Abstract
Advancing age and loss of bone mass and strength are closely linked. Elevated osteoblast and osteocyte apoptosis and decreased osteoblast number characterize the age-related skeletal changes in humans and rodents. Similar to other tissues, oxidative stress increases in bone with age. This article reviews current knowledge on the effects of the aging process on bone and its cellular constituents, with particular emphasis on the role of reactive oxygen species (ROS). FoxOs, sirtuins and the p53/p66shc signaling cascade alter osteoblast number and bone formation via ROS-dependent and -independent mechanisms. Specifically, activation of the p53/p66shc signaling increases osteoblast/osteocyte apoptosis in the aged skeleton and decreases bone mass. FoxO activation in osteoblasts prevents oxidative stress to preserve skeletal homeostasis. However, while defending against stress FoxOs bind to β-catenin and attenuate Wnt/T-cell cell factor transcriptional activity and osteoblast generation. Thus, pathways that impact longevity and several diseases of ageing might also contribute to age-related osteoporosis.
Introduction
With advancing age, the amount of bone resorbed by the osteoclasts is not fully restored with bone deposited by the osteoblasts and this imbalance leads to bone loss. Thus, aging and osteoporosis are intimately linked. The decline in whole bone strength is due to reductions in trabecular and cortical bone density, decreased cortical thickness and a marked increase in cortical porosity.1,2 Loss of cancellous bone mass in humans starts in the third decade while cortical bone begins to decline after the age of 50.3 In women, the loss of bone occurs at a faster rate after the menopause, attesting to the adverse role of estrogen deficiency on bone mass and its contribution to the acceleration of skeletal involution with age. The critical pathogenetic mechanism leading to age-related skeletal fragility is impaired bone formation due primarily to insufficient number of osteoblasts.4,5 The defective osteoblast number in the aging skeleton has been attributed to a decrease in the number of mesenchymal stem cells, defective proliferation/differentiation of progenitor cells or diversion of these progenitors toward the adipocyte lineage, as well as to increased apoptosis.6 However, it remains unclear which of these processes has a causal role in mediating the decreased bone formation with age.
Loss of bone mass and strength in rodents with advancing age, similar to humans, is associated with an increase in the prevalence of apoptotic osteoblasts and osteocytes and a corresponding decrease in osteoblast number and bone formation rate.7,8,9,10 Increased intracortical porosity, associated with intense remodeling activity, is also a feature of murine bone aging.11,12 In contrast, the number of osteoclast in cancellous bone is diminished with age in line with a concomitant decrease in Receptor activator of nuclear factor-kappaβ (NF-kβ) ligand (RANKL) levels in the bone marrow plasma.7,13 Thus, aging exerts opposite effects on bone remodeling in the cancellous and cortical bone compartments. Despite their clinical relevance, the mechanisms underlying skeletal aging remain unclear. This article reviews the role of evolutionary conserved age-related mechanisms on bone and its cellular constituents.
Role of reactive oxygen species in bone
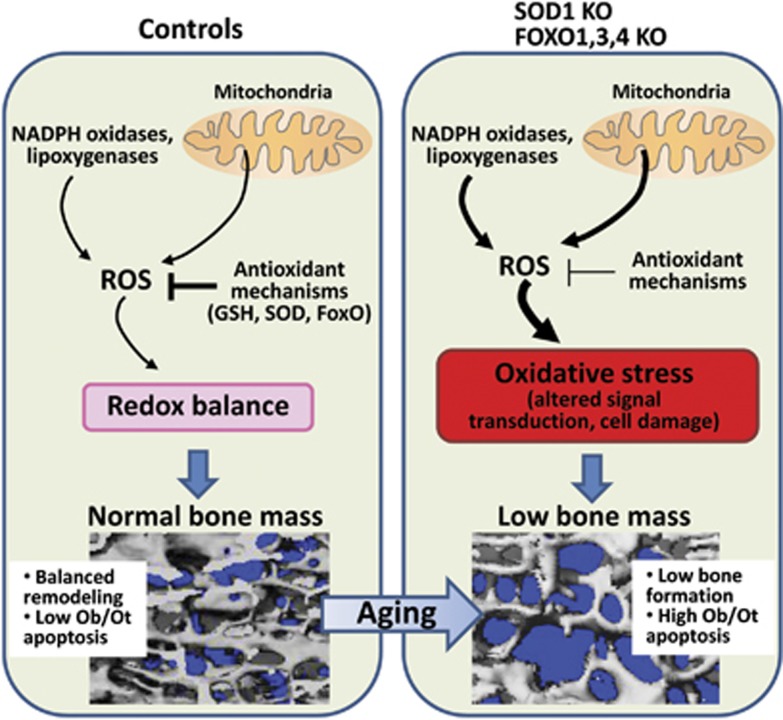
Progressive-free radical damage has been considered a key component in the tissue degeneration associated with aging and the skeleton in no exception.14,15 Indeed, the levels of reactive oxygen species (ROS) increase in bone with age and sex steroid deficiency.7,16,17 The majority of cellular ROS are generated by the mitochondrial electron transport chain during normal metabolism. ROS can also be produced in other cellular compartments by nicotinamide adenine dinucleotide phosphate oxidase oxidases, cyclooxygenases, lipoxygenases and other enzymes. To prevent oxidative stress, cells utilize diverse mechanisms that involve both enzymatic reactions and altered gene transcription. Of the most important antioxidant enzymes, various forms of superoxide dismutase convert superoxide anion to H2O2 and catalase converts H2O2 to water and