This study used a two-stage culture system to efficiently produce natural killer (NK) cells from human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) in the absence of cell sorting and without need for xenogeneic stromal cells. Although different hESC and iPSC lines had varying efficiencies in hematopoietic development, all cell lines tested could produce functional NK cells. This improved method to develop NK cells from human pluripotent stem cells provides a system for clinical-scale expansion of antitumor lymphocytes and a genetically amenable platform to study human NK cell development.
Keywords: Immunotherapy, Lymphocytes, Pluripotent stem cells, Hematopoietic cells, Hematopoiesis
Abstract
Adoptive transfer of antitumor lymphocytes has gained intense interest in the field of cancer therapeutics over the past two decades. Human natural killer (NK) cells are a promising source of lymphocytes for anticancer immunotherapy. NK cells are part of the innate immune system and exhibit potent antitumor activity without need for human leukocyte antigen matching and without prior antigen exposure. Moreover, the derivation of NK cells from pluripotent stem cells could provide an unlimited source of lymphocytes for off-the-shelf therapy. To date, most studies on hematopoietic cell development from human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) have used incompletely defined conditions and been on a limited scale. Here, we have used a two-stage culture system to efficiently produce NK cells from hESCs and iPSCs in the absence of cell sorting and without need for xenogeneic stromal cells. This novel combination of embryoid body formation using defined conditions and membrane-bound interleukin 21-expressing artificial antigen-presenting cells allows production of mature and functional NK cells from several different hESC and iPSC lines. Although different hESC and iPSC lines had varying efficiencies in hematopoietic development, all cell lines tested could produce functional NK cells. These methods can be used to generate enough cytotoxic NK cells to treat a single patient from fewer than 250,000 input hESCs/iPSCs. Additionally, this strategy provides a genetically amenable platform to study normal NK cell development and education in vitro.
Introduction
Human natural killer (NK) cells provide an important arm of the innate immune system by producing cytokines and killing virally infected and malignant cells. Whereas antitumor T-cell responses require a priming phase and are human leukocyte antigen (HLA)-restricted, mature NK cells effectively kill malignant cells without any prior exposure. Both T cell- and NK cell-based adoptive immunotherapies have been used to treat patients with refractory malignancies [1–3]. A major hindrance to expanded use of these therapies is the need for cell processing and donor selection. Therefore, a standardized, off-the-shelf therapeutic with a potent antitumor response could be used to treat thousands of patients with refractory malignancies. Although T-cell development from human pluripotent stem cells (hPSCs) has been reported, it remains relatively inefficient [4, 5]. In contrast, the use of human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) to generate NK cells with antitumor and antiviral capacity is routine [6–8]. NK cells derived from hESCs and iPSCs possess a mature phenotype, secrete cytokines, and are cytotoxic against both hematologic and solid malignancies in vitro and in vivo [6–8]. However, previous work on NK cell generation from both hESCs and iPSCs has relied on the use of murine stromal cell layers and sorting of small numbers of hESC/iPSC-derived hematopoietic progenitor cells [6–8]. Although the use of murine stromal layers does not absolutely prohibit clinical translation (if master cells banks are used), the use of culture systems that eliminate xenogeneic cells provides more defined conditions for NK cell development. Elimination of murine stromal support also provides an improved developmental model to study receptor-ligand interactions driving NK cell licensing. Here, we describe an efficient system for the development of functional NK cells from both hESCs and iPSCs as well as an improved method suitable for clinical translation.
Materials and Methods
Maintenance and Stromal Cell-Mediated Differentiation of hESCs/iPSCs
hESCs (H9 and H1) and iPSCs (UCBiPS7, NHDF-iPS, BJ1-iPS) were maintained on mouse embryonic fibroblasts (MEFs). Stromal cell-mediated differentiation of hematopoietic progenitors on M210-B4 (American Type Culture Collection, Manassas, VA, http://www.atcc.org) cells was done as previously described [8–10]. Briefly, undifferentiated hESCs or iPSCs were placed on M210-B4 in medium containing RPMI 1640 (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), 15% defined fetal bovine serum (FBS) (HyClone, Logan, UT, http://www.hyclone.com), 2 mM l-glutamine (Cellgro, Manassas, VA, http://www.cellgro.com), 1% nonessential amino acids (Invitrogen), 1% penicillin/streptomycin (Invitrogen), and 0.1 mM β-mercaptoethanol (Invitrogen). Medium was changed three times per week, and after 18–21 days cells were harvested for CD34+CD45+ progenitor cell enrichment [8]. One hundred thousand CD34+CD45+ cells were placed onto EL08-1D2 stroma with 1 ml of NK cell initiating cytokines (interleukin [IL]-3, IL7, IL-15, stem cell factor [SCF], and fms-like tyrosine kinase receptor-3 ligand [FLT3L], all from Peprotech, Rocky Hill, NJ, http://www.peprotech.com). NK cell cultures we refreshed with 0.5 ml of cytokine-containing medium every 4–5 days. Mature NK cells were measured at 28–35 days of culture on EL08-1D2.
