The effect of donor age on induced pluripotent stem cell (iPSC) lines and on the cells redifferentiated from these iPSCs was examined. iPSCs were derived from vaginal fibroblasts from women with pelvic organ prolapse. Donor age did not appear to affect reprogramming and cell mitotic activity in fibroblasts redifferentiated from iPSCs, and donor age differences were not observed in the iPSCs using standard senescence markers.
Keywords: p53, Induced pluripotent stem cells, Reprogramming
Abstract
We aimed to derive induced pluripotent stem cell (iPSC) lines from vaginal fibroblasts from older women with pelvic organ prolapse. We examined the effect of donor age on iPSCs and on the cells redifferentiated from these iPSCs. Vaginal fibroblasts were isolated from younger and older subjects for reprogramming. iPSCs were generated simultaneously using an excisable polycistronic lentiviral vector expressing Oct4, Klf4, Sox2, and cMyc. The pluripotent markers of iPSCs were confirmed by immunocytochemistry and quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Spectral karyotyping was performed. The ability of the iPSCs to differentiate into three germ layers was confirmed by embryoid body and teratoma formation. Senescence marker (p21, p53, and Bax) expressions were determined by qRT-PCR and Western blot. The iPSCs were redifferentiated to fibroblasts and were evaluated with senescence-associated β-galactosidase (SA) activity and mitotic index using time-lapse dark-field microscopy. iPSCs derived from both the younger and older subjects expressed pluripotency markers and showed normal karyotype and positive teratoma assays. There was no significant difference in expression of senescence and apoptosis markers (p21, p53, and Bax) in iPSCs derived from the younger subject compared with the older subject. Furthermore, fibroblasts redifferentiated from these iPSCs did not differ in SA activity or mitotic index. We report successful derivation of iPSCs from women with pelvic organ prolapse. Older age did not interfere with successful reprogramming. Donor age differences were not observed in these iPSCs using standard senescence markers, and donor age did not appear to affect cell mitotic activity in fibroblasts redifferentiated from iPSCs.
Introduction
Pelvic floor disorders (PFDs) are a group of degenerative conditions that include urinary and fecal incontinence and pelvic organ prolapse (POP). The pathophysiology of PFDs is multifactorial. It includes atrophy of the urethral rhabdosphincter and smooth muscle, changes in connective tissues, and loss of innervation and pelvic muscle mass. Vaginal birth trauma and aging are thought to be major contributors to PFD [1]. Current treatment is primarily surgical. However, nearly 30% of treated women experience a relapse [2], suggesting a clear need for new preventive and treatment strategies.
Small clinical studies have explored the feasibility of stem cell therapy for treatment of urinary incontinence using adult stem cells such as muscle-derived stem cells (MDSCs) and adipose-derived stem cells (ADSCs) [3, 4]. Carr and colleagues [5] demonstrated treatment efficacy of autologous MDSC injections into the urethra for stress urinary incontinence in a blinded, randomized study involving 29 patients. Animal data with both MDSCs and ADSCs have also shown optimistic results with increases in urethral pressure after stem cell or differentiated myoblast injections [6–12]. These promising clinical data suggest that stem cell therapy may be a viable noninvasive therapy for urinary incontinence and for other forms of PFD [13, 14]. Because these therapies are still in their early explorative stages, the optimum stem cell type, stage of differentiation of the cells to be used, and ultimate composition of differentiated cells for reinjection have not been defined.
The reprogramming of somatic cells to induced pluripotent stem cells (iPSCs) has opened new possibilities to restore tissues that are deficient because of aging or degenerative disease. The advantage of pluripotent stem cells (human embryonic stem cell [hESC] lines and iPSCs) over the adult stem cells is that they are pluripotent, allowing differentiation into all cell types in tissues (muscle, nerves, blood vessels, and connective tissues) for full restoration of tissues with different types of cells. Additionally, data suggest that cellular reprogramming has the ability to counteract the mechanisms of cellular aging, which brings the cells to a self-renewing, rejuvenated state. If iPSCs are to be considered for autologous stem cell therapies for PFD or other degenerative conditions that are associated with aging, the effect of cellular senescence must be defined. Here we report on the feasibility of iPSC derivation from fibroblasts of older women affected with PFD. We also examine donor age-related effects on the derived iPSCs and whether these differences are carried over to the cells differentiated from these cell lines.
Activation of oncogenes, shortened telomeres, DNA damage, oxidative stress, and mitochondrial dysfunction are all associated with cell senescence. Tumor suppressor protein p53 is known to play an important role in senescence and apoptotic responses to dysfunctional telomeres [15] and genotoxic stress [16]. When p53 is activated, it triggers p21 and Bax activation, which mediate different aspects of p53 action on senescence and apoptosis, respectively [17]. Data on adult stem cells such as human mesenchymal stem cells (MSCs) indicate that p53 levels increase with age, mirroring cumulative levels of stress [18], and both p53 and p21 were found to be upregulated in MSCs from older donors [19]. However, studies on senescence-related mitochondrial/oxidative stress pathway and telomere size in iPSCs suggest that cellular reprogramming into iPSCs can modulate these mechanisms toward a rejuvenated state [20, 21]. We evaluate this by examining the p53 signaling pathway gene expressions in the iPSCs derived from patients with PFD. To examine donor age effect on the cells redifferentiated from iPSCs, we examined senescence-associated β-galactosidase (SA) activity, a specific biomarker for senescence [22], and mitotic index, the fraction of cells in mitosis in at any given time [23].
Materials and Methods
Tissue Collection
The Institutional Review Board of the Stanford University School of Medicine and the Stanford University Stem Cell Research Oversight committee approved this study. Written informed consent was obtained from each subject. Vaginal tissues were collected for fibroblast culture because these are the tissues affected by PFD. The vaginal wall tissues were collected as previously described [24]. We excised approximately 1 cm2 of full-thickness, periurethral vaginal tissue 1 cm lateral to the urethrovesical junction (identified by a Foley balloon) from a 78-year-old woman undergoing hysterectomy for pelvic organ prolapse and urinary incontinence. We excised smaller, 0.5-cm2 biopsies of vaginal mucosa from a similar area in a 47-year-old woman undergoing hysterectomy for fibroids. The epithelial layer was removed with a razor blade, and the tissues were washed three times with Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, http://www.invitrogen.com). They were then cut into small pieces (1 mm2) for fibroblast isolation.
Isolation of Primary Vaginal Wall Fibroblast Culture
We isolated fibroblasts from vaginal wall tissues of a 78-year-old woman and a 47-year-old woman and cultured them as described previously [25]. The purity of the cultured fibroblasts was confirmed by immunofluorescence using mouse anti-desmin monoclonal antibody (a smooth muscle cell marker; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), mouse anti-cytokeratin AE1/AE3 monoclonal antibody (an epithelial cell marker; Chemicon, Temecula, CA, http://www.chemicon.com), or mouse anti-vimentin (a fibroblast marker; Sigma-Aldrich) [25]. Fibroblasts isolated from each patient's vaginal wall tissue were cultured in six-well plates (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) in fibroblast culture medium (DMEM with 20% fetal bovine serum and 100 IU/ml penicillin-streptomycin) (Invitrogen).