oxygen. Alternatively, ROS can be detoxified via reactions with thiol-containing oligopeptides, the most abundant of which are glutathione and thioredoxin. Augmented mitochondrial damage with age, however, may result in excessive ROS production that damages proteins, lipids and DNA, leading to cell death.18,19 Oxidative stress results from an imbalance between excessive ROS or other oxidants and the capacity of the cell to build up an effective antioxidant response (Figure 1). Genetic studies using murine models have elucidated that oxidative stress is responsible for age-related tissue damage and various disease states such as diabetes, cardiovascular diseases, cancer and neurodegeneration. In mice, the loss of bone caused by gonadectomy is prevented by antioxidants.7,20,21 In addition, overexpression of the antioxidant thioredoxin-1 attenuates oxidative stress, as shown by 8-hydroxydeoxyguanosine (one of the major products of DNA oxidation) staining in bone tissue including osteoblasts, and prevents the decline in bone formation following streptozotocin-induced diabetes in mice.22 Further support for a deleterious role of oxidative stress in bone is provided by the evidence that both osteoblast numbers and bone formation are decreased in mice treated with the pro-oxidant buthionine sulfoximine,21 and that murine models of premature aging associated with oxidative damage exhibit osteoporosis.23,24 Importantly, mice with global deletion of the antioxidant gene Sod1 exhibit low bone mass, which worsens with age.25 The skeletal features of the Sod−/− mouse are similar to the aged skeleton of a normal mouse and include decreased trabecular and cortical bone mass, lower osteoblast and osteoclast numbers associated with decreased expression of RANKL in bone (Figure 1). Moreover, Sod1-deficient osteoblasts have higher ROS levels associated with increased apoptosis. Administration of the antioxidant vitamin C normalized bone mass, ROS levels and osteoblast lifespan in Sod−/− mouse.
Figure 1. The role of ROS in age-related skeletal involution.
(Left panel) In young normal mice, ROS produced in the mitochondria or in the cytoplasm are scavenged by several cellular antioxidants, resulting in redox homeostasis. This leads to balanced remodeling of the skeleton, low osteoblast (Ob) and osteocyte (Ot) apoptosis and normal bone mass. (Right panel) Antioxidant deficiency, as a result of superoxide dismutase (SOD)1 or FoxO1, 3, 4 deletion, leads to elevated levels of ROS and oxidative stress that, in turn, promote altered signal transduction and cell damage. Thus, SOD1 or FoxO1, 3, 4 KO mice exhibit premature skeletal aging characterized by low bone formation, high osteoblast and osteocyte apoptosis and low bone mass.
At the cellular level, oxidative stress decreases osteoblast and osteocyte lifespan, as highlighted by the evidence that administration of antioxidants abrogates osteoblast and osteocyte apoptosis in the bone of ovariectomized or aged mice.17 In addition to its role in apoptosis, oxidative stress may inhibit osteoblast formation.26 Indeed, the Wnt signaling pathway—critical for osteoblastogenesis—is attenuated by oxidative stress in vitro. Inhibition of Wnt/β-catenin signaling by ROS is mediated by the FoxO transcription factors, as described in detail below. Other mechanisms that contribute to the inhibition of Wnt signaling by ROS include dephosphorylation and activation of GSK-3β, which is required for β-catenin degradation, and increased expression of the Wnt signaling inhibitor Dkk1.27,28,29,30
Osteocytes—former osteoblasts buried in the bone matrix—are long-lived cells and are thus more prone to suffer the ravaging effects of aging. Osteocytes orchestrate bone resorption and formation via production of RANKL and sclerostin, respectively, in response to hormonal and mechanical stimuli.31 The elevated osteocyte apoptosis with age is associated with diminished levels of RANKL and sclerostin protein in bone.7,13 Thus, oxidative stress or other age-intrinsic mechanisms may alter bone remodeling via decreasing the number and, most probably, the synthetic capacity of osteocytes. Why these mechanisms have a different overall effect on the rate of remodeling in cancellous versus cortical bone, remains unclear.
