sakai N. Suppression by Chai-hu-gui-zhi-tang of the development of liver lesions induced by N-nitrosomorpholine in Sprague-Dawley rats. Cancer Lett. 2000;152:31–36. doi: 10.1016/s0304-3835(99)00429-2. [DOI] [PubMed] [Google Scholar]
- 118.Nose M, Tamura M, Ryu N, Mizukami H, Ogihara Y. Sho-saiko-to and Saiko-keisi-to, the traditional Chinese and Japanese herbal medicines, altered hepatic drug-metabolizing enzymes in mice and rats when administered orally for a long time. J Pharm Pharmacol. 2003;55:1419–1426. doi: 10.1211/0022357021873. [DOI] [PubMed] [Google Scholar]
- 119.Su SB, Xie MJ, Sawabu N, Motoo Y. Suppressive effect of herbal medicine saikokeishito on acinar cell apoptosis in rat spontaneous chronic pancreatitis. Pancreatology. 2007;7:28–36. doi: 10.1159/000101875. [DOI] [PubMed] [Google Scholar]
- 120.Su SB, Motoo Y, Xie MJ, Taga H, Sawabu N. Antifibrotic effect of the herbal medicine Saiko-keishi-to (TJ- 10) on chronic pancreatitis in the WBN/ Kob rat. Pancreas. 2001;22:8–17. doi: 10.1097/00006676-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 121.Motoo Y, Su SB, Xie MJ, Taga H, Sawabu N. Effect of herbal medicine Saiko-keishi-to (TJ-10) on rat spontaneous chronic pancreatitis: comparison with other herbal medicines. Int J Pancreatol. 2007;27:123–129. doi: 10.1385/IJGC:27:2:123. [DOI] [PubMed] [Google Scholar]
- 122.Su SB, Motoo Y, Xie MJ, Sakai J, Taga H, Sawabu N. Expression of pancreatitis-associated protein (PAP) in rat spontaneous chronic pancreatitis: effect of herbal medicine Saiko-keishi-to (TJ-10). Pancreas. 1999;19:239–247. doi: 10.1097/00006676-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 123.Sakaguchi M, Goto K, Ichiki H, Hattori N, Iizuka A, Yamamoto M, Takeda S, Ishige A, Aburada M, Yasuda M, et al. Effects of Byakko-ka-ninjin-to on salivary secretion and bladder function in rats. J Ethnopharmacol. 2005;102:164–169. doi: 10.1016/j.jep.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 124.Aburada T, Ikarashi N, Kagami M, Ichikawa Y, Sugitani M, Maniwa A, Ueda H, Toda T, Ito K, Ochiai W, et al. Byakkokaninjinto prevents body water loss by increasing the expression of kidney aquaporin-2 and skin aquaporin-3 in KKAy mice. Phytother Res. 2011;25:897–903. doi: 10.1002/ptr.3358. [DOI] [PubMed] [Google Scholar]
- 125.Yanagi Y, Yasuda M, Hashida K, Kadokura Y, Yamamoto T, Suzaki H. Mechanism of salivary secretion enhancement by Byakkokaninjinto. Biol Pharm Bull. 2008;31:431–435. doi: 10.1248/bpb.31.431. [DOI] [PubMed] [Google Scholar]
- 126.Kimura M, Kimura I, Chem FJ. Combined potentiating effect of byakko-ka-ninjin-to, its constituents, rhizomes of Anemarrhena asphodeloides, tomosaponin A-III, and calcium on pilocarpine-induced saliva secretion in streptozocin-diabetic mice. Biol Pharm Bull. 1996;19:926–931. doi: 10.1248/bpb.19.926. [DOI] [PubMed] [Google Scholar]
- 127.Tohda C, Sugahara H, Kuraishi Y, Komatsu K. Inhibitory effect of Byakko-ka-ninjin-to on itch in a mouse model of atopic dermatitis. Phytother Res. 2000;14:192–194. doi: 10.1002/(sici)1099-1573(200005)14:3<192::aid-ptr609>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 128.Tatsumi T, Yamada T, Nagai H, Terasawa K, Tani T, Nunome S, Saiki I. A Kampo formulation: Byakko-kaninjin- to (Bai-Hu-Jia-Ren-Sheng-Tang) inhibits IgE-mediated triphasic skin reaction in mice: the role of its constituents in expression of the efficacy. Biol Pharm Bull. 2001;24:284–290. doi: 10.1248/bpb.24.284. [DOI] [PubMed] [Google Scholar]