Lentivirus Production and Infection
A single polycistronic lentiviral vector, encoding Oct4, Klf4, Sox2, and cMyc flanked by loxP sites (a gift from Dr. Mostoslavsky, Boston, MA) was produced in 293-FT (Invitrogen) packaging cells [26, 27]. 293FT cells maintained in DMEM with 10% fetal bovine serum (mouse embryonic fibroblast [MEF] growth medium), supplemented with 0.5 mg/ml Geneticin (Invitrogen), were allowed to expand until reaching 85%–90% confluence. One day prior to infection, fresh antibiotic-free MEF medium was added to the cells. For each 175-cm2 flask, 293FT cells were transfected with DNA plasmid mixture carrying 210 μg of pSTEMCCA, 10 μg of pTAT, 10 μg of pREV, 10 μg of pGAG/POL, and 21 μg of pVSVG. The transfection was facilitated by 120 μl of Lipofectamine 2000 (Invitrogen) and 15 ml of Opti-MEM (Invitrogen) for 6 hours and then replaced with 18 ml of fresh MEF medium without antibiotics. Viral supernatants were collected 48 hours after transfection by spinning and passing through a Millex-HV 0.45-μm filter unit (Millipore, Billerica, MA, http://www.millipore.com). The viral supernatants were concentrated by ultracentrifugation (Optima L-80 XP; Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com) at 17,100 rpm for 2.2 hours at 20°C and resuspended in fresh media to obtain 100× concentration.
Fibroblasts (100,000 per well) were plated in a six-well plate 2 days before infection. The fibroblasts were transduced one time with concentrated viral supernatant supplemented with polybrene (8 μg/ml) for 16–20 hours. The viral supernatants were washed with PBS and replaced with fresh fibroblast culture medium and changed every day. Seven days post-transduction, the cells were trypsinized, counted, and seeded into 10-cm dishes, preplated with irradiated mouse embryonic fibroblasts (MEF feeders). Induced pluripotent stem (iPS) colonies were manually picked 25–31 days post-transduction.
Maintenance Culture of iPSCs
iPSCs were clonally expanded and maintained on irradiated MEF feeders in hESC medium consisting of DMEM/F12 (Ham) medium with GlutaMAX supplemented with 20% knockout serum replacer, 0.1 mM nonessential amino acid, 100 IU penicillin-streptomycin, 0.1 mM β-mercaptoethanol, and 10 ng/ml recombinant human basic fibroblast growth factor (Invitrogen). The cells were passed every 5–7 days with collagenase IV (Invitrogen). We focused on three iPSC clones from each patient.
Immunofluorescence and Alkaline Phosphatase Staining
iPSCs grown on MEF feeder or embryoid bodies grown in the four-well chamber slides (Nunc, Rochester, NY, http://www.nalgenunc.com) were fixed in 4% paraformaldehyde for 30 minutes, permeabilized with 0.5% Triton X-100 in phosphate-buffered saline (PBS) for 30 minutes, and then blocked in 1% bovine serum albumin, 5% goat serum in PBS + 0.02% Tween (PBS-T) for 2 hours. The cells were incubated with primary antibodies at 4°C overnight; primary antibodies used were rabbit anti-Nanog (IgG, 1/500; Abcam, Cambridge, U.K., http://www.abcam.com), rabbit anti-Oct4 (IgG, 1/500; Abcam), rabbit anti-Sox2 (IgG, 1/500; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), rabbit anti-hTert (IgG, 1/500; Santa Cruz Biotechnology), rat anti-SSEA3 (IgG, 1/300; Millipore), mouse anti-SSEA4 (1/300; Millipore), mouse anti-TRA-1–60 (IgM, 1/300; Millipore), mouse anti-TRA-1–81 (IgM, 1/300; Millipore), mouse anti-α-fetoprotein (IgG, 1/200; Millipore), rabbit anti-βIII-tubulin (IgG, 1/200; Millipore), mouse anti-α-smooth muscle actin (IgG, 1/200; Millipore), mouse anti-vimentin (IgG, 1/40; Sigma-Aldrich), and mouse anti-cytokeratin (IgG; Dako, Glostrup, Denmark, http://www.dako.com). After washing with PBS-T, the cells were incubated with the secondary antibodies for 1 hour at room temperature. The secondary antibodies used were Alexa 488-conjugated goat anti-rabbit IgG (1/500), goat anti-mouse IgG (1/300; Invitrogen), Alexa 594-conjugated goat anti-rat IgM, or anti-mouse IgM (1/300; Invitrogen). 4′,6-Diamidino-2-phenylindole was used to stain the nuclei. Alkaline phosphatase staining was performed for 30 minutes at room temperature in the dark using the Vector Red Alkaline Phosphatase Substrate Kit I (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) according to the manufacturer's protocol.
In Vitro Differentiation of iPSC-Embryoid Body Formation
For embryoid body (EB) formation, the iPSCs were detached with collagenase IV (200 U/ml) and washed with EB differentiation medium consisting of DMEM/F12 (Ham), GlutaMAX, 20% FBS, 0.1 mM nonessential amino acids, 100 IU/ml penicillin-streptomycin (Invitrogen), and 0.1 mM β-mercaptoethanol (Millipore). The cells were cultured in suspension in ultralow-attachment six-well plates containing differentiated medium for 8 days (Corning Enterprises, Corning, NY, http://www.corning.com). The cells were then transferred into gelatin-precoated six-well plates and cultured in differentiated medium until beating cardiomyocytes (10–15 days) were observed. Differentiated cells were trypsinized and transferred into four-well chamber slides for immunostaining.
In Vitro Differentiation of iPSCs Toward Fibroblast-Like Cells
Floating EBs from three iPSC clones of each cell line were suspended in the native rat tail type I collagen (Sigma-Aldrich) solution (0.75 mg/ml) as described by Togo et al. [28] and cultured in a six-well plate with the basal medium (1:1 of differentiation medium and DMEM/F12 [Ham] medium) for 21 days in type I collagen gels. The gels were dissolved with 1 mg/ml collagenase at 37°C for 1 hour. The resulting cells containing EBs were cultured in the DMEM with 20% FBS, 100 IU/ml penicillin-streptomycin. The cellular biomarkers (vimentin, cytokeratin, and α-smooth muscle actin) were determined by immunocytochemistry.