However, ROS are not always harmful. ROS produced in a regulated manner during cellular metabolism have beneficial roles as mediators in signaling and defense processes including vasorelaxation, angiogenesis, production of erythropoietin and destruction of bacteria by macrophages.32,33 In addition, recent work has elucidated that ROS generation by the mitochondria is a causal factor in adipocyte differentiation.34 Importantly, formation and activation of osteoclasts requires ROS.35 In line with this, several different antioxidants prevent the increased bone resorption that follows loss of sex steroids.20 RANKL stimulates ROS production that, in turn, is required for osteoclastogenesis. The plasma membrane-associated nicotinamide adenine dinucleotide phosphate oxidase oxidases Nox1 and Nox2 are an important source for the ROS required in osteoclastogenesis. Specifically, RANKL increases ROS through a TRAF6/Rac1/Nox1-dependent pathway in osteoclasts.36,37 Mitochondrial biogenesis and ROS production are also essential for the bone resorbing function of osteoclasts and the bone loss caused by estrogen deficiency.38 Moreover, the mitochondria-targeted antioxidant MitoQ suppresses osteoclastogenesis.39 This evidence illustrates the requirement for ROS produced by different cellular compartments in osteoclastogenesis. Taken together, these findings support the contention that ROS have an important role in skeletal homeostasis and that oxidative stress contributes to the adverse effects of aging on bone mass and strength, via actions in osteoclasts and osteoblasts.
Pathways in skeletal aging
The molecular mechanisms that connect aging to age-related diseases are not well understood. A large effort of aging research has focused on genes and signaling pathways that control the rate of aging, the majority of which was initially identified in lower organisms. In particular, FOXO, sirtuins and p66shc proteins can alter diseases of aging in mammals and might have a role in age-related skeletal involution.
FoxO transcription factors.
Initial interest in FoxO transcription factors stemmed from their role in longevity in Caenorhabditis elegans and Drosophila.40,41 Genetic work in mice has also shown that FoxOs can reduce the impact of age-related tissue damage and several pathologies including neurodegeneration, metabolic diseases and cancer.42 These actions underscore the critical role of FoxOs in defense against oxidative stress.43 Mammalian FoxO1, 3 and 4 activity is inhibited by growth factors and the insulin signaling pathways and stimulated by nutrient depletion and a plethora of ROS-induced post-translational modifications.44 Recent studies have uncovered a fundamental role for FoxOs in skeletal homeostasis. Combined somatic deletion of FoxO1, 3 and 4 in adulthood resulted in increased oxidative stress in bone and bone loss at both cancellous and cortical sites45 (Figure 1). The decreased bone mass was due to deficient bone formation and resulted from decreased osteoblast number and increased osteoblast apoptosis. In contrast, mice with a gain of function of FoxO3 in osteoblasts exhibited decreased oxidative stress and osteoblast apoptosis, as well as increased osteoblast number, bone formation rate and bone mass. In line with the contention that FoxOs exert a critical role in defense against oxidative stress in osteoblasts, deletion of FoxO1 in col1a1-expressing cells decreased osteoblast numbers and bone mass as a consequence of increased oxidative stress.46 FoxO1 deletion also attenuated protein synthesis in osteoblasts, leading to diminished levels of glutathione and collagen1. FoxO1 stimulates protein synthesis by partnering with ATF4—a transcription factor that is an integral component of the pathway controlling amino acid import that protects against stress.46 Beside ATF4, FoxOs interact with other transcription factors and coactivators that are critical for osteoblast formation. Indeed, FoxO1 binds to Runx2 in osteoblasts, as well as in prostate cancer cells.47 This association is independent of the DNA-binding activity of FoxO1 and can repress or stimulate Runx2 activity.
As mentioned earlier, one of the main inhibitors of FoxO transcriptional activity is insulin. It has recently been shown that insulin signaling in osteoblasts is a positive regulator of postnatal bone acquisition.48,49 Mice lacking the insulin receptor in osteoblasts had reduced trabecular bone volume secondary to decreased bone formation and lower osteoblast numbers. These mice had also reduced osteoclast numbers in bone. Thus, insulin acting via the insulin receptor, similar to the actions FoxOs, in osteoblasts has a positive effect on bone formation. This work strongly suggests that targets other than inhibition of FoxOs are responsible for the increased bone mass promoted by insulin signaling. In contrast, insulin signaling in osteoblasts favors bone resorption by preventing the stimulatory action of FoxO1 on Opg.49 These lines of evidence clearly show that FoxOs have critical functions in skeletal homeostasis.
FoxO-Wnt signaling cross-talk.
