Abstract
The recent discovery of neural stem cells (NSCs) in the adult mammalian brain has fostered a plethora of translational and preclinical studies to investigate future therapeutic approaches for the cure of neurodegenerative diseases. These studies are finally at the clinical stage, and some of them are already under way. The definition of a bona fide stem cell has long been the object of much debate focused on the establishment of standard and univocal criteria to distinguish between stem and progenitor cells. It is commonly accepted that NSCs have to fulfill two basic requirements, the capacity for long-term self-renewal and the potential for differentiation, which account for their physiological role, namely central nervous system tissue homeostasis. Strategies such as immortalization or reprogramming of somatic cells to the embryonic-like stage of pluripotency indicate the relevance of extensive self-renewal ability of NSCs either in vitro or in vivo. Moreover, the discovery of stem-like tumor cells in brain tumors, such as gliomas, accompanied by the isolation of these cells through the same paradigm used for related healthy cells, has provided further evidence of the key role that self-renewal plays in the development and progression of neurodegenerative diseases and cancer. In this review we provide an overview of the current understanding of the self-renewal capacity of nontransformed human NSCs, with or without immortalization or reprogramming, and of stem-like tumor cells, referring to both research and therapeutic studies.
Keywords: Cancer, Stem cells, Stem cell expansion, Stem cell transplantation, Neural stem cell
This review provides an overview of the current understanding of the self-renewal capacity of nontransformed human neural stem cells, with or without immortalization or reprogramming, and of stem-like tumor cells, referring to both research and therapeutic studies.
Introduction
Neural stem cells are the most primitive cells in the central nervous system (CNS). Since most mature neural cells, with particular reference to neurons, are very specialized cells and are quite sensitive to environmental changes, such as oxygen conditions or excitotoxic molecules, the importance of neural stem cells (NSCs) in sustaining the development and homeostasis of nervous tissue is essential [1, 2]. The slow replenishment of degenerating cells with newly generated neuronal cells under physiological conditions in vivo has suggested that NSCs basically rest in a state of quiescence, which allows them to maintain a balance between the ability to undergo self-renewal and to differentiate without depleting the stem pool. When dividing, the NSC gives rise to other neural stem cells and/or to transient-amplifying cells called progenitors, which display a decreased proliferative potential and which progressively acquire a more restricted differentiation capacity into neurons, astrocytes, and oligodendrocytes. The dynamic equilibrium between self-renewal and differentiation is critical to both the maintenance of the stem cell pool and active neurogenesis. In this regard, a key role in the regulation of stem cell behavior is played by the niche [3], the CNS-specific compartment where somatic adult neural stem cells reside and are self-maintained throughout life. The isolation and characterization of multipotent NSCs from multiple locations within the mammalian brain has represented one of the most significant advancements in neuroscience and has provided accruing evidence of endogenous NSC potential to respond to neurological injuries [4]. Thus far, germinative zones have been identified in the subgranular zone (SGZ) of the hippocampus [5], the olfactory bulb [6–10], the subventricular zone (SVZ) surrounding the ventricles [11], and the subcallosal zone underlying the corpus callosum [12]. Recent evidence has reported the presence of active neurogenesis even in the adult cerebellum [13]. Among this evidence is the fact that the SVZ is the adult brain region with the highest neurogenic rate from which NSCs were first isolated [14, 15] and characterized. Following brain injury, NSC proliferation in the SVZ is enhanced to provide novel neural precursors migrating to the lesion [16, 17], thus suggesting that the mechanisms regulating self-renewal are pivotal to NSC-mediated tissue repair.
Self-Renewal of NSCs
The self-renewal of NSCs is currently explained by two main theories: the stochastic model and the deterministic model. According to the former, the probability that a stem cell divides symmetrically into two stem cells or into two differentiating progenitors is the same [18]. Conversely, the deterministic model is based on the predetermination of the chances that a stem cell will self-renew or differentiate by the occurrence of extrinsic events. According to this theory, which is the most reliable in vivo, a stem cell can divide asymmetrically into one stem cell and one progenitor cell, or into one stem cell and one apoptotic cell, in response to specific environmental cues, such as the extracellular matrix, cytokines, oxygen, chemokines, and so forth [19–22]. However, the precise mechanism by which NSCs and progenitor cells self-renew and generate neurons at the same time is unclear. Because adult NSCs maintain a glial identity resembling that of embryonic radial glia [17], many studies suggest that the principles elucidated during development can be considered valid also for adult neurogenesis. During early neurogenesis of the embryonic mouse brain, most NSC divisions are symmetric to expand the NSC pool and to establish a stable niche compartment. With the progression of neurogenesis from the dorsal and ventral telencephalon, an increasing fraction of asymmetric divisions gives rise to progenitor cells (radial glia), which migrate to form the neocortical layers. An active reorientation of the mitotic spindle, according to vertical, horizontal, or oblique axes, has been proposed as being responsible for the asymmetric segregation of specific transcription factors or regulators with cell-fate determining function, although the precise correlation between spindle orientation and the kinetics of stem cell division remains unclear [22]. In the mammalian brain, as in Drosophila, heterotrimeric G proteins have been identified as master regulators of the mitotic spindle orientation, whereas multiple factors called segregation determinants, such as Numb and Numblike [23–26] and the ubiquitin ligase protein Neuralized (Neur) [27], have been shown to be involved in the regulation of asymmetric versus symmetric divisions. Whereas most NSC divisions in vitro seem to be asymmetric [28], in the adult NSC niche, self-renewal and multipotency are mutually regulated through the secretion of molecules by surrounding nonstem cells [29–31]. Hence, the elucidation of regulatory mechanisms, which underpin the polarized distribution of these factors within the NSCs, could contribute to manipulating the balance between self-renewing and differentiating cells either in vitro or in vivo and target neural precursors for a specific cell fate.
One of the most highly conserved and efficacious mechanisms involved in regulating NSC function and development throughout an individual's lifetime is the family of Wnt and bone morphogenetic protein (BMP), which have broad roles in stem cell biology by regulating the balance between quiescent and actively proliferating cells [32–34]. As a matter of fact, the large family of Wnt proteins regulate neurogenesis [35, 36] by influencing proliferation and lineage decisions of neural progenitor cells and their progeny [37, 38]. Furthermore, BMPs, for their part, cause differentiation of neural progenitors from the subventricular zone and olfactory bulb, inhibiting neurogenesis and promoting exit from the cell cycle, which may be a result of differentiation [39–41]. It has been demonstrated that in vitro cultured NSCs exposed to BMPs show age-dependent disposition in terminal fate choice that mimics the in vivo developmental differentiation process [42]. In addition, self-renewal of NSCs residing either in the SVZ or in the SGZ of the hippocampus is regulated by the dynamic interplay of Notch- and epidermal growth factor (EGF)-activated signaling pathways [43, 44]. In particular, Notch regulates the NSC's identity and self-renewal, whereas EGF receptor modulates proliferation and migration of the transiently amplifying progenitors (neural precursor cells [NPCs]) [34]. Other factors, such as CXCL12, basic fibroblast growth factor, and pigment-epithelium derived factor, are known to contribute to NSC self-maintenance through non-cell-autonomous effects or can modulate the proliferation and differentiation of NPCs [45, 46].