- 129.Kimura I, Nakashima N, Sugihara Y, Fu-jun C, Kimura M. The antihyperglycaemic blend effect of traditional Chinese medicine byakko-ka-ninjin-to on alloxan and diabetic KK-CA(y) mice. Phytother Res. 1999;13:484–488. doi: 10.1002/(sici)1099-1573(199909)13:6<484::aid-ptr485>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 130.Fan H, Shen L, Tang Q, Xiong P, Shou Z, Liao Y, Liang L, Chen X. Effect of Wumeiwan on cytokines TNF-alpha, IL-6, IL-8, IL-10 and expression of NF-kappaBp65 in rats with ulcerative colitis. J Huazhong Univ Sci Technol Med Sci. 2009;29:650–654. doi: 10.1007/s11596-009-0523-4. [DOI] [PubMed] [Google Scholar]
- 131.Fan H, Qiu MY, Mei JJ, Shen GX, Liu SL, Chen R. Effects of four regulating-intestine prescriptions on pathology and ultrastructure of colon tissue in rats with ulcerative colitis. World J Gastroenterol. 2005;11:4800–4806. doi: 10.3748/wjg.v11.i31.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen FP, Chen FJ, Jong MS, Tsai HL, Wang JR, Hwang SJ. Modern use of Chinese herbal formulae from Shang- Han Lun. Chin Med J (Engl) 2009;122:1889–1894. [PubMed] [Google Scholar]
- 133.Xiang YZ, Shang HC, Gao XM, Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008;22:851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 134.An X, Zhang AL, Yang AW, Lin L, Wu D, Guo X, Shergis JL, Thien FC, Worsnop CJ, Xue CC. Oral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic review. Respir Med. 2011;105:165–176. doi: 10.1016/j.rmed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y, Saito H, Nishiyama N. Improving effects of DX-9386, a traditional Chinese medicinal prescription, on thymectomy-induced impairment of learning behaviors in mice. Biol Pharm Bull. 1994;17:1199–1205. doi: 10.1248/bpb.17.1199. [DOI] [PubMed] [Google Scholar]
- 136.Choi JH, Kim HM, Song YS, Park SG, Kim JJ, Lee CK. Anti-aging effects Saccharomyces fermented modified Kyungohkgo extract on skin. Korean J Herbol. 2007;22:219–225. [Google Scholar]
- 137.Scheid V, Bensky D, Ellis A, Barolet R. Chinese herbal medicine: fomulas and strategies. 3rd ed. Estland Press; Seatle: 2009. [Google Scholar]
- 138.Panax ginseng. Monograph. Altern Med Rev. 2009;14:172–176. [PubMed] [Google Scholar]
- 139.Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 140.Siow YL, Gong Y, Au-Yeung KK, Woo CW, Choy PC, O K. Emerging issues in traditional Chinese medicine. Can J Physiol Pharmacol. 2005;83:321–334. doi: 10.1139/y05-029. [DOI] [PubMed] [Google Scholar]
- 141.Cho BH. The politics of herbal drugs in Korea. Soc Sci Med. 2000;51:505–509. doi: 10.1016/s0277-9536(99)00492-x. [DOI] [PubMed] [Google Scholar]
- 142.Yu F, Takahashi T, Moriya J, Kawaura K, Itoh T, Morimoto S, Yamaguchi N, Kanda T. Traditional Chinese medicine and Kampo: a review from the distant past for the future. J Int Med Res. 2006;34:231–239. doi: 10.1177/147323000603400301. [DOI] [PubMed] [Google Scholar]