β-Catenin has an indispensable role in promoting T-cell cell factor (TCF)/lymphoid enhancer-binding factor transcription. In addition, β-catenin is an essential co-activator of FoxOs.16,50 Oxidative stress induces the association of FoxOs with β-catenin, and β-catenin is required for the stimulation of FoxO target genes in osteoblasts and other cell types. Moreover, the interaction between the β-catenin ortholog, BAR-1 and the FoxO ortholog DAF-16 is required for oxidative stress-induced expression of the DAF-16 target gene sod-3, resistance to oxidative damage and lifespan extension in C. elegans.50 Oxidative stress induced by H2O2 promotes FoxO-mediated transcription at the expense of Wnt/TCF-mediated transcription and osteoblast differentiation.16 Similar to oxidative stress, nutrient deprivation promotes the association of β-catenin with FoxOs, rather than with TCF, to modulate the gluconeogenic response in the liver.51 In view of the role of β-catenin on cell proliferation/differentiation and osteoblast generation, not only during development but also postnatally,52,53 and the role of FoxOs in defense against stress, the ability of FoxOs to sequester β-catenin might restrict the proliferation/differentiation of osteoblast progenitors and contribute to the decreased bone formation and loss of bone mass during aging.54 In osteoblasts and osteocytes, β-catenin signaling increases Opg and decreases RANKL expression to attenuate osteoclastogenesis.55,56,57 In line with the inhibitory effect of oxidative stress on β-catenin/TCF-mediated transcription, the expression of Opg and other Wnt-target genes decrease with age in murine bone.16,17 It is unknown whether the decrease in Opg contributes to the increase in cortical bone remodeling seen with age.
Lipid oxidation has a critical role in the development of atherogenesis and, epidemiological evidence shows that atherosclerosis and osteoporosis are linked. Importantly, ROS/FoxO suppression of Wnt signaling may be also the mechanism by which lipid oxidation contributes to the decline in osteoblast number and bone formation that occurs with aging. In the process of lipid oxidation, lipoxygenases, like Alox15, oxidize polyunsaturated fatty acids to form products that bind to and activate PPARγ and generate pro-oxidants like 4-hydroxynonenal.58 Lipid oxidation increases with age in bone and 4-hydroxynonenal, like H2O2, activates FoxOs that in turn attenuate β-catenin/TCF-mediated transcription.59 In addition, TCF-mediated transcription suppresses peroxisome proliferator-activated receptor (PPAR)γ expression,60,61 so that inhibition of β-catenin/TCF transcriptional activity leads to an increase in PPARγ levels. Oxidized polyunsaturated fatty acids promote PPARγ association with β-catenin and induce β-catenin degradation,59,62 thereby further decreasing β-catenin/TCF-mediated transcription. Via these mechanisms, lipid oxidation potentiates oxidative stress and may contribute to the decay in bone formation that occurs with aging. Oxidized lipids also stimulate apoptosis of osteoblastic cells and inhibit bone morphogenetic protein-2-induced osteoblast differentiation via ROS-independent mechanisms.63,64,65
Sirtuins.
Sirtuins are NAD+-dependent deacetylases that slow aging in lower organisms and impact numerous diseases of aging in rodent models.66 Sirtuins mediate a wide range of biological functions, spanning from DNA repair to energy metabolism and oxidative stress responses. These effects have been linked to the deacetylation of many important transcription factors including FoxOs and p53 by Sirt1.67 In several tissues, Sirt1 exerts antioxidant effects by stimulating FoxO transcriptional activity. Moreover, Sirt3—one of the Sirt family member present in the mitochondria—has been recently shown to control the levels of ROS themselves by multiple mechanisms.68 Manipulation of Sirt1 activity, either pharmacologically with resveratrol or genetically, affects bone cells and alters bone mass. In vitro studies have revealed that resveratrol stimulates osteoblastogenesis and attenuates adipogenesis and osteoclastogenesis. The attenuation of adipogenesis and stimulation of osteoblastogenesis in bone marrow mesenchymal cells by resveratrol has been attributed to the inhibitory actions of Sirt1 on PPARγ.69 Resveratrol also upregulates Runx2 gene expression via the Sirt1/FoxO3 axis to promotes osteogenesis of human mesenchymal stem cells.70 In osteoclast progenitors resveratrol attenuates RANKL-induced NF-kβ activation and osteoclast formation.71 In line with these findings is evidence that Sirt1 inhibits NF-kβ signaling by deacetylating RelA/p65.72 Notably, long-term resveratrol administration to mice markedly reduced the signs of aging including loss of bone mass.73 Resveratrol also prevented ovariectomy-induced osteoblast apoptosis and bone loss.74
Physical activity stimulates bone formation and a decrease in mobility with age contributes to the loss of bone mass in humans. Hindlimb unloading in rodents is a commonly used model to mimic wasting disorders of unloading including osteoporosis. Administration of resveratrol to hindlimb suspended rats abrogated the loss of bone mass and strength in this model by preventing bone resorption and stimulating bone formation.75 In line with the effects of resveratrol on bone, heterozygous Sirt1 deletion decreased bone formation and trabecular bone mass in male mice.76 Moreover, targeted deletion of Sirt1 in osteoblasts decreases the bone formation rate and bone volume, whereas targeted deletion in osteoclast precursor cells leads to low bone mass due to increased osteoclast number.77 Thus, Sirt1 controls bone mass by independently regulating osteoblast and osteoclast lineage cells. Whether the actions of Sirt1 in bone cells and bone mass depend on ROS attenuation or modulation of FoxO activity is unknown.
p53–p66shc signaling pathway.