Teratoma Assay
For the teratoma assay, approximately 1 × 106 iPSCs were harvested by collagenase IV treatment and injected subcutaneously into SCID mice (Charles River Laboratories, Wilmington, MA, http://www.criver.com) as described previously [29]. The tumors were dissected 7–9 weeks post-transplantation from the mice and fixed with 4% paraformaldehyde/PBS solution for 16 hours. Paraffin-embedded specimens were cut into 5-μm sections, and hematoxylin and eosin staining was performed. The presence of all three germ layers was detected by light microscope. All animal experiments were performed in accordance with institutional guidelines.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
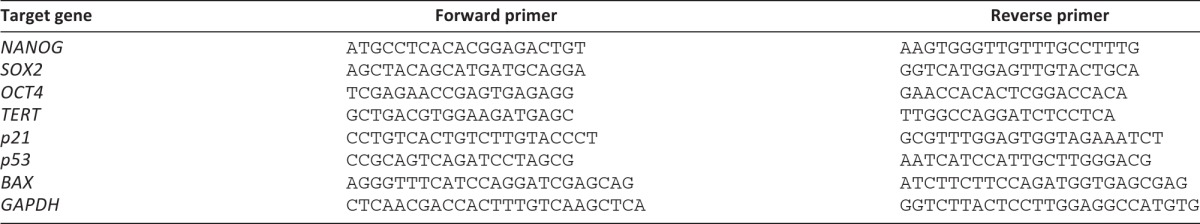
The iPSCs were grown on the Matrigel in 60-mm dishes (BD Biosciences) containing MEF conditioned medium until they were 90% confluent. Total RNA was extracted from cells using the RNeasy kit from Qiagen (Qiagen, Hilden, Germany, http://www.qiagen.com) according to the manufacturer's instructions. The cDNA was generated from 1 μg of total RNA using the SuperScript III First Strand Synthesis System (Invitrogen). PCR primers used to amplify NANOG, SOX2, OCT4, hTERT, p21, p53, BAX, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in Table 1. GAPDH was used as an endogenous reference. Real-time quantification PCR (qPCR) was carried on the Mx3005P Multiplex Quantitative PCR System with MxPro QPCR software (Stratagene, LA Jolla, CA). Brilliant SYBR Green qPCR Master Mix (Stratagene) was used to perform PCR. Real-time qPCR was performed as described previously [30]. The amplifications were done following a 10-minute hot start at 95°C in a three-step protocol with 30 seconds of denaturation (94°C), 1 minute of annealing (55°C), and extension at 72°C for 30 seconds. Forty cycles were performed. Cycle of threshold methods were used for quantification. Relative quantification of the respective gene corrected for the quantity of the normalizer gene (GAPDH) was divided by one normalized control sample value (calibrator) to generate the relative quantification to calibrator. The calculations were done by MxPro QPCR software. The PCR products were loaded on 2% agarose gel to confirm their size.
Table 1.
Primers used for quantitative reverse transcription-polymerase chain reaction
Western Blot Analysis
The iPSCs were grown on Matrigel (BD Biosciences) in 60-mm dishes containing MEF conditioned medium until they were 90% confluent. The cells were washed three times with PBS, lysed with RIPA buffer (50 mM Tris, 150 mM NaCl, 1% NP40, and 0.5% deoxycholate; Roche Diagnostics, Basel, Switzerland, http://www.roche-applied-science.com), and then rotated at 4°C overnight. The cell debris was removed by centrifugation at 14,000 rpm for 30 minutes. Total protein concentrations were determined using the Bradford method (Bio-Rad, Hercules, CA, http://www.bio-rad.com). The samples were subjected to 10% polyacrylamide gels (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). The gels were blotted onto nitrocellulose membranes (Pierce, Rockford, IL, http://www.piercenet.com) in an electrophoretic transfer cell (Bio-Rad). The blots were blocked with 5% nonfat milk at 4°C overnight and then probed with mouse anti-p21 monoclonal antibody (1 μg/ml; Abcam), mouse anti-p53 monoclonal antibody (2.5 μg/ml; Abcam), rabbit anti-Bax polyclonal antibody (1/100; Abcam), or goat anti-GAPDH (1/50,000; Abcam) for 1 hour at room temperature. After washing three times with phosphate-buffered saline with 0.1% Tween, pH 7.4 (PBS-T), the membrane was incubated in 1:5000 dilution of sheep anti-mouse IgG conjugated to HRP or in 1:5000 dilution of donkey anti-rabbit IgG conjugated to HRP or in 1:50,000 dilution of mouse anti-goat IgG conjugated to HRP (GE Healthcare, Little Chalfont, U.K., http://www.gehealthcare.com) for 1 hour at room temperature. This was followed by three washes in PBS-T. The blots were developed by chemiluminescence. The band density was determined by Bio-Rad Quality One Software.
Karyotypic Analysis
Spectral karyotyping was performed as described previously [31]. Briefly, actively growing iPSCs (passage 19) on the Matrigel were treated with 0.1 μg/ml Colcemid solution (Invitrogen) at 37°C for 16 hours. The cells were harvested with 0.05% trypsin for 5 minutes at 37°C. Trypsin activity was inactivated with DMEM containing 10% FBS. The cell pellet was resuspended slowly in prewarmed hypotonic solution containing equal amounts of 0.4% potassium chloride and 0.4% sodium citrate and incubated at 37°C for 7 minutes. After centrifugation, the cells were fixed with Carnoy's solution (methanol:galcial acetic acid, 3:1) for 30 minutes at room temperature. This step was repeated twice. Finally, the cell pellet was resuspended into a small volume of fixative and dropped onto a precleaned slide. Spectral karyotypic (SKY) analysis was performed using a SKY Paint Probe Kit according to the manufacturer's instructions (Applied Spectral Imaging, Vista, CA, http://www.spectral-imaging.com). The metaphase spreads were detected and analyzed using the SkyView Spectral Imaging System (Applied Spectral Imaging, Inc.).
Cytochemical Staining for SA
SA is widely used as a biomarker of cellular senescence. We were unable to clearly identify the SA-positive staining single iPSC because of cell clumping. Therefore, we examined SA in fibroblast-like cells differentiated from EBs derived from the clones of each cell line. The cells at passage 3 were stained with a Senescence Cells Histochemical Staining Kit (Sigma-Aldrich) overnight at 37°C at the manufacturer's suggestion. Stained cells were viewed under a Leica DMIRB inverted microscope at ×200 magnification. The percentage of SA-positive cells was determined by counting the number of blue cells under bright field and then the total number of cells in the same field under phase contrast. Three fields for each well were examined by two examiners. The average numbers were used for data analysis.
Mitotic Activity of Fibroblasts Determined by Time-Lapse Dark-Field Microscopy
We determined the mitotic index, a cell proliferation marker, by time-lapse dark-field microscopy. This technique allowed us to assess cell replication over a period of time without exposure to harmful light or radiation. The single fibroblast-like cells differentiated from three iPSC clones of each cell line in DMEM with 20% FBS were loaded into a four-well plate (BD Biosciences; 200,000 cells per well) coated with Matrigel. The cells were covered with oil. The cells from the older patient were imaged simultaneously with cells from the younger patient in order to minimize experimental variation. The time-lapse imaging was performed on a system setup with two modified Olympus IX-70/71 microscopes equipped with Tokai Hit heated stages, white-light Luxeon, LEDs, and an aperture for dark-field illumination [32]. The time-lapse images were acquired every 5 minutes for 16 hours. Each image field included roughly 130 cells. Quick Time Pro.7 (Apple, Cupertino, CA, http://www.apple.com) and ImageJ (http://rsbweb.nih.gov/ij/download.html) software were used to count the cell numbers of mitosis.