Generation of Spin EBs
In order to generate spin EBs amenable to aggregation, hESCs and iPSCs were passaged in TrypLE Select (Invitrogen) on low-density MEFs (90,000 cells per well) for a minimum of 10 passages. For the spin EB studies, we used an H9 line modified with a green fluorescence protein/firefly luciferase construct [10] for future in vivo experiments. For iPSCs, we tested the UCBiP7 line derived from umbilical cord blood (UCB) CD34+ hematopoietic progenitors (supplemental online Fig. 1). To generate TrypLE adapted hESCs or iPSCs, cultures at approximately 60%–70% confluence were dissociated and filtered through a 70-μm sterile filter. Only pure cultures of hESCs lacking any signs of differentiation were used. Cells were passaged 1:1 on low-density MEFs in regular hESC medium until cellular proliferation allowed passing at more dilute ratios, typically occurring around passage 10. To set up TrypLE passaged hESCs into stage I spin EBs, adapted cells at approximately 70% confluence were dissociated with TrypLE and filtered through a 70-μm filter to remove any clumps. Cells were then counted and placed at a concentration of 3,000 cells per well (100 μl volume) of a round-bottom 96-well plate in BPEL (bovine serum albumin polyvinyl alchohol essential lipids) medium containing SCF (40 ng/ml), vascular endothelial growth factor (20 ng/ml), and bone morphogenic protein 4 (20 ng/ml). BPEL medium was made in 200-ml volumes and contained Iscove's modified Dulbecco's medium (86 ml; Invitrogen), F12 Nutrient Mixture with GlutaMAX I (86 ml; Invitrogen), 10% deionized bovine serum albumin (5 ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 5% polyvinyl alcohol (10 ml; Sigma-Aldrich), linoleic acid (20 μl of 1 mg/ml solution; Sigma-Aldrich), linolenic acid (20 μl of 1 mg/ml solution; Sigma-Aldrich), Synthecol 500× solution (Sigma-Aldrich), α-monothioglyceral (Sigma-Aldrich), protein-free hybridoma mix II (Invitrogen), ascorbic acid (5 mg/ml; Sigma-Aldrich), GlutaMAX I (Invitrogen), insulin-transferrin-selenium 100× solution (Invitrogen), and penicillin/streptomycin (Invitrogen). The outer wells of the plate were filled with sterile water to prevent any evaporation of the medium. Plates were then spin-aggregated at 1,500 rpm for 5 minutes at room temperature and placed undisturbed in a 37°C incubator with 5% CO2. Cells were not removed for at least 3 days to ensure formation of spin EBs in the plates.
NK Cell Differentiation From Spin EBs
At day 11 of spin EB differentiation, 6 wells of a 96-well plate were directly transferred to 1 well of a 24-well plate in NK cell initiating cytokines, as described above. Initially, the 24-well plates contained 100,000 irradiated (3,000 rads) EL08-1D2 cells per well. For completely defined conditions, 6 wells of spin EBs were directly transferred to uncoated 24-well plates. On the day of analysis each well was collected, filtered, and washed.
Flow Cytometry
The following antibodies were used: CD34-APC, CD45-PE, CD31-PE, CD31-APC, CD-73PE, CD43-PE, NKp46-PE, NKp44-PE, CD56-APC, NKG2D-PE, TRAIL-PE, FAS ligand-PE, CD16-PercpCy5.5, and CD117-PercpCy5.5, all from Becton, Dickinson and Company (Franklin Lakes, NJ, http://www.bd.com). CD158a/h-PE, CD158j-PE, CD158i-PE, CD158e1/e2, and CD159a-PE and -APC were obtained from Beckman Coulter (Fullerton, CA, http://www.beckmancoulter.com). CD107aPercpCy5.5 and INF-γ PacBlue were obtained from eBioscience Inc. (San Diego, CA, http://www.ebioscience.com). Flow cytometry was done on a BD FACSCalibur or LSRII (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), and data were analyzed using FlowJo (Tree Star, Ashland, OR, http://www.treestar.com).
Gene Expression
For reverse transcription-polymerase chain reaction (RT-PCR), total RNA was prepared from cells using an RNeasy mini-kit (Qiagen, Valencia, CA, http://www.qiagen.com). Following isolation of total RNA, complementary DNA was made using Superscript III reverse transcriptase (Life Technologies, Grand Island, NY, http://www.lifetech.com). RT-PCR was then performed on the resulting cDNA with the primers and cycle number listed in supplemental online Table 1 using the Peltier Thermal Cycler-200. Annealing temperature was set at 55°C for all primers except OCT4, which had an annealing temperature of 60°C. Products from the PCRs were then separated on a 0.9% agarose gel via electrophoresis.
In Vitro Cytotoxicity
Tumor targets (K562, SVP10, S2013, OPM2, RPMI 8226, U266) were incubated with chromium 51 (51Cr) for 2 hours at 37°C, washed three times, and cocultured with NK cells at the indicated effector to target (E:T) ratios. After a period of 4 hours, cells were harvested and analyzed. Specific 51Cr lysis was calculated using the following equation: Percentage of specific lysis = 100 × (Test release − Spontaneous release)/(Maximal release − Spontaneous release). For redirected antibody-dependent cellular cytotoxicity (ADCC) assays, P815 targets were incubated as described above. Thirty minutes prior to the addition of the NK cells to target cells, effectors were incubated with either isotype control (catalog no. 400153; Biolegend, San Diego, CA, http://www.biolegend.com) or NKp46 antibodies (catalog no. 331904; Biolegend). Following an incubation of 5 hours, cells were harvested and analyzed as described above.
CD107a and Interferon-γ Assays
NK cells were incubated with or without K562 targets at 1:1 effector to target ratios. CD107a-PerCPCy5.5 antibody was added to each well and allowed to incubate for 1 hour. GolgiStop and GolgiPlug (BD Biosciences) were then added to each well and incubated for another 4 hours. At the completion of incubation, cells were washed, stained with CD56-APC, and fixed with 2% paraformaldehyde for 10 minutes on ice. Cells were then permeabilized with 1% saponin for 20 minutes at 4°C, washed, and stained for interferon-γ (IFN-γ).
Stromal Cell Functional Assays
To assess the endothelial and mesenchymal stromal cell (MSC) characteristics of the stroma derived in NK cell cultures, nonadherent cells were first washed away and then the adherent layer was trypsinized and washed. Cells were stained with endothelial (CD31) and MSC (CD73) markers, as well as the hematopoietic (CD45), monocyte/macrophage (CD14, CD15), and dendritic cell (CD11c) markers. To assess the ability of each of the stromal cell layers (EL08-1D2, human umbilical vein endothelial cells [HUVECs], feeder-free stroma) to support NK cell development, each was plated at 100,000 cells per well of a 24-well plate and irradiated (3,000 rads). HUVECs were grown as previously described [9]. Each well was then seeded with 500 CD34+ cells from umbilical cord blood. The no-stroma conditions contained UCB CD34+ cells and medium alone. Cells were then cultured under standard NK cell conditions as described above.