The tumor suppressor protein, p53, is a critical cellular stress sensor that is activated in response to ROS, DNA damage and other adverse stimuli such as oncogene activation and hypoxia. Thus, it is not surprising that p53 phosphorylation increases in the bone of male and female mice with aging or sex steroid deficiency.7,17 Depending on the degree of activation, p53 promotes growth arrest and repair, apoptosis or cellular senescence.78 Genetically engineered mouse models of hyperactive mutant p53 have elucidated a role for activated p53 in organismal ageing. Such mice display early ageing phenotypes, including osteoporosis.23,79 In contrast, p53 null mice display a high bone mass phenotype, due to increased osteoblast number and bone formation rate.80 The negative effect of p53 on osteoblast generation has been attributed to the repression of Runx2 and Osterix expression.80,81 In addition, p53 activity in osteoblasts attenuates osteoclast generation by decreasing M-CSF expression.80 Importantly, p53 activation downstream of ROS was associated with decreased osteoblast proliferation in a murine model of FoxO1 deficiency in osteoblasts,46 suggesting that the activation of p53 in aged bone might contribute to the decrease in osteoblast numbers and bone mass.
P53 is not only activated by ROS but can also modulate ROS levels. Indeed, in response to mild levels of stress, p53 can stimulate an antioxidant response program to prevent oxidative damage. However, when the stress source persists and the cellular repair process fails, prolonged p53 activation promotes cellular senescence or apoptosis by elevating ROS levels and this response is believed to be intrinsically linked to aging.82 Indeed, the telomere theory of ageing postulates that progressive loss of telomere function triggers chronic activation of p53, implicating telomeres in the pathogenesis of age-related disorders.43 Telomeres are DNA/nucleoprotein complexes at chromosome ends that function to preserve chromosomal integrity. Recent work has elucidated that p53, activated in response to telomere shortening, can lead to defects in mitochondrial biology and increased ROS, which are mediated by p53-induced PPAR coactivator 1α and PPAR coactivator 1β repression.83 These findings provide a link between nuclear and mitochondrial dysfunction with age. It is currently unknown whether the pro-oxidant actions of p53 have any role in bone. Interestingly, defects in telomere maintenance, similar to p53 activation, impair osteoblast differentiation and promote osteoporosis in mice,6 suggesting that a telomere dysfunction/p53/ROS pathway might also be operative in osteoblast progenitor cells.
P53 stimulate ROS production via several mechanisms including an increase in p66shc protein abundance. p66Shc is an isoform of Shc that has an important role in mitochondrial ROS generation and translation of oxidative signals into apoptosis.84 Specifically, oxygen-derived free radicals activate protein kinase C-β isoform to induce Ser36 phosphorylation of p66Shc and translocation of the protein from the cytosol to mitochondria. In the mitochondria, p66Shc functions as a redox enzyme to amplify the production of H2O2 and promote apoptosis.85 In line with these findings, either p53-null or p66shc-null cells are relatively resistant to oxidant-induced apoptosis and exhibit lower endogenous ROS production and reduced oxidative damage to DNA as compared to wild-type cells. The phosphorylation of p53 and p66Shc increases in bone with age, along with increased ROS and osteoblast apoptosis.7 Conversely, apoptosis and p66Shc phosphorylation in aged mice decrease with antioxidant administration.17 Mice with global p66Shc deficiency have lower ROS levels in bone and higher bone mass.86 In addition, p66shc-deficient osteoblasts exhibit increased resistance to oxidative stress.87 Overall, these lines of evidence suggest that p53 and p66shc might be causally involved in age-related skeletal involution via ROS-dependent and -independent mechanisms.