Results
Generation and Characterization of iPSCs Derived From Fibroblasts of Younger and Older Subjects
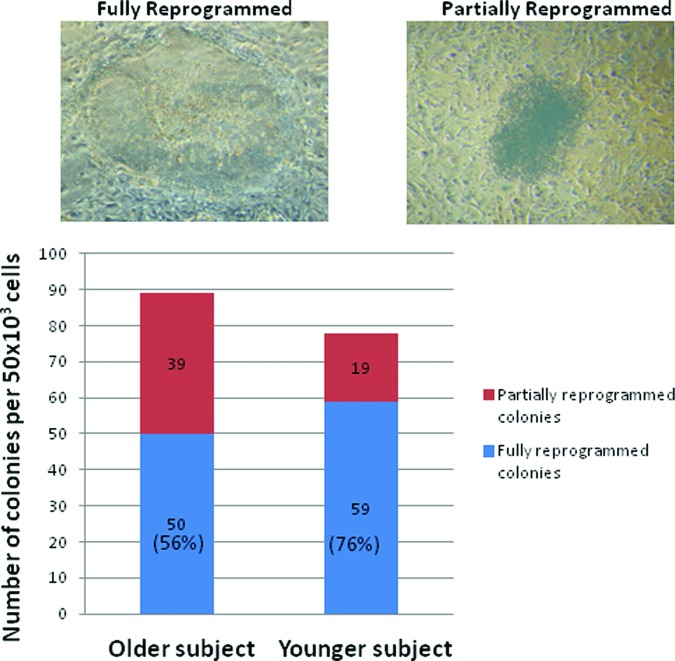
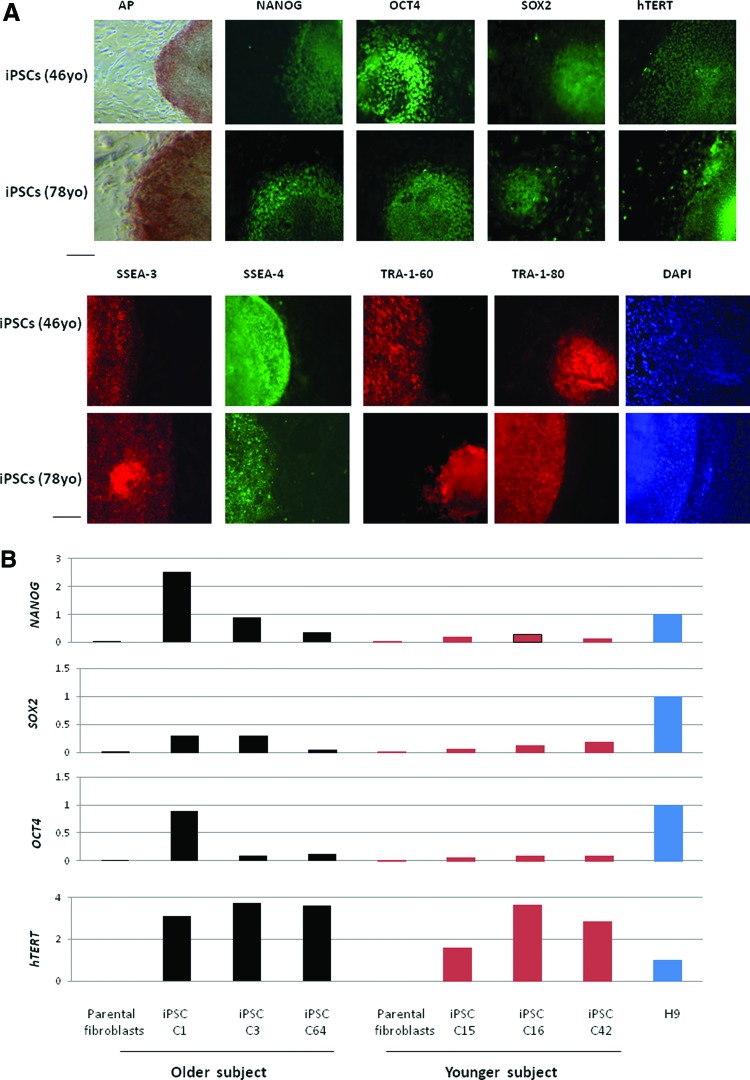
To generate iPSCs, we simultaneously transduced the fibroblasts, which were isolated from the vaginal wall of a 78-year-old woman with POP and a 47-year-old woman without POP, with a single polycistronic lentiviral vector, encoding Oct4, Klf4, Sox2, and cMyc transgenes flanked by loxP sites. The iPS colonies were picked up at day 25 post-transduction. Reprogramming of somatic cells to iPSCs was more efficient in the younger subject (76% fully reprogramming colonies) compared with the older subject (56% fully reprogramming colonies; Fig. 1). The iPSC clones from these two cell lines were maintained and expanded on irradiated MEF cells. They expressed alkaline phosphatase and were positive for pluripotency markers: NANOG, OCT4, SOX2, hTERT, SSEA-3, SSEA-4, Tra-1–60, and Tra-1–80 (Fig. 2A, 2B). Quantitative real-time RT-PCR using the primers designed to recognize the genes of pluripotency markers (Table 1) showed that the mRNA expression levels of NANOG, SOX2, OCT4, and hTERT in these two iPSC lines were increased relative to their parental fibroblast controls. H9 hESCs were used as a positive control. When compared with H9 hESCs, there was no significant difference in increased expression of pluripotency marker gene expressions between the iPSC lines.
Figure 1.
Counts of colonies with human embryonic stem (hES) cell-like morphology and non-hES cell-like colonies from transduction of 50 × 103 vaginal wall fibroblasts isolated from a 78-year-old woman with pelvic organ prolapse and a 47-year-old control woman. The number of colonies was counted at day 31 after transduction. The data are the averages of two individual plates of each subject.
Figure 2.
Characterization of iPSC lines. (A): Patient-specific iPSC lines derived from vaginal wall fibroblasts of 78- and 46-year-old subjects expressed AP. Immunofluorescence staining of the iPSCs also showed expression of pluripotency markers, including NANOG, OCT4, SOX2, hTERT, SSEA-3, SSEA-4, Tra-1–60, and Tra-1–80. DAPI staining is shown on the right and indicates the total cell content per image. Mouse embryonic fibroblasts surrounding human colonies served as internal negative controls for immunohistochemistry staining. Scale bars = 100 μm. (B): Quantitative real-time reverse transcription-polymerase chain reaction (PCR) assay for mRNA expression level of pluripotency-associated genes in two iPSC lines (passage 9) derived from the young and older donors and their respective parental fibroblasts. The expression level of endogenous NANOG, SOX2, OCT4, and hTERT genes was increased in both iPSC lines (passage 9) relative to their respective parental fibroblast controls. Three clones (passage 9) from each cell line were analyzed (C1, C3, and C64 were derived from the older donor; C15, C16 and C42 were derived from the younger donor). PCRs were normalized against internal control (glyceraldehyde-3-phosphate dehydrogenase). The H9 clone was used as a positive control and a calibrator for PCR. Abbreviations: AP, alkaline phosphatase; DAPI, 4′,6-diamidino-2-phenylindole; iPSC, induced pluripotent stem cell; yo, years old.