Recent studies have highlighted the novel definition of the SVZ as a neurovascular niche consisting of two specific sublocalized compartments within the SVZ niche: the apical ependymal niche, which lines the ventricles and consists of ciliated ependymal cells intercalated with stem cells; and, proximal to it, the basal vasculature niche, a rich plexus of blood vessels [47]. Endothelial cells have been shown to secrete factors that enhance self-renewal and neuron generation from progenitor cells [48], suggesting that the vascular niche harbors mostly activated progenitors and suggesting the concept of a niche-dependent self-renewal. NSCs spontaneously arrest to proliferate and differentiate with increasing distance from the niche or, under restrictive conditions, they even die. This behavior is likely due to the absence of factors promoting self-renewal and also to the presence of signals fostering NSCs toward differentiation (Fig. 1).
Figure 1.
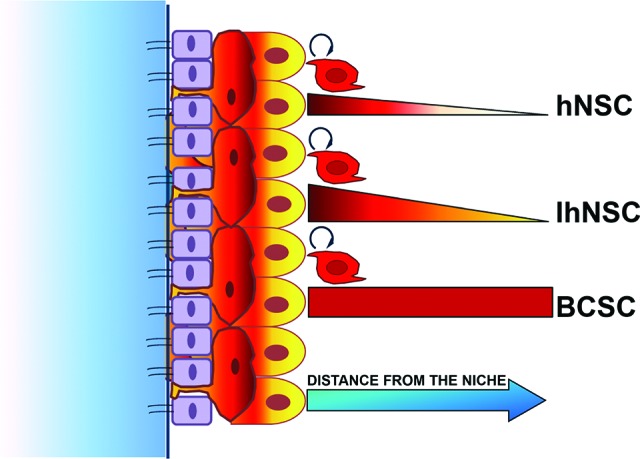
Model of the niche-dependent self-renewal capacity of neural stem cells and BCSCs. Untransformed cells, namely hNSCs or IhNSCs, which display a growth factor-dependent self-renewal in vitro, are able to self-renew in vivo only if implanted in proximity of a stem niche area, whereas BCSCs maintain a cell-autonomous self-renewal capacity both in vitro and in vivo. Red bars measure self-renewal capacity of hNSCs, IhNSCs, and cancer stem-like cells by two parameters: the kinetic and the long-term maintenance. In particular, red color gradations correlate with proliferation rate, which is higher in IhNSCs and BCSCs than in hNSC. The thickness of the red bars indicates the maintenance (for BCSCs) or the shortening (for hNSC and IhNSC) of self-renewal capacity, dependent on the distance from the niche. Abbreviations: BCSC, brain cancer stem cell; hNSC, human neural stem cell; IhNSC, immortalized human neural stem cell.
Isolation of Bona Fide NSCs Ex Vivo
Following the acceptance of the existence of multipotent NSCs in the adult mammalian brain able to self-renew and differentiate in vitro [14, 49], questions arose about specific selection criteria to establish stable NSC lines for either basic research or therapeutic purposes. NSCs are characterized by the expression of markers such as Sox2, glial fibrillary acidic protein (GFAP), nestin, and Musashi 1 and 2, but no specific combination of markers has so far been identified to definitely distinguish an NSC from an NPC. Much debate is now focused on the marker CD133 (also called prominin-1). It is currently unclear which portion of the CD133 population defines the relationship of CD133 to somatic NSCs and the selectivity of this relationship. This transmembrane glycoprotein, with a largely unknown function, has been used as one of the major markers, although it has been shown that clonogenic multipotent cells are also found in populations negative for CD133 [50–53]. However, lineage negativity remains as important as other criteria in identifying bona fide NSCs; indeed, cerebellar NSC have been isolated as CD133+ and lin− (lack of lineage marker expression) cells [13]. Alternatively, the neurosphere assay is based on the self-renewal capacity of NSCs, which is dependent on the presence of the mitogenic factors fibroblast growth factor 2 and EGF [54, 55], and it was developed as a procedure for the isolation and expansion of NSCs in vitro. Under these culture conditions, NSCs grow in suspension as floating cell clusters, called neurospheres, which are heterogeneously composed of stem, progenitor, and differentiating cells. A caveat of this method is that actively proliferating progenitors also generate neurospheres in vitro [56–58]. The neural-colony forming cell assay [59], by which only large colonies are enumerated as clonally derived from the extensive proliferation of NSCs, has been designed to exclude the possibility that an NSC population is composed exclusively of transient-amplifying progenitors.
Alternatively to the neurosphere assay, a number of groups have established NSC cultures on adhesive substrates mimicking the extracellular environment in the stem cell niche [60–62]. However, when establishing a renewable source of NSCs in vitro, expansion of NSCs on adhesion should be carefully evaluated. Monolayer cultures present the advantage of appearing as very homogeneous cell populations thanks to the continuous exposure of all the cells to the culture medium, but the adhesion substrates are molecules expressed in vivo by the extracellular matrix of the niche, such as laminin or collagen, which alter the expression of key receptors associated with proliferation, adhesion, migration, and differentiation of progenitor cell subtypes [19, 20, 63, 64]. Under these conditions, long-term proliferation of transient progenitors is most likely facilitated, and a neural progenitor culture can be erroneously mistaken for a neural stem cell culture. Soluble factors (such as growth factors, cytokines, and chemokines) and cell density have to be considered [19, 65–68].
Embryonic Stem-Derived NSCs
During development, before the formation of the neural stem cell niches, the cells of the inner cell mass (ESCs) generate the three germinal layers of embryos and will then give rise to tissue-restricted stem cells [69]. Starting from these pluripotent ESCs, it is possible to test, in vitro, the conditions by which ESCs can be induced to generate a specific population of tissue-restricted stem cells able to grow and differentiate. Several protocols have been described for the derivation of a neural progeny from ESCs, some of them allowing for a long-term expansion of transient progenitors followed by terminal differentiation upon changes in culture conditions. In summary, three methods have been reported to generate neural progenitor cells from ESCs. The first method leads to the formation of embryoid bodies (EBs) [70]; the second generates neural rosettes under adhesion conditions and by transient supplementation of Noggin to the culture medium, without passing through the EB stage [71]; and the third is based on the progressive differentiation of EBs into neural rosettes under sequential exposure to retinoic acid, Sonic hedgehog (Shh), and cAMP [72]. These protocols have been widely used to originate a variety of differentiated neural cell types (such as astrocytes; oligodendrocytes; and glutamatergic, GABAergic, and dopaminergic neurons), thus providing a basal platform of tools for preclinical studies. Nevertheless, the characterization of the in vitro-derived neural progenitors still remains elusive, and their single-cell clonogenic potential over time needs further investigation.