Artificial Antigen-Presenting Cell Expansion
To expand hESC-derived NK cells from EL08-1D2 or feeder-free conditions, each was placed in culture with clone 9.mbIL-21 artificial antigen-presenting cells (aAPCs) [11]. For the first week of culture, aAPCs were irradiated with 10,000 rads and added to NK cells at a ratio of 2:1 (day 0). The following stimulations with aAPCs (every 7 days) were at a 1:1 ratio. Cultures were fed three times per week (RPMI 1640, 15% FBS, 1% penicillin/streptomycin, 50 U/ml interleukin-2), maintaining cell counts at 250,000 cells per milliliter for optimal expansion.
Statistical Analysis
Differences between groups were compared using Student t test post hoc analysis or ANOVA using Prism 4 (GraphPad Software, San Diego, CA). Results were considered significant at p values of .05 or less.
Results
hESC- and iPSC-Derived Hematopoietic Progenitor Cells Can Develop Into NK Cells
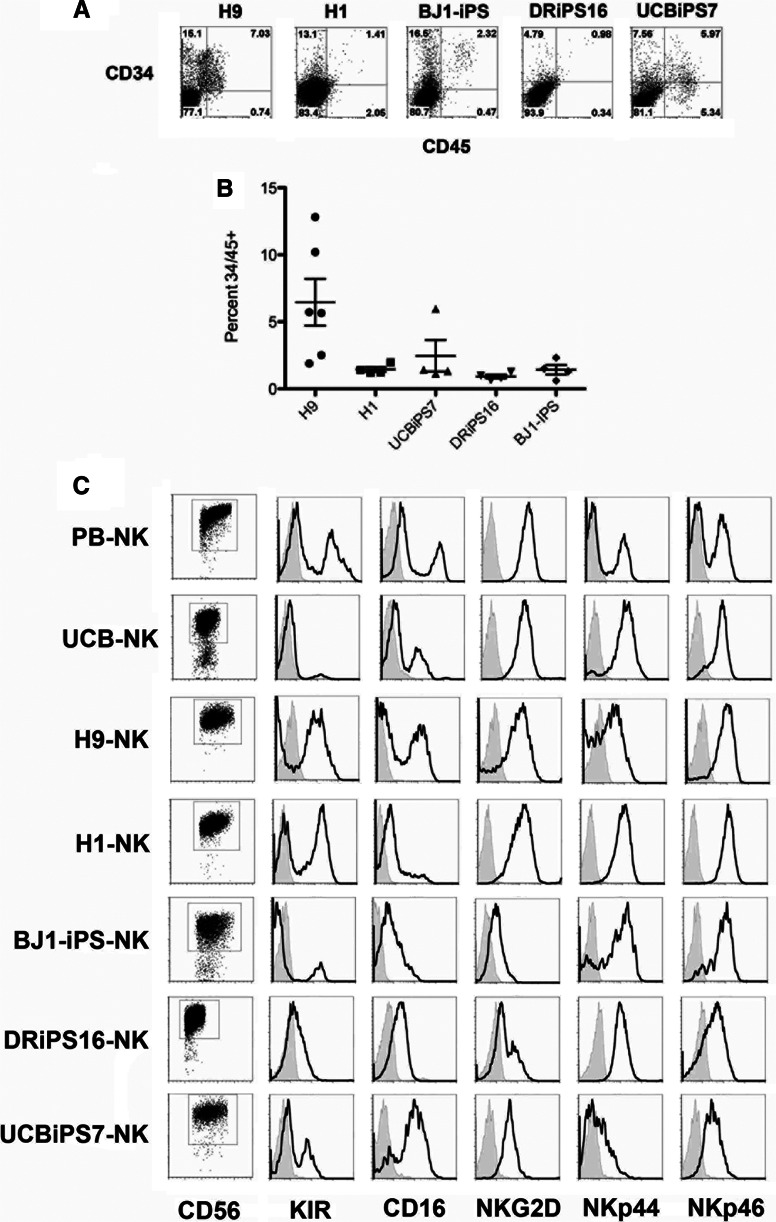
Initial studies used a stromal cell coculture method [6–8] to compare hematopoietic and NK cell developmental potential of two different hESC lines (H1 and H9) and three different iPSC lines (BJ1-iPS12, UCBiPS7, and DRiPS16). UCBiPS7 and DRiPS16 were derived and characterized in our laboratory (supplemental online Fig. 1). For this method, hESCs or iPSCs are cultured on M210-B4 stromal cells in medium containing only FBS. Over a period of 3 weeks, all hESC and iPSC lines generated hematopoietic progenitor cells coexpressing CD34 and CD45 (Fig. 1). Whereas the H9 cells gave rise to the highest percentage of hematopoietic progenitor cells expressing CD34 and CD45 (6.46 ± 1.75%), other hESC and iPSC lines yielded consistently lower numbers: 1.45 ± 0.18% for H1 hESCs, 2.46 ± 1.71% for UCBiPS7, 0.92 ± 0.14% for DRiPS16, and 1.43 ± 0.35% for the BJ1-iPS line (Fig. 1B). These numbers are similar to what we and others have previously shown, where the efficiency of hematopoietic development using the stromal cell-based system is relatively limited [12, 13]. After demonstrating that different iPSC lines gave rise to varying numbers of hematopoietic progenitor cells, we generated NK cells from each of the hESC/iPSC-derived CD34+CD45+ cell populations. Here, CD34+CD45+ cells were sorted and cultured in conditions known to support human NK cell development, including the murine stromal cell line EL08-1D2 and cytokines (SCF, FLT3L, IL-15, IL-7, IL-3) [8] for 4 weeks. Although distinct lines of hESCs or iPSCs gave rise to varying frequencies of hematopoietic progenitor cells, each cell line was able to produce phenotypically mature and functional NK cells. Both hESC- and iPSC-derived NK cells consist of a homogeneous population of cells expressing CD56, killer immunoglobulin-like receptors (KIRs), CD16, NKp44, NKp46, and NKG2D (Fig. 1C). Also, NK cells from all five hESC/iPSC populations were able to kill tumor cells similarly to NK cells isolated from peripheral blood (PB-NK) (supplemental online Fig. 2). These results demonstrated that although individual hESCs and iPSCs have reproducible differences in their ability to derive hematopoietic progenitor cells, each was capable of making mature, cytolytically active NK cells.