In Vitro and In Vivo Differentiation of iPSC Lines
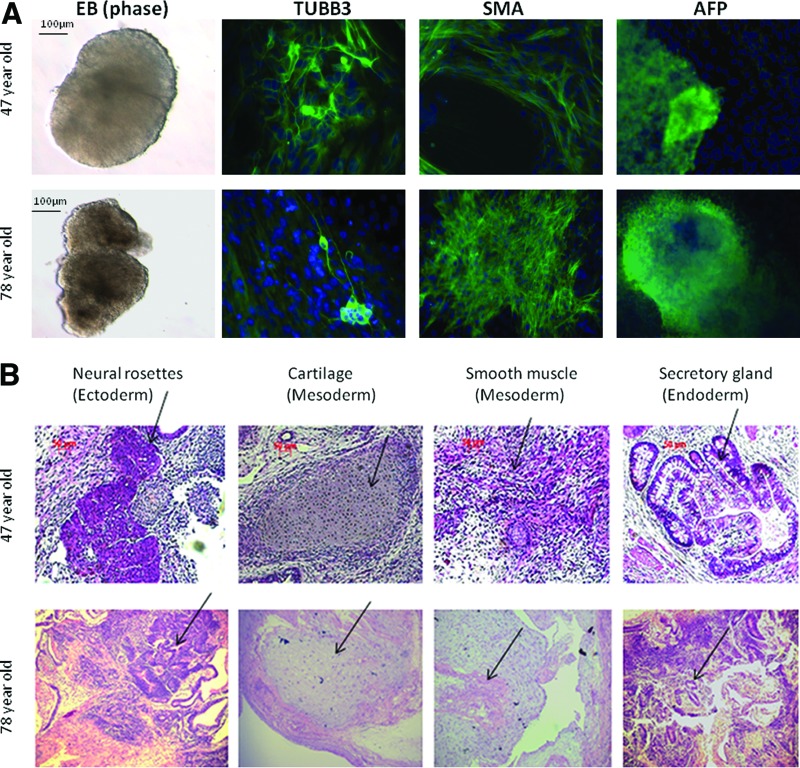
iPSCs from both of the lines were able to spontaneously differentiate into derivatives of the three germ layers in vitro. The EBs derived from these two cell lines showed positive staining for βIII-tubulin, an ectodermal marker; α-smooth muscle actin (SMA), a mesoderm marker; and α-fetoprotein, an endodermal marker (Fig. 3A).
Figure 3.
In vitro and in vivo differentiation of induced pluripotent stem cell (iPSC) lines. (A): Immunofluorescence staining of differentiated markers (overlayered with 4′,6-diamidino-2-phenylindole) on 28-day attached EBs. Ectoderm marker βIII-tubulin, mesoderm marker SMA, and endoderm marker α-fetoprotein were detected in EBs. EBs derived from H9 clone were used as a positive control. Scale bars = 100–200 μm. (B): The formation of teratoma was evaluated using passage 15 iPSCs implanted subcutaneously. Hematoxylin- and eosin-stained teratoma sections were detected by light microscopy. Scale bars = 200 μm. The tissues from each of the three germ layers, including neural rosettes (ectoderm), cartilage and smooth muscle (mesoderm), and secretory gland (endoderm), are shown. Abbreviations: AFP, α-fetoprotein; EB, embryoid body; SMA, α-smooth muscle actin; TUBB3, βIII-tubulin.
Furthermore, these iPSCs were able to form teratomas in vivo after subcutaneous injection into SCID mice. Histological analysis revealed that the teratomas were comprised of tissues from each of the three germ layers (Fig. 3B).
Effects of Donor Age on p53 Pathway in iPSCs
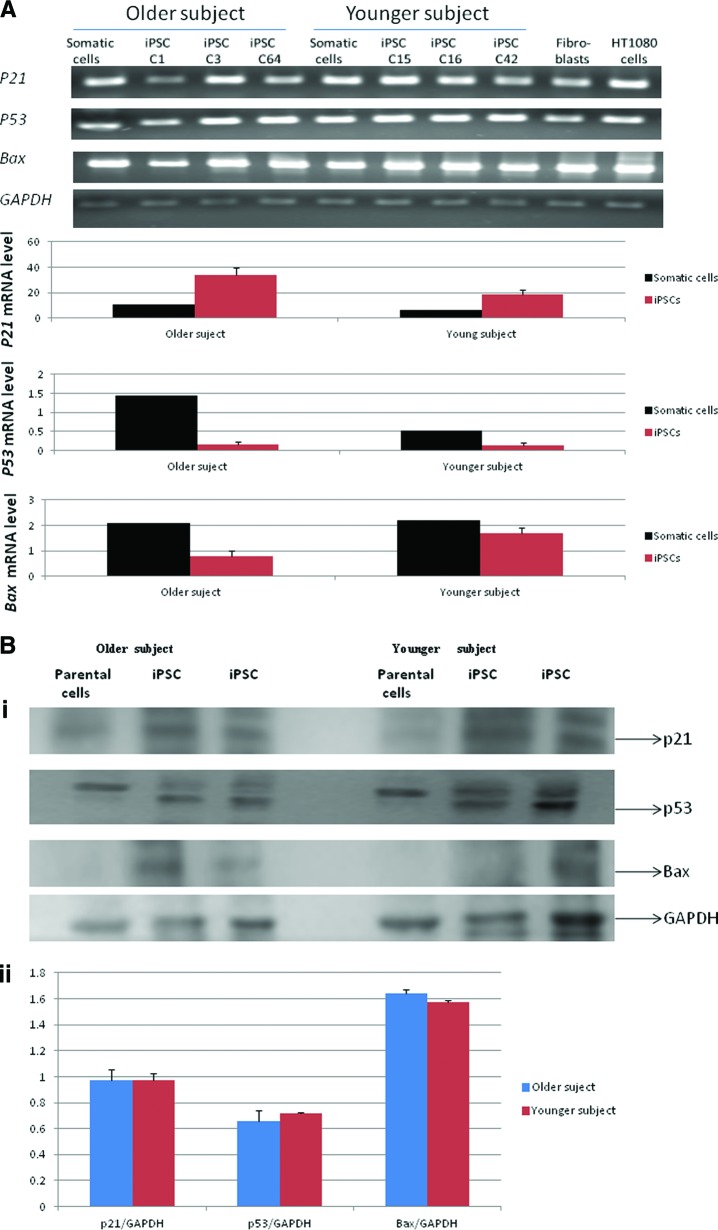
To test the effect of age on p53 pathway gene expression, we examined the expression of p53, p21, and BAX by quantitative real-time RT-PCR from three iPSC clones of each cell line. The expression level of p53, p21, and BAX in the three iPS clones derived from the older subject did not differ significantly compared with the three clones derived from the younger subject (Fig. 4A). This was confirmed by the protein expression levels of p53, p21, and BAX (Fig. 4B).