Fetal and Adult NSCs
Somatic NSCs can be retrieved from the fetal neural tissue (8–12 weeks postconception) or from the neurogenetic regions of the adult brain (described above), and they can extensively self-renew in vitro as neurospheres and differentiate into neuronal and macroglial cells without altering their functional properties over passaging. Given the slow-dividing feature of adult NSCs in the mammalian brain, the isolation, in vitro, of stable human NSC lines still remains infeasible. Sanai et al. were able to isolate NSCs from different regions of the SVZ of a cadaveric autoptic brain, but their expansion in vitro was far from generating a number of cells amenable to preclinical studies [73]. Conversely, human NSCs have been successfully isolated from the telencephalic-diencephalic region [55] or from the SGZ [9] of the fetal human brain. Their self-renewal features have been widely characterized (Table 1).
Table 1.
NSC basal properties
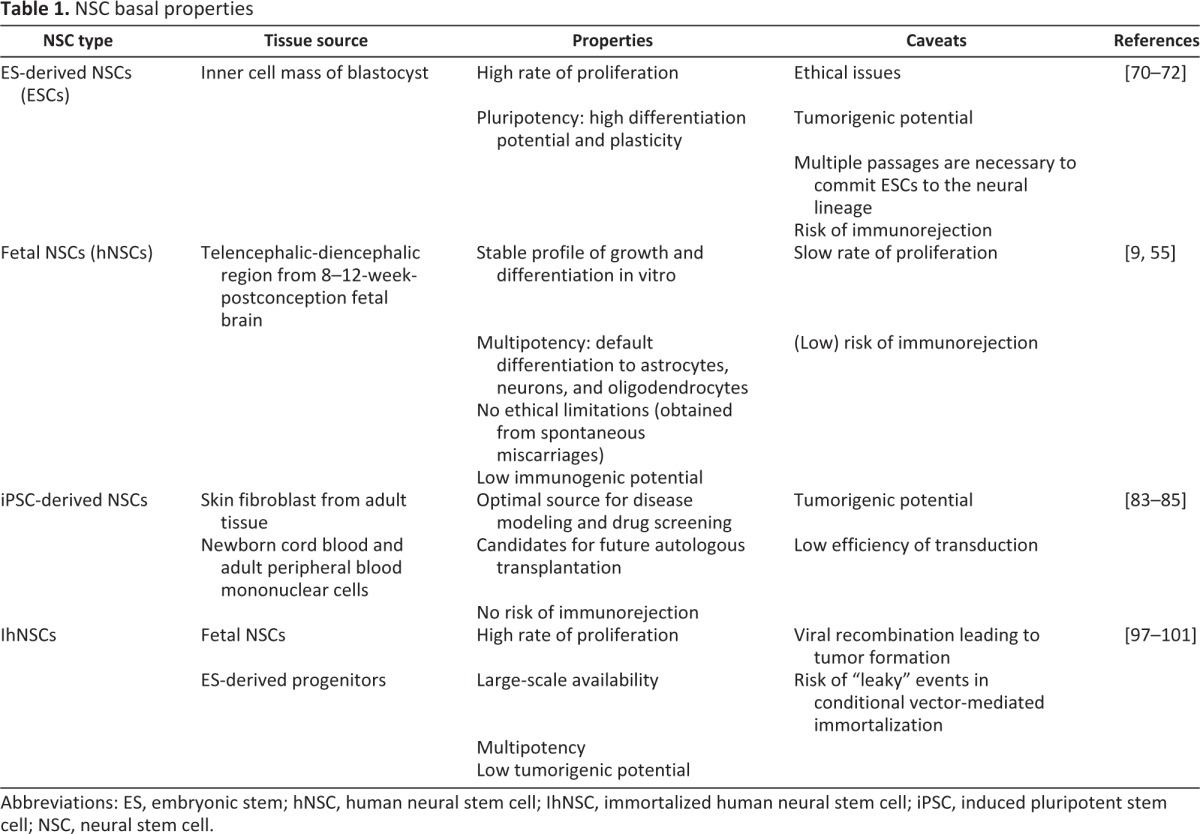
Abbreviations: ES, embryonic stem; hNSC, human neural stem cell; IhNSC, immortalized human neural stem cell; iPSC, induced pluripotent stem cell; NSC, neural stem cell.
Induced Pluripotent Stem Cells
Renewable sources of normal human neural cells with the functional characteristics expected of bona fide NSCs have significantly facilitated basic studies on human neurogenesis, as well as drug discovery. Their use in clinical applications will most likely eliminate the need for fetal human tissue [74]. A major obstacle to the progression of neural transplantation from the experimental level to clinical applications is the source of donor material [75]. In addition to the significant moral and ethical issues surrounding the procurement of human fetal tissue, other parameters, such as age, storage, viability, and contamination, must be standardized, making elective surgery difficult to schedule [76]. To further compound the problem, multiple fetuses are usually required for a single transplant, thereby introducing heterogeneity in the donor tissue and increasing the probability of immunological rejection or contamination [76]. Awareness of these difficulties has driven the search for alternative donor sources. Thus, immortalized brain precursors [77], xenogenic tissue [78, 79], and genetically engineered cells [80] have been used in experimental neural transplantation, although autologous transplantation of non-neural stem cells or of somatic cell-derived NSCs (induced pluripotent stem cells [iPSCs]) actually represents the most desirable solution in overriding most of the limitations described above.
Recently, iPSCs have been proposed as an alternative source of neural cells, since they share embryonic stem characteristics (i.e., self-renewal) and the potential to differentiate into any somatic cell type, and they could provide an escape from the risk of immunorejection. OCT4, SOX2, and NANOG are among the few of the most notable primitive master genes involved in the maintenance of the undifferentiated phenotype, through the simultaneous activation of genes promoting proliferation and repression of genes promoting cell cycle arrest and differentiation [81]. The combinatory overexpression of the four master genes OCT4, SOX2, C-MYC, and KLF4 has successfully reprogrammed adult human fibroblasts to the pluripotent stage [82], thus triggering a series of exciting studies mostly aimed at reprogramming somatic cells from patients affected by genetic or sporadic neurodegenerative diseases. Indeed, reprogramming of fibroblasts from two elderly amyotrophic lateral sclerosis (ALS) patients has allowed the generation of motor neurons potentially available for autologous transplantation [83, 84]. Moreover, a recent study by Chou et al. [85] has successfully established a method to generate integration-free human iPSCs from blood mononuclear cells. iPSCs currently offer multiple advantages: first, the ability to derive stem cells from skin fibroblasts could override the limit imposed by the need for human embryos to generate ESCs; second, with respect to traditional stem cells, they are able to generate neurons, astrocytes, and oligodendrocytes from adult patients; third, they provide the opportunity to elucidate how different cell types may be involved in a specific pathobiology through either cell-autonomous or non-cell-autonomous effects; fourth, they can be exploited to identify and characterize the cellular and biomolecular mechanisms that underpin the development of a chronic or progressive disorder; and finally, they represent an optimal tool for the discovery of novel drugs and high-throughput screenings [86].