Figure 1.
Derivation of functional NK cells from human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs). (A): CD34+CD45+ progenitors derived from hESCs (H1, H9) or iPSCs (BJ1-iPS, DRiPS16, UCBiPS7) following 21 days on M210-B4 stroma. (B): Differentiation efficiencies of hESCs and iPSCs, at least four separate experiments for each line. (C): NK cells derived from hESCs, iPSCs (BJ1-iPS, NHDF-iPS, UCB-iPS), or UCB CD34+ cells or isolated from adult peripheral blood (PB-NK). Histogram plots are gated on CD56+ events. KIR plots used a cocktail of KIR antibodies (CD158a/h, CD158e1/e2, and CD158i). Similar to PB-NKs, hESC- and iPSC-derived NK cells expressed markers of functionally mature NK cells (CD16, NKG2D, NKp44, NKp46, CD161). Histograms are representative of at least three individual experiments. Abbreviations: iPS, induced pluripotent stem; KIR, killer immunoglobulin-like receptor; NK, natural killer; PB, peripheral blood; UCB, umbilical cord blood.
Enhanced Generation of Progenitor Cells Eliminates Cell Sorting in the Derivation of hPSC-Derived NK Cells
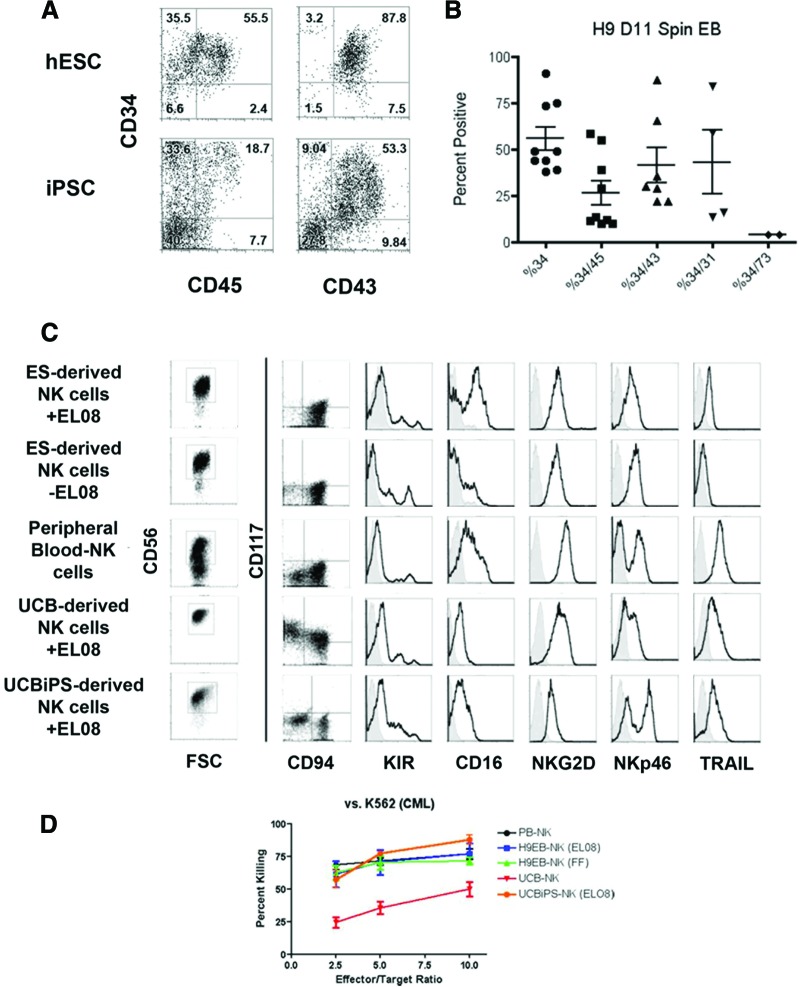
In an effort to better understand specific stimuli required to mediate derivation of NK cells from hESCs or iPSCs and to improve culture efficiency, we took a stepwise approach to translate these methods to completely feeder-free and serum-free culture system. First, undifferentiated hESCs and iPSCs were supported to produce hematopoietic progenitor cells using a “spin EB” method [14, 15]. Here, defined numbers (3,000 cells) of undifferentiated hESCs (H9) or iPSCs (UCBiPS7) were spin aggregated in serum-free medium in a 96-well format (supplemental online Fig. 3A). Over a period of 11 days these cultures demonstrated hematopoietic cell development and proliferation (supplemental online Fig. 3B). Not only did this method remove the need for M210-B4 murine stroma, we found that it allowed higher frequency and more consistent generation of hematopoietic progenitor cells from both hESCs and iPSCs. For hESCs we found that a majority of the hematopoietic cells expressed CD34 (55.9 ± 6.4%), with many coexpressing CD43 (41.8 ± 9.51%) or CD45 (26.2 ± 6.6%) (Fig. 2A, 2B). iPSCs also generated CD34+ cells (12.06 ± 5.40%) and CD45+ cells (3.20 ± 1.43%), although typically fewer than hESCs. This method provides significant improvement over the M210-B4 stromal-based system (Fig. 1) for both hESCs and iPSCs.