Figure 4.
Expression of senescence-associated genes in iPSC lines. (A): Quantitative real-time reverse transcription-polymerase chain reaction (PCR) for p21, p53, and Bax genes indicated no difference (p > .05) in the expression of senescence-associated genes in three induced pluripotent stem clones of each cell line. The mRNA expression level is shown as the average of three clones of each cell line (passage 9). C1, C3, and C64 were derived from the older donor; C15, C16, and C42 were derived from the younger donor). The reactions were normalized against internal control (GAPDH). Fibroblasts isolated from the vaginal wall of a 34-year-old subject were used a calibrator for real-time PCR. HT1080 cells were used a positive control. (Bi): Representative Western blots of p53, p21, and Bax from two clones (passage 9) from each cell line were analyzed. GAPDH was used a loading control. (Bii): The average expression levels of three clones (passage 9) derived from an older and a younger donor indicate no significant difference between groups. Abbreviations, GAPDH, glyceraldehyde-3-phosphate dehydrogenase; iPSC, induced pluripotent stem cell.
Effects of Donor Age on SA Activity and Cell Mitotic Index in the Fibroblasts Redifferentiated From iPSC Lines
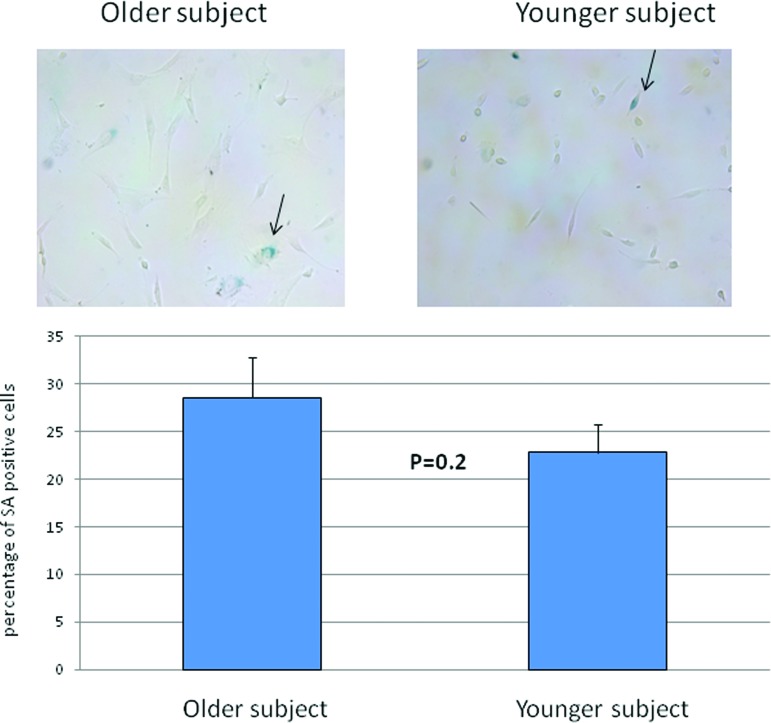
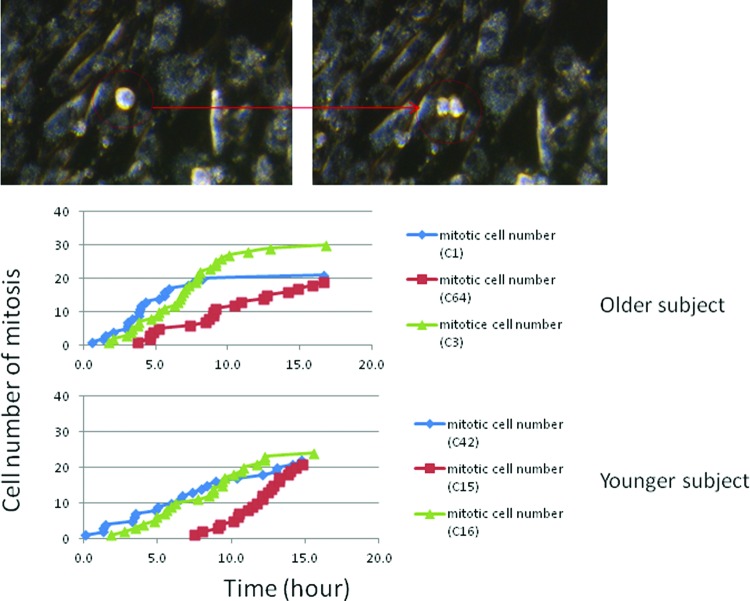
The fibroblast-like cells derived from EBs (three clones from each cell line) stained with vimentin (marker for fibroblasts), cytokeratin (marker for epithelial cells), and α-SMA showed that more than 95% cells were positive for vimentin. Less than 5% of the redifferentiated cells were positive for α-SMA, and they were entirely negative for cytokeratin (Fig. 5). We stained these cells for SA activity and showed no difference between the older and younger cell lines (Fig. 6). The mitotic index, which was determined by the numbers of cells in mitosis at a given time, in the fibroblast redifferentiated from the older donor iPSC line (20–30 cells per 16 hours) was similar to that of the fibroblasts differentiated from the younger donor iPSC lines (20–25 cells per 16 hours) over a 16-hour time course (Fig. 7).
Figure 5.
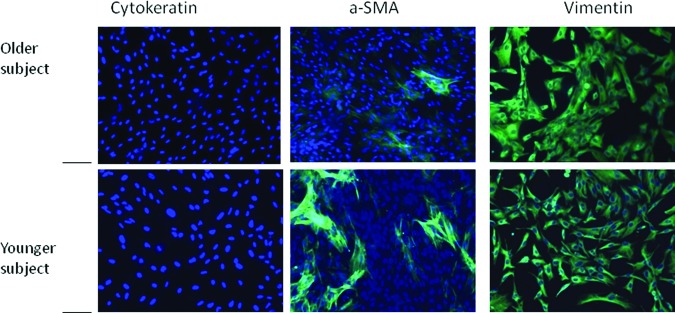
Immunocytochemistry of redifferentiated fibroblasts in monolayers from embryoid bodies cultured in collagen type 1 gel for 26 days. At passage 2, cellular biomarkers were analyzed by immunohistochemistry. Top: older donor; bottom: young donor. Less than 5% of the cells were cytokeratin-positive and α-SMA-positive, and more than 95% of the cells were vimetin-positive. Scale bars = 200 μm. Abbreviation: a-SMA, α-smooth muscle actin.
Figure 6.