One roadblock to the promotion of these cells to the clinical stage is represented by the genetic manipulation implied by the procedure, that, in principle, can drive the iPSCs to final tumorigenic modifications (Table 1). Accruing studies are currently investigating novel protocols for a higher grade of safety [87], so that, besides the traditional Yamanaka reprogramming, other methodologies are currently used: recombinant protein reprogramming [88, 89], consisting of administering each reprogramming factor to the cells as recombinant proteins, and cut-in/cut-out reprogramming by the use of the piggyBac transposon [89], synthetic RNA [90], and episomal DNA method [91]. However, when considering iPSCs as a source for autologous transplantation in human patients affected by neurodegenerative disorders, it still has to be determined to what extent the original affected microenvironment in the tissue from which iPSCs are derived is able to condition the novel regenerated cells and whether these are particularly sensitive when implanted back into the patient. Alternatively to full reprogramming of terminally committed cells into a pluripotent primitive stage, direct conversion without reverting cells to a pluripotent state is being used to generate neurons and NSCs [92, 93].
Immortalized Stem Cells
Several clonal, genetically homogeneous human neural stem cell (hNSC) lines have been obtained by genetic perpetuation methods [94–96]. Taking advantage of their nontransformed nature, human origin, multipotency, fast but conditional growth, unlimited availability, and suitability for molecular manipulation, these cell lines offer a great opportunity for the development of cell replacement and/or gene transfer-based therapies, such as using assays for pharmacological studies, drug discovery, and investigation of specific intracellular regulatory pathways, which require large amounts of human brain cells to be generated in a rapid and reproducible fashion. In particular, we have used stem cell lines from the telencephalic-diencephalic region of the human fetal brain as a source for generating cell lines immortalized with V-MYC or with the single-mutated variant C-MYC T58A, which could be routinely available for extensive studies aimed at elucidating the mechanisms of regulation of NSC development [97, 98]. Immortalized human neural stem cells (IhNSCs) provide an available source of human neural stem cells for pilot experiments of transplantation into animal models of neurodegenerative diseases [60, 61, 99, 100]. Recent studies have shown that conditional induction of V-MYC gene expression suffices to enhance the self-renewal of neural progenitors derived from fetal human brain with no tumorigenic potential either in vitro or in vivo [101].
CNS Cancer Stem Cells
One of the most prominent topics in the field of cancer biology and therapy is that transformed stem/precursor cells, with the cardinal properties of stem cells, such as the ability of self-renewal and to generate large numbers of progeny, are responsible for the origin and maintenance of solid malignancies [102–104]. The idea that transformed stem cells initiate tumors was initially confirmed in the 1990s, based on studies of acute myeloid leukemia [105, 106], and has been strengthened by findings related to breast cancer [107]. A similar involvement of tumor stem cells in brain cancer was also supported by the fact that neural stem cells are nestin- and GFAP-positive precursors [108] and the discovery that, when targeted to nestin- or GFAP-positive cells, alterations of critical G1 arrest regulatory pathways cause the onset of high-grade gliomas [106, 109]. The ensuing view that tumor stem cells underpin the development and/or maintenance of brain cancer has been confirmed, once and for all, by their identification in tumors of the central nervous system [102, 110–113]. Like stem cells in normal tissues, these brain cancer stem-like cells are relatively rare but have the capacity to establish and maintain glioma tumors at the clonal level and thus are thought to be tumor-initiating cells (Fig. 2) [114, 115]. It is currently unknown whether the molecular and functional characteristics of these cancer stem cells are a reflection of their origin from mutated normal NSCs or, rather, they emerge from the acquisition of stem-like features by more mature CNS cells following transformation [116–120].
Figure 2.
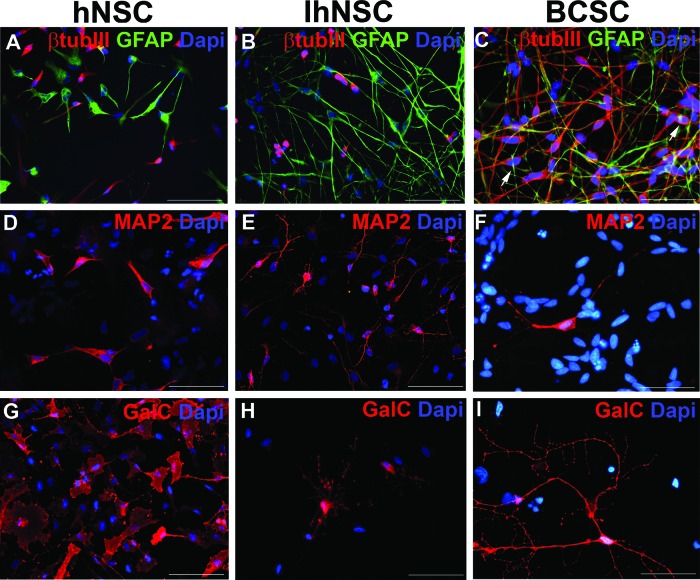
Expression of differentiation neural markers by normal (hNSC), v-myc-immortalized (IhNSC), and human glioblastoma (hGBM)-derived stem-like cells. hNSCs, IhNSCs, and hGBM-derived stem-like cells (indicated as BCSCs) were differentiated onto an adhesive substrate in the absence of mitogenic factors for 10 days. (A–C): Immunostaining showing the definite segregation of the early neuronal marker βtubIII (red) and the astroglial marker GFAP in hNSCs (A) and IhNSCs (B) and, conversely, a partial colocalization of the two markers in BCSCs (arrows in [C]). (D–I): Immunostaining of hNSCs, IhNSCs, and BCSCs with antibodies recognizing late neuronal marker MAP2 (D–F) and oligodendrocyte marker GalC (G–I). Total nuclei are shown by Dapi staining (blue). Scale bars = 50 μm. Abbreviations: βtubIII, β-tubulin III; BCSC, brain cancer stem cell; Dapi, 4′,6-diamidino-2-phenylindole; GalC, galactocerebroside C; GFAP, glial fibrillary acidic protein; hNSC, human neural stem cell; IhNSC, immortalized human neural stem cell; MAP2, microtubular-associated protein 2.
By the application of the same culture system developed for neural stem cells (i.e., neurosphere assay), long-term expanding stable lines were isolated, propagated, and characterized in a reproducible fashion from human high-grade gliomas, named human glioblastomas (hGBMs) [55, 111]. Upon differentiation, these hGBM stem-like cells gave rise to the three major neural lineages in a way that is rather similar to NSCs [55], and they demonstrated self-renewal capacity. These cells also bear tumor-specific features, such as an exacerbated growth rate, highly unbalanced karyotypes with a high degree of hyperdiploidy and telomerase reactivation, a dysfunctional segregation of neural markers, and the activation of an aberrant differentiation program (Fig. 2). Nonetheless, following either subcutaneous or intracranial implantation, hGBM stem-like cells displayed the essential requirement for a cancer stem cell, that is, the capability of generating new tumors. Importantly, when injected into the brains of immunocompromised mice, the resulting tumors recapitulated the morphology, genotype, and gene expression patterns of primary glioblastomas (GBMs) and had extensive migratory and infiltrative capacity [114], thus being superior pathological models of human disease. These findings indicate that the in vitro cells, tentatively called hGBM stem-like cells or stem-like tumor initiating cells, faithfully preserve the in vivo key features of human pathology.