Figure 2.
Derivation of functional NK cells in feeder-free conditions. (A): Spin EBs generated higher frequencies of hematopoietic progenitors cells from hESCs and iPSCs than coculture on murine stromal cells. At day 11 of spin EB culture, individual EBs were collected, dissociated, and stained with antibodies against CD34, CD43, and CD45. (B): Quantification of the high level of blood progenitors produced in the spin EB system. Percentages of cells expressing CD34 alone or in combination with CD45, CD43, CD31, and CD73 are shown. Each dot represents results from a separate experiment. Lines indicate mean ± SEM. (C): CD56+ NK cells derived from hESCs (with or without EL08-1D2 feeders), PB-NKs, or iPSCs (UCBiPS7). hESC- and iPSC-derived NK cells formed a CD117−CD94+ homogeneous, mature population similar to activated PB-NKs. Each also expressed various effector molecules, including KIR, CD16, NKG2D, NKp46, and the apoptosis-inducing ligand TRAIL. Histograms are representative of at least three independent experiments. (D): Cytotoxicity assay against the leukemic cell line K562 (n = 4 per cell type). hESC- and iPSC-derived NK cells killed K562 cells similarly to activated PB-NK cells and significantly better than UCB-NK cells (p = .0054). Data are represented as mean ± SEM. Abbreviations: CML, chronic myelogenous leukemia; ES, embryonic stem; hESC, human embryonic stem cell; iPSC, induced pluripotent stem cell; KIR, killer immunoglobulin-like receptor; NK, natural killer; PB, peripheral blood; UCB, umbilical cord blood.
We next tested the ability of the hematopoietic progenitor cells generated in the spin EB system to derive NK cells. To do this we directly transferred spin EBs, without dissociation of sorting, into NK cell initiating conditions containing cytokines and EL08-1D2 stroma. As previously shown, this stage II culture system provides reliable development of NK cells over a period of 4 weeks [7, 8]. Similar to stromal cell coculture-derived progenitor cells, spin EB-derived hematopoietic progenitors acquired NK cell surface markers culminating in a mature NK cell phenotype (Fig. 2C). Indeed, we found that spin EB-derived cells differentiate into a homogeneous population of CD56+ NK cells expressing CD94 in the absence of CD117 [8]. Additionally, these cells expressed high levels of KIRs, CD16, NKG2D, NKp46, and TRAIL. They also have a gene expression profile consistent with the NK cell lineage. They expressed ID2, E2A, and E4BP4 (supplemental online Fig. 4). They did not express the B cell lineage-specific transcription factor PAX5. Also, in contrast to their parent lines, hESC- and iPSC-derived NK cells did not express the pluripotency factor OCT4. Spin EB-derived NK cells (from both hESCs and iPSCs) not only had the correct phenotype and genotype but killed K562 tumor cells at a similar level to PB-NK cells and were more cytotoxic than UCB-derived NK cells (Fig. 2D). Additionally, we demonstrated specific killing mediated by the activating receptor, NKp46, expressed on the hESC- and iPSC-derived NK cells. Similar to PB-NK cells, hESC- and iPSC-derived NK cells could be triggered, through a reverse ADCC assay, to kill P815 targets (supplemental online Fig. 5). These data demonstrate that not only does the spin EB approach provide a feeder-free system to generate high numbers of hematopoietic progenitor cells, but that these progenitors do not require sorting to differentiate into phenotypically mature NK cells with cytotoxic function similar to activated PB-NK cells.