Comparison of SA activity in the fibroblasts differentiated from the older-donor and young-donor induced pluripotent stem cells by cytochemistry. No significant difference was observed between the two cell lines. Arrows indicate SA-positive staining fibroblasts. Abbreviation: SA, senescence-associated β-galactosidase.
Figure 7.
The proliferation of the fibroblasts redifferentiated from the older-donor and younger-donor iPSCs was measured by mitotic activity with a time-lapse dark-field microscope during a 16-hour time course (150 cells per field at time 0). The cells in mitosis (red arrow) were counted with Quick Time Pro7 and ImageJ software. The fibroblasts of C1, C3, and C64 were redifferentiated from three clones of iPSCs of the older donor; the fibroblasts of C15, C16, and C42 were redifferentiated from three clones of iPSCs of the younger donor. The number of cells in mitosis in the older donor (20–30 cells per 16 hours) was similar to that in the younger donor (20–25 cells per 16 hours). The experiments with the same color curve were imaged simultaneously.
Discussion
Cell-based therapy using patient-specific iPSCs presents an alternative approach in the treatment of urinary incontinence and pelvic floor disorders. In contrast to surgery, where the repair is based on mechanical support of already deficient and aged tissues, stem cell therapy holds the promise of restoring many of the cellular components of the urethral continence mechanism. The prevalence of PFDs, such as urinary incontinence and pelvic organ prolapse, increases with age. Therefore, many of the patients seeking treatment will be older. With respect to PFD, there are no data on reprogramming of somatic cells from older patients, and there are scant data about the effect of donor age on the basic characteristics of iPSCs and their potential in regenerative applications. The present study reports derivation of iPSCs from vaginal fibroblasts from older women with PFD. We also examine whether there are donor age-related effects in our iPSCs and whether these differences are carried over to the cells redifferentiated from these iPSC lines.
Although reprogramming of somatic cells to iPSCs was more efficient in the younger subject (76% fully reprogramming colonies) compared with the older subject (56% fully reprogramming colonies), the iPSCs derived from the vaginal wall fibroblasts of both the older and younger women expressed the embryonic stem cell markers detected by reverse transcription-polymerase chain reaction and immunocytochemical staining. They were all pluripotent with respect to their ability to differentiate into the three germ layers in vitro (embryonic bodies) and in vivo (teratomas). Both iPSC lines showed normal karyotype (data shown in supplemental online Fig. 1).
When the tumor suppressor protein p53 is activated, it triggers p21 and Bax to mediate different aspects of p53 action on senescence and apoptosis, respectively [17]. We previously documented that the expression of apoptotic proteins, Bad and Bax, in vaginal wall tissues of women with severe PFD was higher compared with the control group [33]. These data suggest that there is increased apoptotic activity or sensitivity to induction of apoptosis in the vaginal wall tissues of women with PFD. Therefore, we characterized the effect of donor age on the iPSCs derived from the older woman with PFD with respect to p53 signaling pathway and apoptosis. We did not find significant differences in the mRNA and protein expression of p53, p21, and Bax between iPSCs from younger and older PFD donors, consistent with other reports of cellular rejuvenation after reprogramming [21, 34, 35].
Human iPSC lines have been derived from a variety of somatic cell types and characterized. However, few studies examined the proliferative capability, a key parameter of cell function, of the cells redifferentiated from the iPSCs. Feng et al. [36] observed significantly increased apoptosis, limited growth and expansion capability, and substantially decreased hematopoietic colony-forming capability in the hemangioblasts differentiated from human iPSCs (hiPSCs). Likewise, reduced proliferation was documented in cardiomyocytes differentiated from hiPSCs [37]. In contrast, Lapasset et al. [21] recently reported that fibroblasts derived from hiPSC from older donors proliferated at the same rate as young fibroblasts. These studies show inconsistent data on the behavior of cells redifferentiated from iPSCs. To investigate whether donor-age effects are carried over to the cells differentiated from the iPSC lines from donors affected with PFD, we examined cellular senescence (senescence-associated β-galactosidase activity) and mitotic activity of the fibroblasts redifferentiated from each iPSC line.
The activity of SA has been used as a marker for aged cells because it increases with aging of fibroblasts both in vitro and in vivo [38]. The number of SA-positive fibroblast-like cells differentiated from the iPSCs of the older donor was similar to that of young donor. Mitotic index, a proliferation marker [23], was analyzed using time-lapse dark-field imaging analysis over 16 hours. In previous experiments, we observed that the mitotic rate of the vaginal wall fibroblasts from older women with PFD (age >60 years old) was significantly lower than the rate of the vaginal wall fibroblasts from young women (age <50 years old; unpublished data). This is consistent with published reports on the effects of donor age on fibroblasts, where population doubling level and mitotic index were observed to have an inverse relationship with donor age [39, 40]. However, this age-associated effect on the mitotic rate was not observed in the fibroblasts redifferentiated from the iPSCs derived from the old donor. The mitotic rate of cells redifferentiated from iPSCs was similar between the younger and older subjects.
The above observations suggest that reprogramming vaginal fibroblasts from older patients with PFD into iPSCs and subsequent redifferentiation can yield “rejuvenated” cells. This is consistent with the recent documentation that skin fibroblasts from a centenarian can be reprogrammed into iPSCs with aging markers that are indistinguishable from human embryonic stem cells and that the iPSCs are able to redifferentiate into rejuvenated cells [21]. Likewise, iPSCs have been successfully reprogrammed from two older patients with type 2 diabetes. These iPSC clones acquired a rejuvenated state with elongated telomeres and decreased senescence-related gene expression and oxidative stress signaling [34]. We note that, similar to these and other studies, the sample size in our study is small. Because of this, we cannot generalize our observations to conclude that cellular rejuvenation occurs through iPSC reprogramming of cells from older patients with PFD. We plan on future studies with a larger sample size to confirm our observations.
Conclusion
The decline in tissue regenerative capacity caused by age-related changes in adult stem cells has been observed in animal and human studies [41–43]. Because of this, iPSC technologies should be considered for treatment of pelvic floor disorders. We report successful derivation of iPSCs from vaginal fibroblasts from older women with PFD. We did not detect donor age-related changes in the iPSC lines derived from women with PFD or in the fibroblasts redifferentiated from these iPSCs. Similar to reports from iPSCs derived from the older subject, our observations suggest that the effects of aging may be “erased” by the reprogramming of fibroblasts from donors with PFD into iPSCs. These results support further exploration of iPSC therapies for pelvic floor disorders.
Acknowledgments
This work was supported by a grant from the Stanford University School of Medicine (to B.C.) and by a grant from the California Institute for Regenerative Medicine (CL-00518; to R.A.R.P.).