The complementary parallelism between the healthy behavior of wild-type NSCs and the uncontrolled expansion of transformed cancer stem-like cells has paved the way to the identification and the study of future cancer therapies. Administration of epigenetic factors, which are able to dampen or, hopefully, to arrest tumor progression, represents the most feasible strategy. The role of stem cells in the origination (i.e., initiation) of gliomas remains controversial, but the identification of brain cancer stem-like cells has led to new and specific cellular targets for therapeutic intervention in primary brain cancers, raising the possibility that specifically killing or blocking the proliferative potential of the tumor-initiating stem cells may increase treatment efficacy. Furthermore, it has stimulated the development of new hGBM cancer stem-like cell-based preclinical experimental models with the potential to improve mechanistic and preclinical therapeutic research [104, 121].
Nonetheless, the absence of maturity in the knowledge of somatic NSC biology and the transition of stem cell progeny into functional cells has stymied the study and characterization of the complexity of cellular interactions in human gliomas, making it difficult to develop targeted therapies if a specific target is still missing. Despite this controversial issue, however, signaling pathways that regulate self-renewal and cell fate in normal neural stem cells have been shown to be active and associated with oncogenesis in cancer stem cells of GBMs and brain tumors [122–124]. In addition to the well-known NOTCH, SHH, and WNT, tumor suppressor genes such as PTEN (phosphatase and tensin homolog on chromosome 10) and TP53 (tumor protein p53) have been implicated in the uncontrolled self-renewal of brain cancer stem-like cells, which might generate tumors that are resistant to conventional therapies [125, 126]. Therefore, it has been suggested that therapeutic agents targeting these pathways might effectively deplete cancer stem-like cell populations in GBM as well [127–129].
An alternative approach is to activate specific differentiation pathways in a small population of tumor-initiating stem-like cells that drive proliferation and resistance, causing them to lose their stem and proliferative qualities. This regimen would make tumors less aggressive and more sensitive to cytotoxic treatment [130].
A member of the bone morphogenetic protein family, BMP4, has been reported to be a potential heterogeneous/targeted strategy that appears to block tumor initiation and progression in a xenogenic in vivo murine model [42]. In culture and in vivo, the delivery of this protein to hGBM stem-like cells blocks proliferation and induces the cells to differentiate, as opposed to killing them [131]. This result is similar to the outcome of exposing somatic NSC to BMPs, which induces their differentiation down an astrocyte pathway [40].
Finally, it has also been documented that endogenous NSCs are naturally attracted to gliomas [132–134] and have the ability to migrate into the tumor mass, even contributing to the tumor bulk. As a matter of fact, Aboody et al. from the City of Hope's Department of Neurosciences demonstrated this inherent propensity (also known as tropism) of neural stem cells to home in on invasive tumor cells [135], even migrating from the opposite side of the brain or across the blood-brain barrier, and they harnessed the tumor tropism of neural stem cells to deliver therapeutic agents to invasive tumor sites. Because of the attraction of NSCs to gliomas, NSCs can be used as cellular vehicles for the delivery of therapeutic agents [135, 136]. In 2010, the FDA approved a phase I clinical trial for NSC-mediated therapy of high-grade gliomas, by a genetically modified human NSC line, generated by Seung U. Kim (Division of Neurology, University of British Columbia), which delivers a prodrug-activating enzyme (cytosine deaminase) to brain tumor sites. It is imperative to design new strategies based upon a better understanding of the signaling pathways that control aspects of self-renewal and survival, both in normal and cancer stem cells, to identify novel therapeutic targets.
Therapeutic Relevance of NSCs
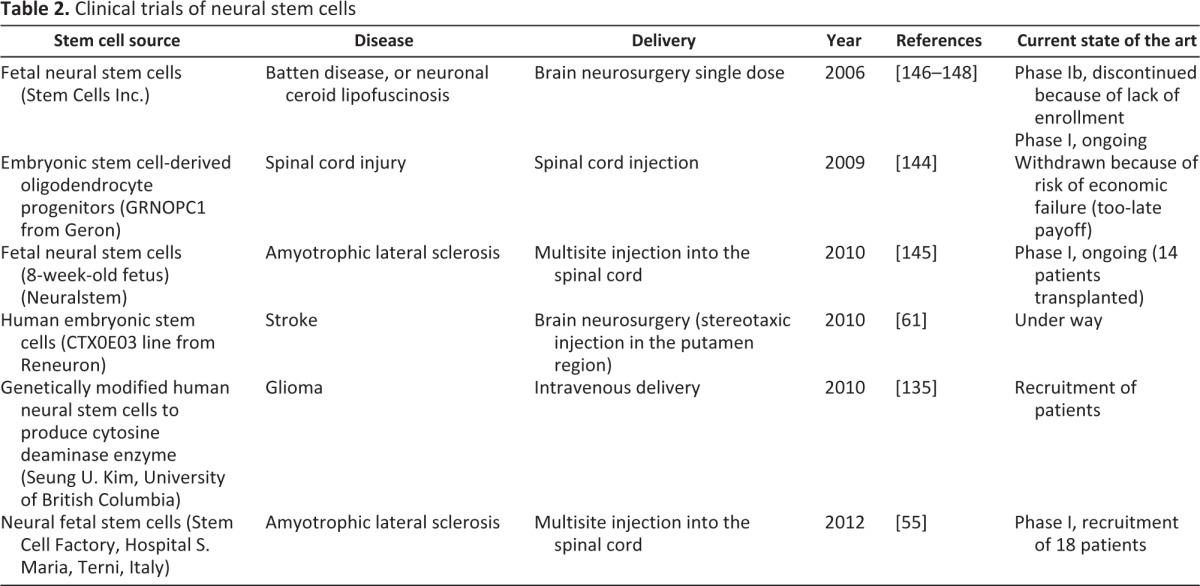
As of now, thanks to the development of paradigms for the isolation and expansion of NSCs ex vivo from different tissue sources, NSCs have been transplanted in different animal models as a tool for the cure of neurodegenerative diseases [137–140]. Irrespective of their specific etiology, neurodegenerative disorders eventually lead to loss or functional alteration of mature cells of the brain parenchyma. Symptoms vary significantly, depending on many parameters, such as age of onset, region of the brain, the type of cells being damaged, and the origin and nature of the noxa—genetic, toxic, traumatic, ischemic, hemorrhagic, infectious, or immunological and inflammatory—to highlight the major examples. Several studies have demonstrated that, following brain injury, endogenous NSC proliferation is enhanced, and soon after it is followed by an increased migration of progenitors from the niche to the lesion site [16, 17]. However, given the inherent (and perhaps functionally essential) resilience of the postnatal and, particularly, adult mammalian brain, in addition to new cells of preexisting circuitry, repair of the damaged