hPSC-Derived Stroma Support NK Cell Development from Hematopoietic Progenitor Cells
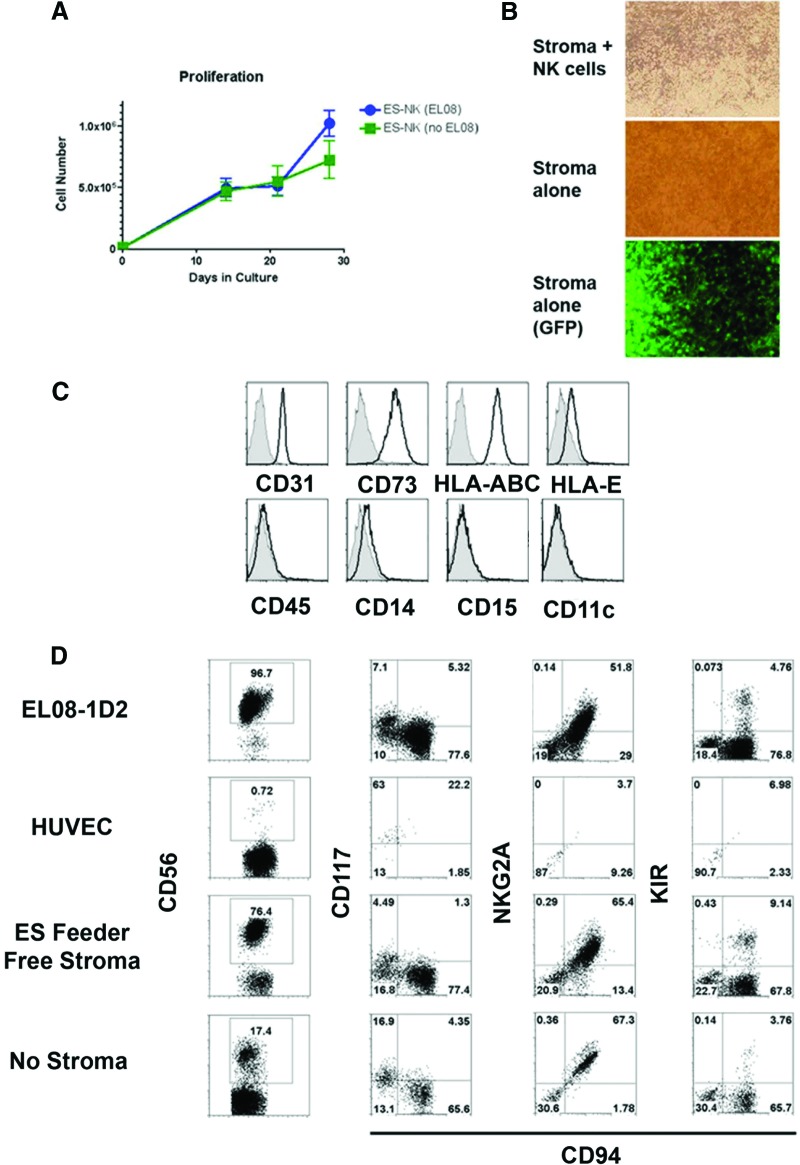
To more completely define the conditions required for NK cell development from hESCs and iPSCs, we next tested spin EB-derived cells in a feeder-free and serum-free stage II system containing NK cell promoting cytokines (IL-3, IL-7, IL-15, SCF, FLT3L) without EL08-1D2 or other exogenous stromal cells (supplemental online Fig. 3A). Within the first 2 weeks following transfer, there was proliferation of nonadherent hematopoietic cells from the spin EBs at a similar level to what is seen with EL08-1D2 stroma (Fig. 3A). Additionally, we found these cells started to produce their own adherent cells in culture (Fig. 3B). We have previously demonstrated development of endothelial cells (ECs) and MSCs from hESCs [9, 16]. Here, we demonstrated both these cell types (CD34+CD31+ ECs and CD34+CD73+ MSCs) (supplemental online Fig. 6) are routinely produced in the spin EB cultures. As nonhematopoietic cells such as ECs and MSCs that reside in the bone marrow are known to support NK cells in vivo, we hypothesized that these adherent cells could efficiently support growth of NK cells from hESCs in vitro [17, 18]. Notably, we found the spin EB stromal cell layers express high levels of CD31 and CD73 (Fig. 3C) in addition to MHC class I molecules (HLA-A,B,C and HLA-E) known to be important for NK cell development and acquisition of KIRs [19]. Additionally, these spin EB-derived stromal cells support the development of NK cells from UCB CD34+ cells similar to EL08-1D2 stroma and more efficiently than human umbilical vein endothelial cells or cytokines alone (Fig. 3D).
Figure 3.
Human pluripotent stem cell-derived stroma support development of mature NK cells. (A): Human embryonic stem cell (hESC)-derived NK cells proliferated in the presence or absence of murine EL08-1D2 stromal cells. At 4 weeks of culture with or without EL08 cells, the stromal cells provided 56.8-fold expansion and feeder-free cells expanded 40.4-fold. n = 4 for each condition. (B): hESC-derived stroma following 2 weeks of NK cell culture. Cells were imaged at ×40 magnification. The hematopoietic cells were then washed away and cells were reimaged to evaluate only the stromal layer. The stromal cells also expressed GFP, indicating their hESC origin (the parent H9 line used is GFP+). (C): Stroma from feeder-free conditions expressed surface antigens typical of both endothelial (CD31) and mesenchymal stromal cells but did not express the pan-hematopoietic marker CD45 or myeloid markers (CD14, CD15, CD11c). (D): Stroma derived from feeder-free conditions supported the development of NK cells from umbilical cord blood CD34+ hematopoietic stem cells. Each stromal layer (EL08-1D2, HUVEC, feeder-free stroma) was evaluated at day 28 for the presence of NK cells (CD56). A no-stroma, cytokine-only condition is also shown. CD56+ events were then evaluated for the expression of CD117, CD94, NKG2A, and KIR (n = 2). Abbreviations: ES, embryonic stem; GFP, green fluorescence protein; HUVEC, human umbilical vein endothelial cell; KIR, killer immunoglobulin-like receptor; NK, natural killer.