Hormonal control of bone mass via modulation of ROS
An increase in the production of endogenous glucocorticoids with age, as well as enhanced sensitivity of bone cells to glucocorticoids represents another age-associated pathogenetic mechanism of involutional osteoporosis.88 Glucocorticoids are strong inhibitors of bone formation by stimulating osteoblast apoptosis and decreasing osteoblast numbers. Mice with osteoblast/osteocyte-specific transgenic expression of 11β-HSD2, the enzyme that inactivates glucocorticoids, are partially protected from the adverse effects of aging on osteoblast and osteocyte apoptosis, bone formation rate and strength. Old 11β-HSD2 transgenic mice are also protected from the decreased bone vasculature volume and osteocyte lacunar-canalicular fluid observed in the aged wild-type controls. This, and the evidence that dehydration of bone decreases strength, suggests that endogenous glucocorticoids contribute to skeletal fragility in old age via cell autonomous effects on osteoblasts and osteocytes. In addition to their pro-apoptotic actions in mature cells, glucocorticoids attenuate osteoblast generation. Suppression of PI3K/Akt/GSK3β signaling and upregulation of the Wnt inhibitor DKK1 have been implicated in the attenuation of Wnt signaling and osteoblastogenesis by glucocorticoids.89,90,91
Recently, we and others have found that glucocorticoids stimulate ROS production and FoxO activity.92,93,94 Specifically, activation of a PKCβ/p66shc/JNK signaling cascade is responsible for the pro-apoptotic effects of glucocorticoids on osteoblastic cells. In addition, activation of FoxOs, secondary to the attenuation of Akt by glucocorticoids is a cell-autonomous mechanism of Wnt/β-catenin antagonism that contributes to the adverse effects of glucocorticoids on osteoblastogenesis.94 This work suggests that at least some of the deleterious actions of glucocorticoids on bone might be mediated by ROS.
Similar to aging, gonadectomy promotes an increase in ROS and the phosphorylation of p53 and p66shc in bone.26 Estradiol, dihydrotestosterone or antioxidants completely reverse the effects of gonadectomy on these markers of oxidative stress. More strikingly, administration of antioxidants is as effective as sex steroid replacement in preventing the loss of BMD in gonadectomized female or male mice. P66Shc is an essential mediator of the effects of oxidative stress, not only on apoptosis but also on NF-kβ activation and the expression of cytokines like tumor necrosis factor-α and interleukin 6 in osteoblastic cells.87 Sex steroids antagonize all these effects of oxidative stress by preventing PKCβ-induced p66Shc phosphorylation via a mechanism that requires extracellular signal-regulated kinases.7 Importantly, the DNA-binding function of estrogen receptor α is not required for the antioxidant effects of estrogens on bone.95
Consistent with the requirement of ROS for osteoclast generation and the in vivo evidence that sex steroids prevent bone loss via antioxidant effects, estradiol or dihydrotestosterone attenuate osteoclastogenesis and stimulate osteoclast apoptosis by a mechanism that involves activation of glutathione and thioredoxin reductases and inhibition of NF-kβ.7,20 Hence, an increase in endogenous glucocorticoids and the loss of sex steroids may contribute to age-related bone loss, at least in part, by increasing oxidative stress.
Summary and conclusions
Advancing age is a major risk factor for the decline in bone mass and strength and, consequently, the rise in the incidence of bone fractures. Indeed, it is expected the incidence of osteoporotic-related fractures in the United States will reach 3 million annually by 2025.96 Skeletal aging in the murine model closely recapitulates age-related skeletal changes in humans, including decreased osteoblast number as well as decreased bone mass and strength, and is associated with increased oxidative stress. Anabolic agents that promote osteoblast generation, such as intermittent parathyroid hormone or anti-sclerostin antibody administration, are efficacious in reversing age-related bone loss in rodents and in humans.97 Interestingly, parathyroid hormone attenuates oxidative stress in the aged murine skeleton, suggesting that the antioxidant properties of parathyroid hormone might contribute to its efficacy in treating age-related osteoporosis.17 In line with their pro-oxidant and deleterious role in other tissues, activation of p53 and p66shc in bone with age or following sex steroid depletion increases osteoblast and osteocyte apoptosis and decreases bone mass. On the other hand, ROS stimulate the FoxO family of transcription factors and sirtuins to combat oxidative stress and maintain skeletal homeostasis. These studies suggest that evolutionary conserved pathways that mediate the age-related degeneration of several tissues, as well as organismal longevity, also have a role in the aging skeleton. Elucidation of the molecular mechanisms affected by aging in bone cells should reveal novel drug targets for the prevention/treatment of age-related skeletal involution.
Acknowledgments
I am grateful to Charles O'Brien, Robert Jilka, Haibo Zhao, Robert Weinstein and Stavros Manolagas for sharing ideas on the subject and for reviewing the manuscript. This work was supported by the National Institutes of Health Grants R01 AR056679 (to MA) and P01 AG13918 (to SM).
Footnotes
The author declares no conflict of interest.