- 143.Leung KF, Liu FB, Zhao L, Fang JQ, Chan K, Lin LZ. Development and validation of the Chinese Quality of Life Instrument. Health Qual Life Outcomes. 2005;3:26. doi: 10.1186/1477-7525-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kishida Y, Nishii T, Inoue T, Nishida S, Arimitsu J, Yoshikawa H, Sugano N. Juzentaihoto (TJ-48), a traditional Japanese herbal medicine, influences hemoglobin recovery during preoperative autologous blood donation and after hip surgery. Int J Clin Pharmacol Ther. 2009;47:716–721. doi: 10.5414/cpp47716. [DOI] [PubMed] [Google Scholar]
- 145.Wang XQ, Takahashi T, Zhu SJ, Moriya J, Saegusa S, Yamakawa J, Kusaka K, Itoh T, Kanda T. Effect of Hochu-ekki-to (TJ-41), a Japanese herbal medicine, on daily activity in a murine model of chronic fatigue syndrome. Evid Based Complement Alternat Med. 2004;1:203–206. doi: 10.1093/ecam/neh020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nagano T, Itoh H, Takeyama M. Effect of Dai-kenchuto on levels of 3 brain-gut peptides (motilin, gastrin and somatostatin) in human plasma. Biol Pharm Bull. 1999;22:1131–1133. doi: 10.1248/bpb.22.1131. [DOI] [PubMed] [Google Scholar]
- 147.Nagano T, Itoh H, Takeyama M. Effects of Dai-kenchuto on levels of 5-hydroxytryptamine (serotonin) and vasoactive intestinal peptides in human plasma. Biol Pharm Bull. 2000;23:352–353. doi: 10.1248/bpb.23.352. [DOI] [PubMed] [Google Scholar]
- 148.Itoh T, Yamakawa J, Mai M, Yamaguchi N, Kanda T. The effect of the herbal medicine dai-kenchu-to on postoperative ileus. J Int Med Res. 2002;30:428–432. doi: 10.1177/147323000203000410. [DOI] [PubMed] [Google Scholar]
- 149.Kawahara H, Yanaga K. The herbal medicine Dai-Kenchu-To directly stimulates colonic motility. Surg Today. 2009;39:175–177. doi: 10.1007/s00595-008-3810-y. [DOI] [PubMed] [Google Scholar]
- 150.Iwabu J, Watanabe J, Hirakura K, Ozaki Y, Hanazaki K. Profiling of the compounds absorbed in human plasma and urine after oral administration of a traditional Japanese (kampo) medicine, daikenchuto. Drug Metab Dispos. 2010;38:2040–2048. doi: 10.1124/dmd.110.033589. [DOI] [PubMed] [Google Scholar]
- 151.Sato Y, Katagiri F, Inoue S, Itoh H, Takeyama M. Effects of Ninjin-to on levels of calcitonin gene-related peptide and substance P in human plasma. Biol Pharm Bull. 2004;27:2032–2034. doi: 10.1248/bpb.27.2032. [DOI] [PubMed] [Google Scholar]
- 152.Pratico D, Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J Alzheimers Dis. 2004;6:171–175. doi: 10.3233/jad-2004-6209. [DOI] [PubMed] [Google Scholar]
- 153.Parihar MS, Hemnani T. Alzheimer’ s disease pathogenesis and therapeutic interventions. J Clin Neurosci. 2004;11:456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 154.Ichikawa H, Konishi T. In vitro antioxidant potentials of traditional Chinese medicine, Shengmai San and their relation to in vivo protective effect on cerebral oxidative damage in rats. Biol Pharm Bull. 2002;25:898–903. doi: 10.1248/bpb.25.898. [DOI] [PubMed] [Google Scholar]