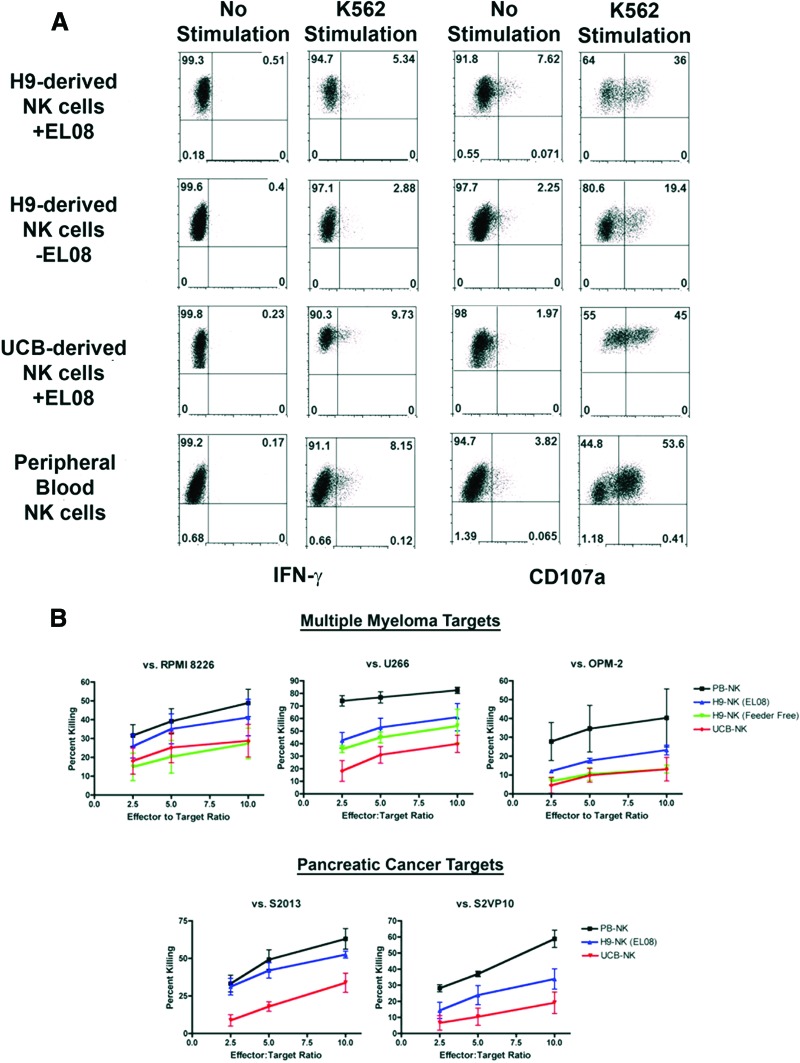
Using these defined conditions with no exogenous stromal cells, NK cells developed in similar numbers and phenotype compared with stage II conditions using the EL08-1D2 stromal cells (Figs. 1C, 2C). These cells expressed a mature NK cell phenotype and were comparable to their stromal-derived counterparts in cytotoxicity assays, indicating proper NK cell education and acquisition of effector function. Spin EB-derived NK cells degranulated, made IFN-γ, and also had activity against diverse tumor targets including pancreatic cancer and multiple myeloma (Fig. 4). These data demonstrate for the first time, successful in vitro derivation of functional, cytotoxic lymphocytes in the absence of any sorting or murine stromal cell support. Avoiding xenogeneic feeder layers provides a novel, genetically amenable system to study human NK cell education, as well as a defined human source for adoptive immunotherapy.
Figure 4.
Spin EB-derived NK cells are functional against a variety of targets. (A): Human embryonic stem cell-derived NK cells in feeder or feeder-free conditions, UCB-derived NK cells, and PB-NK cells were tested against K562 targets for IFN-γ secretion and CD107a expression. Effectors were incubated with targets for 5 hours and analyzed by flow cytometry. (B): Each effector population was also tested against myeloma (RPMI 8226, U266, OPM-2) and pancreatic cancer (S2013, S2VP10) targets using a standard chromium 51 release assay. Data are represented as mean ± SEM. Abbreviations: IFN-γ, interferon-γ; NK, natural killer; PB, peripheral blood; UCB, umbilical cord blood.
Clinical-Scale Expansion of hESC-Derived NK Cells for Antitumor Immunotherapy
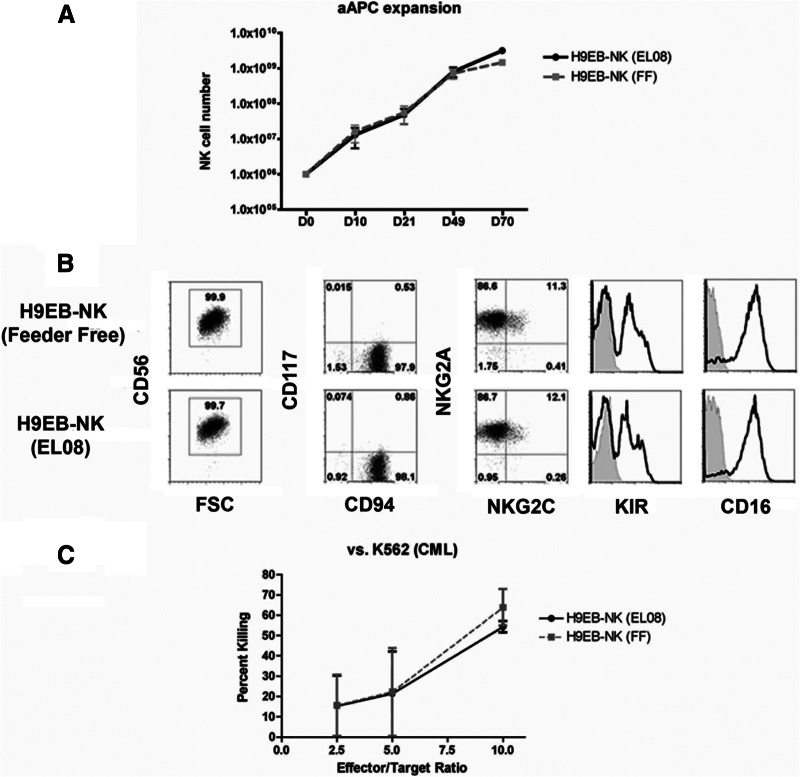
Although this EB-based approach shows marked expansion and clinical feasibility, we next aimed to further enhance the number of NK cells generated through another clinically amenable method. Recently, several groups have used aAPCs to expand T cells or NK cells for adoptive immunotherapy [20]. One major hindrance of this approach is that high levels of in vitro expansion lead to shortening of telomeres and cellular senescence. Denman et al. have generated an aAPC line expressing membrane bound IL-21 (clone 9.mbIL-21) leading to marked expansion of PB-NK cells while maintaining telomere length and in vitro activity [11]. We tested whether these aAPCs could lead to further expansion of hESC-derived NK cells and found that clone 9.mbIL-21 aAPCs mediated an additional 2–3 log expansion of both the EL08-1D2 and feeder-free derived NK cells (Fig. 5A). The aAPC-expanded cells maintained their NK cell phenotype as well as in vitro activity (Fig. 5B, 5C). Additionally, these hESC-derived NK cells could be maintained and continually expanded in culture for more than 2 months. Combined, the spin EB method and aAPC expansion provide a straightforward, translatable approach to generate enough human NK cells from hPSCs for cancer immunotherapy.
Figure 5.