References
- Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 2010;375:1729–1736. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif Tissue Int 1984;36 (Suppl 1): S123–S128. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 2008;23:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res 1978;26:13–17. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Bone-forming cells in clinical conditions. In: Hall BK (ed). Bone The Osteoblast and Osteocyte, Vol 1. Telford Press and CRC Press: Boca Raton, FL, 1990.. pp 351–429. [Google Scholar]
- Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 2011;10:191–197. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 2007;282:27285–27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed FA, Modder UI, Roforth M, Hensen I, Fraser DG, Peterson JM et al. Effects of chronic estrogen treatment on modulating age-related bone loss in female mice. J Bone Miner Res 2010;25:2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res 2002;17:1044–1050. [DOI] [PubMed] [Google Scholar]
- Iida H, Fukuda S. Age-related changes in bone mineral density, cross-sectional area and strength at different skeletal sites in male rats. J Vet Med Sci 2002;64:29–34. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Weiss A, Reznick AZ, Eilam Y, Szydel N, Gershon D. Age-related trend for osteopenia in femurs of female C57BL/6 mice. Compr Gerontol 1987;1:45–51. [PubMed] [Google Scholar]
- Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone 2003;33:387–398. [DOI] [PubMed] [Google Scholar]
- Shahnazari M, Dwyer D, Chu V, Asuncion F, Stolina M, Ominsky M et al. Bone turnover markers in peripheral blood and marrow plasma reflect trabecular bone loss but not endocortical expansion in aging mice. Bone 2012;50:628–637. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956;11:298–300. [DOI] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med 2007;43:477–503. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, O′Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 2007;282:27298–27305. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS et al. Decreased oxidative stress and greater bone anabolism in the aged, as compared to the young, murine skeleton by parathyroid hormone. Aging Cell 2010;9:851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005;120:483–495. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47–95. [DOI] [PubMed] [Google Scholar]
- Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 2003;112:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger CJ, Lean JM, Davies JT, Chambers TJ. Tumor necrosis factor-alpha mediates osteopenia caused by depletion of antioxidants. Endocrinology 2005;146:113–118. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Fujii H, Kitazawa R, Yodoi J, Kitazawa S, Fukagawa M. Thioredoxin-1 overexpression in transgenic mice attenuates streptozotocin-induced diabetic osteopenia: a novel role of oxidative stress and therapeutic implications. Bone 2009;44:936–941. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002;415:45–53. [DOI] [PubMed] [Google Scholar]
- De Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H et al. Premature aging in mice deficient in DNA repair and transcription. Science 2002;296:1276–1279. [DOI] [PubMed] [Google Scholar]
- Nojiri H, Saita Y, Morikawa D, Kobayashi K, Tsuda C, Miyazaki T et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res 2011;26:2682–2694. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 2010;31:266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Chin BR, Lee YH, Kim JH. Involvement of glycogen synthase kinase-3beta in hydrogen peroxide-induced suppression of Tcf/Lef-dependent transcriptional activity. Cell Signal 2006;18:601–607. [DOI] [PubMed] [Google Scholar]
- Lin CL, Wang JY, Ko JY, Surendran K, Huang YT, Kuo YH et al. Superoxide destabilization of beta-catenin augments apoptosis of high-glucose-stressed mesangial cells. Endocrinology 2008;149:2934–2942. [DOI] [PubMed] [Google Scholar]
- Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene 2002;21:878–889. [DOI] [PubMed] [Google Scholar]
- Colla S, Zhan F, Xiong W, Wu X, Xu H, Stephens O et al. The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood 2007;109:4470–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, O'Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res 2012;27:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004;4:181–189. [DOI] [PubMed] [Google Scholar]
- Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 2010;140:517–528. [DOI] [PubMed] [Google Scholar]
- Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 2011;14:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 1990;85:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res 2004;301:119–127. [DOI] [PubMed] [Google Scholar]
- Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005;106:852–859. [DOI] [PubMed] [Google Scholar]
- Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 2009;15:259–266. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Koenigstein A, Joseph J, Sun L, Kalyanaraman B, Zaidi M et al. Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann NY Acad Sci 2010;1192:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden KC. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene 2008;27:2345–2350. [DOI] [PubMed] [Google Scholar]
- Puig O, Mattila J. Understanding Forkhead box class O function: lessons from Drosophila melanogaster. Antioxid Redox Signal 2011;14:635–647. [DOI] [PubMed] [Google Scholar]
- Partridge L, Bruning JC. Forkhead transcription factors and ageing. Oncogene 2008;27:2351–2363. [DOI] [PubMed] [Google Scholar]
- Sahin E, DePinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010;464:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. The FoxO code. Oncogene 2008;27:2276–2288. [DOI] [PubMed] [Google Scholar]