brain tissue by endogenous cell replacement is very limited. One of the current, most valued therapeutic hypotheses is to accomplish neuroregeneration by transplantation of exogenous cells, that is, by cell-mediated therapy [141]. Perhaps counterintuitively, it has now emerged that transplanted cells may actually boost endogenous recovery, besides replacing and partially repopulating damaged areas. One of the most promising approaches in cell therapy may exploit a plethora of healing actions that the introduction of new and healthy cells may elicit on damaged brain tissue. In the current scenario, NSCs may act as a reservoir, providing trophic support to surviving cells and synapses at various levels, perhaps by scavenging toxic compounds in genetic and metabolic disorders, such as Tay-Sachs, Sandoff, Canavan, and Batten diseases, or by releasing trophic factors at the level of post-traumatic or ischemic injury in neurodegenerative diseases, such as ALS [142]. Transplantation experiments in animal models of brain lesions or neurodegenerations have revealed that NSCs are capable of integrating into the host brain and ameliorate functional defects. Local environmental cues produced by the stem niche or by a damaged area, soon after injury, have been identified as promoting exogenous cell survival and migration to the lesion (pathotropism) [143]. Consistently, several studies have shown that donor NSCs also display a tropism intrinsic to the recipient stem niche (homing), matching signals resembling their naive site of origin [100] (Fig. 1). The mechanisms determining the survival and migration of exogenous NSCs into healthy or lesioned adult brain have still to be identified and characterized, but extensive self-renewal of exogenous NSCs appears dependent on their proximity to the recipient stem niche (Fig. 1). Therefore, in view of future clinical applications of NSCs for the cure of neurodegenerative diseases, these findings suggest accurately evaluating the site of transplantation, according to specific therapeutic aims. NSCs are able to provide a multitude of therapeutic effects, and the extent to which and for how long these effects are needed depend on the specific neurological disorder [141]. In addition to the establishment of protocols for the extensive culture of NSC lines ex vivo, the nontumorigenic self-renewal of transplanted cells into the recipient CNS would be an auspicious goal in chronic disorders. Contrary to this, upon acute injury, such as stroke or focal demyelination, morbidity develops within a few days, together with the development of an acute inflammatory reaction. In this case, a limited or even absent self-renewal in vivo of transplanted cells together with an inherent or epigenetically induced tendency to differentiate into the proper phenotype could be the most appropriate therapeutic strategy. The transplantation of multiple subpopulations of neural precursors or of specific NSC-derived progenitors next to the site of injury offers the chance for a rapid and targeted therapeutic effect [100, 143] (Fig. 3). Several clinical trials harnessing various sources of neural stem cells have been started in the last few years (Table 2) [61, 144–148]. These important breakthroughs in the clinical application of NSCs demonstrate that a continuous and standardized clinical-grade source of normal human CNS cells (hNSCs), combining the plasticity of fetal tissue with extensive proliferative capacity and functional stability, is of paramount importance.
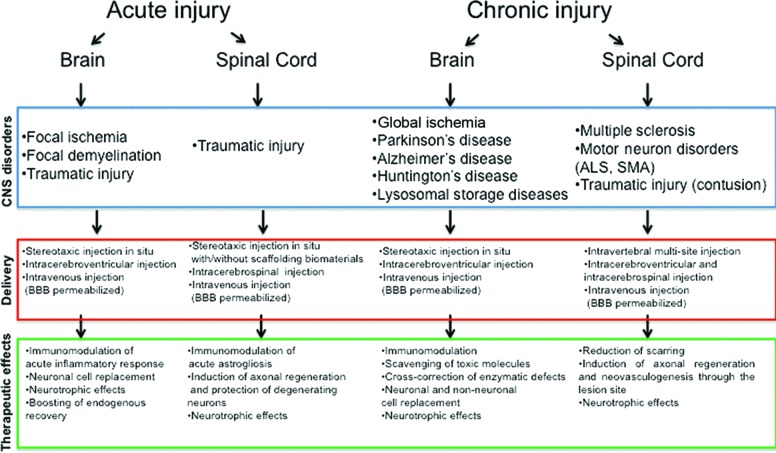
Figure 3.
Neural stem cell-mediated therapeutic effects and their multiple clinical applications. Abbreviations: ALS, amyotrophic lateral sclerosis; BBB, blood-brain barrier; CNS, central nervous system; SMA, spinal muscular atrophy.
Table 2.
Clinical trials of neural stem cells
Conclusion
The development of methods to establish NSC lines in vitro has been one of the main goals of researchers since the discovery of active neurogenesis in the adult mammalian CNS. Current preclinical studies strongly suggest that the therapeutic efficacy of stem cell transplantation mostly relies on NSC-mediated neuroprotection, rather than replacement of damaged cells. For clinical application, it is important that these protective strategies are proven safe and effective in humans. Several clinical trials using human embryonic stem-derived NSCs or fetal NSCs are currently under way, and their outcomes will contribute to improved transplant-based therapies.
Our greatest limitation in treating many neurodegenerative disorders is the lack of understanding of what causes the onset or drives the progression of sporadic and idiopathic pathologies. In this regard, one of the most significant advances in neural stem cell biology has been the use of stem cells for understanding pathobiological mechanisms and for the screening of novel therapeutic drugs. To this end, both ESCs and iPSCs, which can be cultured on a large scale in vitro, have been proven to be optimal candidates.
Acknowledgments
We thank Daniela Ferrari for manuscript revision, Cristina Zalfa for image editing, Alberto Visioli for technical support, and Prof. Angelo Vescovi for valuable suggestions. This work was supported by Fondazione Borgonovo, Fondazione Neurothon ONLUS, Fondazione Cellule Staminali of Terni, Fondazione Milan, and the Italian Association for Cancer Research.