- 155.Wang L, Muxin G, Nishida H, Shirakawa C, Sato S, Konishi T. Psychological stress-induced oxidative stress as a model of sub-healthy condition and the effect of TCM. Evid Based Complement Alternat Med. 2007;4:195–202. doi: 10.1093/ecam/nel080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nishiyama N, Zhou Y, Takashina K, Saito H. Effects of DX-9386, a traditional Chinese preparation, on passive and active avoidance performances in mice. Biol Pharm Bull. 1994;17:1472–1476. doi: 10.1248/bpb.17.1472. [DOI] [PubMed] [Google Scholar]
- 157.Zhang Y, Takashina K, Saito H, Nishiyama N. Anti-aging effect of DX-9386 in senescence accelerated mouse. Biol Pharm Bull. 1994;17:866–868. doi: 10.1248/bpb.17.866. [DOI] [PubMed] [Google Scholar]
- 158.Shin BY, Lee YH, Kim D, Park CS, Lee YW, Cho HJ, Kim DH, Yamamoto Y, Kang DH, Lee S, et al. Ameliorating effect of a herbal medicinal prescription, Kyung-Ok-Ko, on scopolamine-induced memory impairment in mice. J Tradit Med. 2009;26:35–43. [Google Scholar]
- 159.Morimoto Y, Sakata M, Ohno A, Maegawa T, Tajima S. Effects of Byakko-ka-ninjin-to, Bofu-tsusho-san and Gorei-san on blood glucose level, water intake and urine volume in KKAy mice. Yakugaku Zasshi. 2002;122:163–168. doi: 10.1248/yakushi.122.163. [DOI] [PubMed] [Google Scholar]
- 160.Jia L, Zhao Y. Current evaluation of the millennium phytomedicine. Ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem. 2009;16:2475–2484. doi: 10.2174/092986709788682146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16(Suppl):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paker L, Cadenas E. Herbal and traditional medicine. Marcel Dekker; New York: 2004. [Google Scholar]
- 163.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 164.Wood WB, Roh BL, White RP. Cardiovascular actions of Panx ginseng in dogs. Jpn J Pharmacol. 1964;14:284–294. doi: 10.1254/jjp.14.284. [DOI] [PubMed] [Google Scholar]
- 165.Lee KD, Huemer RP. Antitumoral activity of Panax ginseng extracts. Jpn J Pharmacol. 1971;21:299–302. doi: 10.1254/jjp.21.299. [DOI] [PubMed] [Google Scholar]
- 166.Hsu CT. On Panax shinseng Nies, var. Notoginseng Burkill. Jpn J Pharmacol. 1956;6:18–28. [PubMed] [Google Scholar]
- 167.Park JH, Cha HY, Seo JJ, Hong JT, Han K, Oh KW. Anxiolytic-like effects of ginseng in the elevated plusmaze model: comparison of red ginseng and sun ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:895–900. doi: 10.1016/j.pnpbp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 168.Yoo JH, Kwon HC, Kim YJ, Park JH, Yang HO. KG- 135, enriched with selected ginsenosides, inhibits the proliferation of human prostate cancer cells in culture and inhibits xenograft growth in athymic mice. Cancer Lett. 2010;289:99–110. doi: 10.1016/j.canlet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 169.Chen CF, Chiou WF, Zhang JT. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- 170.Hui H, Tang G, Go VL. Hypoglycemic herbs and their action mechanisms. Chin Med. 2009;4:11. doi: 10.1186/1749-8546-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chung JW, Kim YJ, Lee SJ, Hahm KB. Korean Red Ginseng: Qualitative and Quantitative Benefits on Helicobacter pylori Infection. J Ginseng Res. 2010;34:77–88. [Google Scholar]
- 172.Ernst E. Panax ginseng: An Overview of the Clinical Evidence. J Ginseng Res. 2010;34:259–263. [Google Scholar]