Author Contributions
Y.W.: conception and design, provision of the study material, collection and assembly of data, data analysis and interpretation, manuscript writing; P.W.: conception and design, manuscript writing; L.Z.: collection and assembly of data; T.B.: provision of the study material; S.M.P.: data analysis; R.A.R.P.: conception and design, financial support, manuscript writing, final approval of manuscript; B.C.: conception and design, financial support manuscript, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Rortveit G, Daltveit AK, Hannestad YS, et al. Urinary incontinence after vaginal delivery or cesarean section. New Engl J Med. 2003;348:900–907. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Nikolavasky D, Stangel-Wjcikiewicz K, Stec M, et al. Stem cell therapy: A future treatment of stress urinary incontinence. Semin Reprod Med. 2011;29:61–70. doi: 10.1055/s-0030-1268705. [DOI] [PubMed] [Google Scholar]
- 4.Staack A, Rodrguez L. Stem cells for the treatment of urinary incontinence. Curr Urol Rep. 2011;12:41–46. doi: 10.1007/s11934-010-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herschom S, Carr LK, Birch C. Autologous muscle-derived cells as therapy for stress urinary incontinence: A randomized blinded trial. Neurourol Urodyn. 2010;29:243–326. [Google Scholar]
- 6.Corcos J, Loutochin O, Campeau L, et al. Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodyn. 2011;30:447–455. doi: 10.1002/nau.20998. [DOI] [PubMed] [Google Scholar]
- 7.Jack G, Almeida F, Zhang R, et al. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: Implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. 2005;174:2041–2045. doi: 10.1097/01.ju.0000176489.96993.84. [DOI] [PubMed] [Google Scholar]
- 8.Kim S-O, Na H, Kwon D, et al. Bone-marrow-derived mesenchymal stem cell transplantation enhances closing pressure and leak point pressure in a female urinary incontinence rat model. Urol Int. 2011;86:110–116. doi: 10.1159/000317322. [DOI] [PubMed] [Google Scholar]
- 9.Lin G, Wang G, Banie L, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez L, Alfonso Z, Zhang R, et al. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng X, Jack G, Zhang R. Treatment of sui using adipose derived stem cells: Restoration of urethral function (Abstract) J Urol. 2006;175:291. [Google Scholar]
- 12.Zhao W, Zhang C, Jin C, et al. Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur Urol. 2011;59:155–163. doi: 10.1016/j.eururo.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Carr LK, Steele D, Steele S, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:881–883. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 14.Chancellor MB, Yoshimura N, Pruchnic R. Gene therapy strategies for urological dysfunction. Trends Mol Med. 2001;7:301–306. doi: 10.1016/s1471-4914(01)02088-3. [DOI] [PubMed] [Google Scholar]
- 15.Artandi S, Attardi L. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun. 2005;331:881–890. doi: 10.1016/j.bbrc.2005.03.211. [DOI] [PubMed] [Google Scholar]
- 16.Helmbold H, Deppert W, Bohn W. Regulation of cellular senescence by rb2/p130. Oncogene. 2006;25:5257–5262. doi: 10.1038/sj.onc.1209613. [DOI] [PubMed] [Google Scholar]
- 17.Vousden K. Activation of the p53 tumor suppressor protein. Biochim Biophys Acta. 2002;1602:47–59. doi: 10.1016/s0304-419x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 18.Atadja P, Wong H, Garkavtsev I, et al. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci USA. 1995;92:8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stolzing A, Jones E, McGonagle D. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Prigione A, Hossini A, Lichtner B, et al. Mitochondrial-associated cell death mechanisms are reset to an embryonic-like state in aged donor-derived iPS cells harboring chromosomal aberrations. PLoS One. 2011;6:e27352. doi: 10.1371/journal.pone.0027352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapasset L, Milhavet O, Prieur A, et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–2253. doi: 10.1101/gad.173922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H-T, Lee M-J, Chen C-H, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16:582–593. doi: 10.1111/j.1582-4934.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medri L, Volpi A, Nanni O, et al. Prognostic relevance of mitotic activity in patients with node-negative breast cancer. Mod Pathol. 2003;16:1067–1075. doi: 10.1097/01.MP.0000093625.20366.9D. [DOI] [PubMed] [Google Scholar]
- 24.Wen Y, Zhao Y-Y, Polan ML, et al. Effect of relaxin on TGF-beta1 expression in cultured vaginal fibroblasts from women with stress urinary incontinence. Reprod Sci. 2008;15:312–320. doi: 10.1177/1933719108315299. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, Wen Y, Yu X, et al. Elastin metabolism in pelvic tissues: Is it modulated by reproductive hormones? Am J Obstet Gynecol. 2005;192:1605–1613. doi: 10.1016/j.ajog.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Mostoslavsky G, Fabian A, Rooney S, et al. Complete correction of murine artemis immunodeficiency by lentiviral vector-mediated gene transfer. Proc Natl Acad Sci USA. 2006;103:16406–16411. doi: 10.1073/pnas.0608130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommer C, Sommer A, Longmire T, et al. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells. 2010;28:64–74. doi: 10.1002/stem.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Togo S, Sato T, Sugiura H, et al. Differentiation of embryonic stem cells into fibroblast-like cells in three-dimensional type I collagen gel cultures. In Vitro Cell Dev Biol Anim. 2011;47:114–124. doi: 10.1007/s11626-010-9367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne J, Nguyen H, Reijo Pera RA. Enhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblasts. PLoS One. 2009;4:e7118–e7118. doi: 10.1371/journal.pone.0007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen Y, Man WC, Sokol ER, et al. Is alpha2-macroglobulin important in female stress urinary incontinence? Hum Reprod. 2008;23:387–393. doi: 10.1093/humrep/dem370. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen H, Reijo Pera R. Metaphase spreads and spectral karyotyping of human embryonic stem cells. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot5047. pdb.prot5047. [DOI] [PubMed] [Google Scholar]
- 32.Smith Z, Nachman I, Regev A. Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nat Biotechnol. 2010;28:521–526. doi: 10.1038/nbt.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen Y, Ho J, Polan M, et al. Expression of apoptotic factors in vaginal tissues from women with urogenital prolapse. Neurourol Urodyn. 2011;30:1627–1632. doi: 10.1002/nau.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohmine S, Squillace K, Hartjes K, et al. Reprogrammed keratinocytes from elderly type 2 diabetes patients suppress senescence genes to acquire induced pluripotency. Aging. 2012;4:60–73. doi: 10.18632/aging.100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prigione A, Fauler B, Lurz R, et al. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 36.Feng Q, Lu S-J, Klimanskaya I, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geigl J, Langer S, Barwisch S, et al. Analysis of gene expression patterns and chromosomal changes associated with aging. Cancer Res. 2004;64:8550–8557. doi: 10.1158/0008-5472.CAN-04-2151. [DOI] [PubMed] [Google Scholar]
- 40.Kaji K, Ohta T, Horie N, et al. Donor age reflects the replicative lifespan of human fibroblasts in culture. Human Cell. 2009;22:38–42. doi: 10.1111/j.1749-0774.2009.00066.x. [DOI] [PubMed] [Google Scholar]
- 41.Asumda F, Chase PB. Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol. 2011;12:44–44. doi: 10.1186/1471-2121-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rando T. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 43.Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]