Author Contributions
L.D.F.: conception and design, manuscript writing, provision of study material, final approval of manuscript; E.B.: manuscript writing, provision of study material, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Léveillé F, Soriano FX, Papadia S, et al. Excitotoxic insults lead to peroxiredoxin hyperoxidation. Oxid Med Cell Longev. 2009;2:110–113. doi: 10.4161/oxim.2.2.8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 4.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 6.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 7.Bayer SA. Neuron production in the hippocampus and olfactory bulb of the adult rat brain: Addition or replacement? Ann NY Acad Sci. 1985;457:163–172. doi: 10.1111/j.1749-6632.1985.tb20804.x. [DOI] [PubMed] [Google Scholar]
- 8.Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 9.Gage FH, Kempermann G, Palmer TD, et al. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 11.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seri B, Herrera DG, Gritti A, et al. Composition and organization of the SCZ: A large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex. 2006;16(suppl 1):i103–i111. doi: 10.1093/cercor/bhk027. [DOI] [PubMed] [Google Scholar]
- 13.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 17.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Gordon MY, Blackett NM. Routes to repopulation: A unification of the stochastic model and separation of stem-cell subpopulations. Leukemia. 1994;8:1068–1072. discussion 1072–1073. [PubMed] [Google Scholar]
- 19.Ariza CA, McHugh KP, White SJ, et al. Extracellular matrix proteins and astrocyte-derived soluble factors influence the differentiation and proliferation of adult neural progenitor cells. J Biomed Mater Res A. 2010;94:816–824. doi: 10.1002/jbm.a.32741. [DOI] [PubMed] [Google Scholar]
- 20.Kazanis I, Lathia JD, Vadakkan TJ, et al. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa MR, Ortega F, Brill MS, et al. Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development. 2011;138:1057–1068. doi: 10.1242/dev.061663. [DOI] [PubMed] [Google Scholar]
- 22.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res. 2010;316:900–906. doi: 10.1016/j.yexcr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Kuo CT, Mirzadeh Z, Soriano-Navarro M, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li HS, Wang D, Shen Q, et al. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–1118. doi: 10.1016/s0896-6273(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 26.Petersen PH, Zou K, Hwang JK, et al. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- 27.Bhat KM, Gaziova I, Katipalla S. Neuralized mediates asymmetric division of neural precursors by two distinct and sequential events: Promoting asymmetric localization of Numb and enhancing activation of Notch-signaling. Dev Biol. 2011;351:186–198. doi: 10.1016/j.ydbio.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpowicz P, Morshead C, Kam A, et al. Support for the immortal strand hypothesis: Neural stem cells partition DNA asymmetrically in vitro. J Cell Biol. 2005;170:721–732. doi: 10.1083/jcb.200502073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conover JC, Notti RQ. The neural stem cell niche. Cell Tissue Res. 2008;331:211–224. doi: 10.1007/s00441-007-0503-6. [DOI] [PubMed] [Google Scholar]
- 30.García-Verdugo JM, Doetsch F, Wichterle H, et al. Architecture and cell types of the adult subventricular zone: In search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Lim DA, Huang YC, Alvarez-Buylla A. The adult neural stem cell niche: Lessons for future neural cell replacement strategies. Neurosurg Clin N Am. 2007;18:81–92, ix. doi: 10.1016/j.nec.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wexler EM, Paucer A, Kornblum HI, et al. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–1141. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mira H, Andreu Z, Suh H, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 36.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 37.Adachi K, Mirzadeh Z, Sakaguchi M, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 38.Hirabayashi Y, Itoh Y, Tabata H, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 39.Gross RE, Mehler MF, Mabie PC, et al. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 40.Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 41.Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- 42.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 43.Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexson TO, Hitoshi S, Coles BL, et al. Notch signaling is required to maintain all neural stem cell populations: Irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- 45.Kokovay E, Goderie S, Wang Y, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramírez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 47.Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci USA. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louissaint A, Jr, Rao S, Leventhal C, et al. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 49.Ray J, Peterson DA, Schinstine M, et al. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci USA. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Guo T, Zhang L, et al. CD133 and CD44 are universally overexpressed in GIST and do not represent cancer stem cell markers. Genes Chromosomes Cancer. 2012;51:186–195. doi: 10.1002/gcc.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y, Kong W, Falk A, et al. CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS One. 2009;4:e5498. doi: 10.1371/journal.pone.0005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gires O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell Mol Life Sci. 2011;68:4009–4022. doi: 10.1007/s00018-011-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prestegarden L, Enger PO. Cancer stem cells in the central nervous system: A critical review. Cancer Res. 2010;70:8255–8258. doi: 10.1158/0008-5472.CAN-10-1592. [DOI] [PubMed] [Google Scholar]
- 54.Azari H, Rahman M, Sharififar S, et al. Isolation and expansion of the adult mouse neural stem cells using the neurosphere assay. J Vis Exp. 2010;(45):2393. doi: 10.3791/2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vescovi AL, Parati EA, Gritti A, et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds BA, Rietze RL. Neural stem cells and neurospheres: Re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 57.Marshall GP, 2nd, Reynolds BA, Laywell ED. Using the neurosphere assay to quantify neural stem cells in vivo. Curr Pharm Biotechnol. 2007;8:141–145. doi: 10.2174/138920107780906559. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed S. The culture of neural stem cells. J Cell Biochem. 2009;106:1–6. doi: 10.1002/jcb.21972. [DOI] [PubMed] [Google Scholar]
- 59.Deleyrolle LP, Reynolds BA. Identifying and enumerating neural stem cells: Application to aging and cancer. Prog Brain Res. 2009;175:43–51. doi: 10.1016/S0079-6123(09)17504-0. [DOI] [PubMed] [Google Scholar]
- 60.Miljan EA, Sinden JD. Stem cell treatment of ischemic brain injury. Curr Opin Mol Ther. 2009;11:394–403. [PubMed] [Google Scholar]
- 61.Stroemer P, Patel S, Hope A, et al. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair. 2009;23:895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- 62.Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340:471–473. doi: 10.1038/340471a0. [DOI] [PubMed] [Google Scholar]
- 63.Jurga M, Dainiak MB, Sarnowska A, et al. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials. 2011;32:3423–3434. doi: 10.1016/j.biomaterials.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 64.Ortinau S, Schmich J, Block S, et al. Effect of 3D-scaffold formation on differentiation and survival in human neural progenitor cells. Biomed Eng Online. 2010;9:70. doi: 10.1186/1475-925X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang MY, Park CH, Lee SH. Embryonic cortical stem cells secrete diffusible factors to enhance their survival. Neuroreport. 2003;14:1191–1195. doi: 10.1097/00001756-200307010-00001. [DOI] [PubMed] [Google Scholar]
- 66.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 67.Li G, Chen Z, Hu YD, et al. Autocrine factors sustain glioblastoma stem cell self-renewal. Oncol Rep. 2009;21:419–424. [PubMed] [Google Scholar]
- 68.Taupin P, Ray J, Fischer WH, et al. FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28:385–397. doi: 10.1016/s0896-6273(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 69.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 70.Pankratz MT, Li XJ, Lavaute TM, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Itsykson P, Ilouz N, Turetsky T, et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005;30:24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 72.Erceg S, Lainez S, Ronaghi M, et al. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One. 2008;3:e2122. doi: 10.1371/journal.pone.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 74.Brüstle O, McKay RD. Neuronal progenitors as tools for cell replacement in the nervous system. Curr Opin Neurobiol. 1996;6:688–695. doi: 10.1016/s0959-4388(96)80104-8. [DOI] [PubMed] [Google Scholar]
- 75.Björklund A. Neurobiology: Better cells for brain repair. Nature. 1993;362:414–415. doi: 10.1038/362414a0. [DOI] [PubMed] [Google Scholar]