- Ambrogini E, Almeida M, Martin-Millan M, Paik J, dePinho R, Han L et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab 2010;11:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, DePinho RA et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab 2010;11:147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M. Unraveling the role of FoxOs in bone—insights from mouse models. Bone 2011;49:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 2010;142:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010;142:296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MA, Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005;308:1181–1184. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O et al. Wnt signaling regulates hepatic metabolism. Sci Signal 2011;4:ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Liu M, McMahon A, Bringhurst F, Kronenberg H. Pstnatal inactivation of beta-catenin in cells of the osteoblast lineage causes progressive bone loss, ectopic cartilage formation and mesenchymal cell accumulation. J Bone Miner Res 25[S1]S24:2010 Ref type: abstract. [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 2006;133:3231–3244. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol 2007;21:2605–2614. [DOI] [PubMed] [Google Scholar]
- Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H et al. Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 2005;8:751–764. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem 2005;280:21162–21168. [DOI] [PubMed] [Google Scholar]
- Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB et al. Osteocyte Wnt/{beta}-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 2010;30:3071–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem 2008;283:15539–15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased PPAR\{gamma\} expression. J Biol Chem 2009;284:27438–27448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, MacDougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J Biol Chem 2007;282:14515–14524. [DOI] [PubMed] [Google Scholar]
- Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci USA 2009;106:5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Pradeep A, Wong L, Rana A, Rana B. Peroxisome proliferator-activated receptor gamma activation can regulate beta-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. J Biol Chem 2004;279:35583–35594. [DOI] [PubMed] [Google Scholar]
- Brodeur MR, Brissette L, Falstrault L, Ouellet P, Moreau R. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med 2008;44:506–517. [DOI] [PubMed] [Google Scholar]
- Klein BY, Rojansky N, Ben Yehuda A, Abou-Atta I, Abedat S, Friedman G. Cell death in cultured human Saos2 osteoblasts exposed to low-density lipoprotein. J Cell Biochem 2003;90:42–58. [DOI] [PubMed] [Google Scholar]
- Huang MS, Morony S, Lu J, Zhang Z, Bezouglaia O, Tseng W et al. Atherogenic phospholipids attenuate osteogenic signaling by BMP-2 and parathyroid hormone in osteoblasts. J Biol Chem 2007;282:21237–21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 2010;5:253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 2007;404:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Guarente L. The SirT3 divining rod points to oxidative stress. Mol Cell 2011;42:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backesjo CM, Li Y, Lindgren U, Haldosen LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res 2006;21:993–1002. [DOI] [PubMed] [Google Scholar]
- Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML et al. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res 2011;26:2552–2563. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem 2011;286:11492–11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004;23:2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 2008;8:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JL, Yang CY, Zhao M, Kuo ML, Yen ML. Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J Biol Chem 2007;282:19385–19398. [DOI] [PubMed] [Google Scholar]
- Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J 2011;25:3646–3660. [DOI] [PubMed] [Google Scholar]
- Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I et al. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology 2011;152:4514–4524. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Zainabadi K, Lwin ST, Elefteriou E, Munoz S, Moore MM et al. The longevity gene SIRT-1 independently controls both osteoblast and osteoclast function. J Bone Miner Res 2008;23[S1]:S28 Ref type: abstract. [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol 2007;8:275–283. [DOI] [PubMed] [Google Scholar]
- Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev 2004;18:306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol 2006;172:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE et al. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol 2006;172:909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging 2010;2:471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011;470:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinei M, Berniakovich I, Beltrami E, Migliaccio E, Fassina A, Pelicci P et al. P66Shc signals to age. Aging 2009;1:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005;122:221–233. [DOI] [PubMed] [Google Scholar]
- Bartel SM, Han L, Iyer S, Warren A, Bradsher RW, Shelton RS et al. Deletion of the redox amplifier p66shc decreases ROS production in murine bone and increases osteoblast resistance to oxidative stress and bone mass. J Bone Miner Res 2011;26[S1]:S85 Ref type: abstract. [Google Scholar]
- Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC. Oxidative stress stimulates apoptosis and activates NF-B in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol Endocrinol 2010. ;24:2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien C et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 2010;9:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 2007;13:156–163. [DOI] [PubMed] [Google Scholar]
- Smith E, Frenkel B. Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3beta-dependent and -independent manner. J Biol Chem 2005;280:2388–2394. [DOI] [PubMed] [Google Scholar]
- Wang FS, Ko JY, Yeh DW, Ke HC, Wu HL. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology 2008;149:1793–1801. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–948. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004;117:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC. Glucocorticoids and tumor necrosis factor (TNF) alpha increase oxidative stress and suppress WNT signaling in osteoblasts. J Biol Chem 2011;286:44326–44335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Martin-Millan M, Ambrogini E, Bradsher R III, Han L, Chen XD et al. Estrogens attenuate oxidative stress, osteoblast differentiation and apoptosis by DNA binding-independent actions of the ERalpha. J Bone Mineral Res 2010;25:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007;22:465–475. [DOI] [PubMed] [Google Scholar]
- Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab 2012;97:311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]