- 76.Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci. 1996;19:102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- 77.Whittemore SR, Snyder EY. Physiological relevance and functional potential of central nervous system-derived cell lines. Mol Neurobiol. 1996;12:13–38. doi: 10.1007/BF02740745. [DOI] [PubMed] [Google Scholar]
- 78.Deacon T, Schumacher J, Dinsmore J, et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson's disease. Nat Med. 1997;3:350–353. doi: 10.1038/nm0397-350. [DOI] [PubMed] [Google Scholar]
- 79.Zawada WM, Cibelli JB, Choi PK, et al. Somatic cell cloned transgenic bovine neurons for transplantation in parkinsonian rats. Nat Med. 1998;4:569–574. doi: 10.1038/nm0598-569. [DOI] [PubMed] [Google Scholar]
- 80.Rosenberg MB, Friedmann T, Robertson RC, et al. Grafting genetically modified cells to the damaged brain: Restorative effects of NGF expression. Science. 1988;242:1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- 81.Silva J, Nichols J, Theunissen TW, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 83.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 84.Singh Roy N, Nakano T, Xuing L, et al. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 85.Chou BK, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nizzardo M, Simone C, Falcone M, et al. Human motor neuron generation from embryonic stem cells and induced pluripotent stem cells. Cell Mol Life Sci. 2010;67:3837–3847. doi: 10.1007/s00018-010-0463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 88.Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Topp S, Gallivan JP. Emerging applications of riboswitches in chemical biology. ACS Chem Biol. 2010;5:139–148. doi: 10.1021/cb900278x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng L, Hansen NF, Zhao L, et al. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell. 2012;10:337–344. doi: 10.1016/j.stem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lujan E, Chanda S, Ahlenius H, et al. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vierbuchen T, Wernig M. Direct lineage conversions: Unnatural but useful? Nat Biotechnol. 2011;29:892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flax JD, Aurora S, Yang C, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 95.Sah DW, Ray J, Gage FH. Bipotent progenitor cell lines from the human CNS. Nat Biotechnol. 1997;15:574–580. doi: 10.1038/nbt0697-574. [DOI] [PubMed] [Google Scholar]
- 96.Villa A, Snyder EY, Vescovi A, et al. Establishment and properties of a growth factor-dependent, perpetual neural stem cell line from the human CNS. Exp Neurol. 2000;161:67–84. doi: 10.1006/exnr.1999.7237. [DOI] [PubMed] [Google Scholar]
- 97.De Filippis L, D Ferrari, Nodari Rota L, et al. Immortalization of human neural stem cells with the c-myc mutant T58A. PLoS One. 2008;3:e3310. doi: 10.1371/journal.pone.0003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Filippis L, Lamorte G, Snyder EY, et al. A novel, immortal, and multipotent human neural stem cell line generating functional neurons and oligodendrocytes. Stem Cells. 2007;25:2312–2321. doi: 10.1634/stemcells.2007-0040. [DOI] [PubMed] [Google Scholar]
- 99.Martínez-Serrano A, Bjorklund A. Immortalized neural progenitor cells for CNS gene transfer and repair. Trends Neurosci. 1997;20:530–538. doi: 10.1016/s0166-2236(97)01119-3. [DOI] [PubMed] [Google Scholar]
- 100.Rota Nodari L, Ferrari D, Giani F, et al. Long-term survival of human neural stem cells in the ischemic rat brain upon transient immunosuppression. PLoS One. 2010;5:e14035. doi: 10.1371/journal.pone.0014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim KS, Lee HJ, Jeong HS, et al. Self-renewal induced efficiently, safely, and effective therapeutically with one regulatable gene in a human somatic progenitor cell. Proc Natl Acad Sci USA. 2011;108:4876–4881. doi: 10.1073/pnas.1019743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clarke MF, Fuller M. Stem cells and cancer: Two faces of Eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 103.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 104.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 105.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 106.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 107.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 109.Holland EC, Hively WP, Gallo V, et al. Modeling mutations in the G1 arrest pathway in human gliomas: Overexpression of CDK4 but not loss of INK4a-ARF induces hyperploidy in cultured mouse astrocytes. Genes Dev. 1998;12:3644–3649. doi: 10.1101/gad.12.23.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huntly BJ, Gilliland DG. Cancer biology: Summing up cancer stem cells. Nature. 2005;435:1169–1170. doi: 10.1038/4351169a. [DOI] [PubMed] [Google Scholar]
- 111.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 112.Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 113.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 114.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 115.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 116.Assanah M, Lochhead R, Ogden A, et al. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Doetsch F, Verdugo JM, Caille I, et al. Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J Neurosci. 2002;22:2255–2264. doi: 10.1523/JNEUROSCI.22-06-02255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 119.Kondo T, Raff MC. A role for Noggin in the development of oligodendrocyte precursor cells. Dev Biol. 2004;267:242–251. doi: 10.1016/j.ydbio.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 120.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 121.Rich JN, Bao S. Chemotherapy and cancer stem cells. Cell Stem Cell. 2007;1:353–355. doi: 10.1016/j.stem.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 122.Eberhart CG. Even cancers want commitment: Lineage identity and medulloblastoma formation. Cancer Cell. 2008;14:105–107. doi: 10.1016/j.ccr.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shiras A, Chettiar ST, Shepal V, et al. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- 124.Taylor DL, Jones F, Kubota ES, et al. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci. 2005;25:2952–2964. doi: 10.1523/JNEUROSCI.4456-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abou-Kheir WG, Hynes PG, Martin PL, et al. Characterizing the contribution of stem/progenitor cells to tumorigenesis in the Pten-/-TP53-/- prostate cancer model. Stem Cells. 2010;28:2129–2140. doi: 10.1002/stem.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen R, Nishimura MC, Bumbaca SM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 127.Bar EE, Chaudhry A, Farah MH, et al. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol. 2007;170:347–355. doi: 10.2353/ajpath.2007.060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 129.Takebe N, Harris PJ, Warren RQ, et al. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 130.Dirks PB. Cancer: Stem cells and brain tumours. Nature. 2006;444:687–688. doi: 10.1038/444687a. [DOI] [PubMed] [Google Scholar]
- 131.Lee D, Park C, Lee H, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 134.Glass R, Synowitz M, Kronenberg G, et al. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 136.Oh MC, Lim DA. Novel treatment strategies for malignant gliomas using neural stem cells. Neurotherapeutics. 2009;6:458–464. doi: 10.1016/j.nurt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maragakis NJ. Stem cells and the ALS neurologist. Amyotroph Lateral Scler. 2010;11:417–423. doi: 10.3109/17482968.2010.489116. [DOI] [PubMed] [Google Scholar]
- 138.Park DH, Eve DJ, Chung YG, et al. Regenerative medicine for neurological disorders. ScientificWorldJournal. 2010;10:470–489. doi: 10.1100/tsw.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schwarz SC, Schwarz J. Translation of stem cell therapy for neurological diseases. Transl Res. 2010;156:155–160. doi: 10.1016/j.trsl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 140.Taupin P. Neurogenesis, NSCs, pathogenesis and therapies for Alzheimer's disease. Front Biosci (Schol Ed) 2011;3:178–190. doi: 10.2741/s143. [DOI] [PubMed] [Google Scholar]
- 141.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders: Time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10(suppl):S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 143.Kelly S, Bliss TM, Shah AK, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alper J. Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol. 2009;27:213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- 145.Raore B, Federici T, Taub J, et al. Cervical multilevel intraspinal stem cell therapy: Assessment of surgical risks in Gottingen minipigs. Spine (Phila Pa 1976) 2011;36:E164–E171. doi: 10.1097/BRS.0b013e3181d77a47. [DOI] [PubMed] [Google Scholar]
- 146.Taupin P. Therapeutic potential of adult neural stem cells. Recent Pat CNS Drug Discov. 2006;1:299–303. doi: 10.2174/157488906778773670. [DOI] [PubMed] [Google Scholar]
- 147.Taupin P. Adult neural stem cells, neurogenic niches, and cellular therapy. Stem Cell Rev. 2006;2:213–219. doi: 10.1007/s12015-006-0049-0. [DOI] [PubMed] [Google Scholar]
- 148.Taupin P. HuCNS-SC (StemCells) Curr Opin Mol Ther. 2006;8:156–163. [PubMed] [Google Scholar]