NK cell expansion with aAPCs. (A): Cultures containing aAPCs and 1 × 106 human embryonic stem cell-derived NK cells (EL08-1D2 or feeder-free) were evaluated for NK cell expansion at days 10, 21, 49, and 70 (days 10 and 21, n = 4; days 49 and 70, n = 2). (B): Following 3 weeks of expansion both the EL08-1D2 and feeder-free NK cell cultures maintained pre-expansion phenotype and were similar to expanded peripheral blood-NK cells. Each contained pure cultures of CD56+ NK cells that remained CD94+CD117−. Each expressed high levels of KIR, CD16, and NKG2A with a small percentage of the cells expressing NKG2C (n = 3). (C): Expanded NK cells maintained their in vitro function. Each was tested in a standard chromium 51 release cytotoxicity assay against K562 targets (n = 3 for each). Data are represented as mean ± SEM. Abbreviations: aAPC, artificial antigen-presenting cell; CML, chronic myelogenous leukemia; FSC, forward scatter; KIR, killer immunoglobulin-like receptor; NK, natural killer.
Discussion
These studies demonstrated a feeder-free system that can be used to generate large numbers of cytotoxic NK cells for clinical translation. NK cells derived in this feeder-free system had a genotype and phenotype similar to those grown using murine stromal cells [7, 8]. Similar to our previous reports, we saw high levels of effector molecules expressed on the surface of NK cells from hESCs and iPSCs, including both KIR and CD16. Notable differences in the level of KIR and CD16 expression between the stromal-based and feeder-free method were likely due to developmental kinetics. When using the M210-B4 stromal-based method it is necessary to enrich the population for CD34+/CD45+ progenitor cells prior to NK cell differentiation as it gives rise to low frequencies (2%–10%) of these cells (Fig. 1). Although direct transfer of spin EBs allowed successful NK cell development, it may take longer for optimal CD16 and KIR acquisition because of the lower starting percentage (26.2 ± 6.6%; Fig. 2) of progenitor cells. However, there are no intrinsic differences between NK cells derived using either method as it can be demonstrated that they still acquire a high level of KIR and CD16 expression following expansion with aAPCs. Additionally, both stromal-based and feeder-free NK cells are functionally similar as they kill tumor targets at equivalent levels.
With the improved efficiency and defined components of this system, clinical translation of hESC/iPSC-derived cells becomes feasible. Current adoptive NK cell-based immunotherapy uses an NK cell containing clinical product (typically comprising approximately 50% NK cells) consisting of approximately 2 × 107 cells per kilogram [2]. Our methods without the aAPCs would provide this number of NK cells from approximately 13 × 106 undifferentiated hESCs or iPSCs (approximately one six-well plate). Using the aAPCs would mean that fewer than 106 undifferentiated hESCs/iPSCs would be required per patient at current NK cell doses. This process can be used to produce substantially more NK cells starting from a single, homogeneous and well-characterized starting cell population than can be done with individual apheresis donors used for PB-NK cells. Additionally, these methods decrease the amount of cell processing compared with that of peripheral blood, which requires depletion with anti-CD3 antibodies against T-cells to prevent graft-versus-host disease and anti-CD20 antibodies against B cells to prevent passenger lymphocyte syndrome. Neither T cells nor B cells are present in our cultures [21]. Using the expanding knowledge of KIRs and allo-reactivity, NK cells from diverse genetic backgrounds could be generated to create the optimal NK cell “superdonor,” a concept recently established in a large cohort of subjects indicating that particular KIR haplotypes (centromeric B/B) are optimal in clearing residual leukemia in patients undergoing allogeneic hematopoietic stem cell transplantation [22]. Improved treatment of patients with other tumors may also be feasible with these hESC- and iPSC-derived cells that have cytolytic activity against ovarian, pancreatic, breast cancer, prostate cancer, and myeloma cells [8] (Fig. 4 and data not shown). Treatment of HIV or other chronic viral infections may also be possible [7]. Additionally, it may be possible to engineer hESCs and iPSCs with antitumor and antiviral chimeric antigen receptors to provide an off-the-shelf product of targeted lymphocytes for immunotherapies [23–26].
Clinical translation of hESC- and iPSC-derived cells continues to be steadily advancing. Indeed, investigators have shown the delivery of retinal pigment epithelial cells derived from hESCs are safe, and may be effective, in patients with a form of macular degeneration [27]. Clinical use of hESC/iPSC-derived hematopoietic cells has been of keen interest for more than a decade [21]. Strictly considering cell number, the ability to create enough hESC-derived NK cells for therapy is more feasible than the number of cells needed to generate 1 unit of red blood cells (RBCs) (1012 RBCs per unit). Studies on more efficient derivation of human iPSCs using nonintegrating methods more suitable for clinical translation are also advancing [28]. Therefore, our ability to now produce large numbers of cytotoxic NK cells means the prospect hESC- and iPSC-derived hematopoietic products for diverse clinical therapies can be realized in the not-too-distant future.
Conclusion
Our data demonstrate an improved method to develop NK cells from human pluripotent stem cells. Using a stepwise approach, we were able to transition to a completely defined system amenable to clinical translation. We also demonstrated that hESC-derived progenitors produce their own adherent stromal cells supporting NK cell development. This system not only provides a system for clinical-scale expansion of antitumor lymphocytes but a genetically amenable platform to study human NK cell development.
Acknowledgments
We thank Minh Hong, Michael Lepley, Patrick Walsh, Amanda Guiassis, and Allison Bock for their technical assistance with this work. This work was supported by NIH/NHLBI R01-HL77923 (D.S.K.) and by NIH MSTP Grant T32 GM008244 (D.A.K.), Stem Cell Biology Training Grant T32 (D.A.K.) (T32HD060536), the Leukemia Research Fund of the University of Minnesota Cancer Center, the William L. and Blanche Hughes Foundation, and the European Regional Development Fund, Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123).
Author Contributions
D.A.K.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Z.N. and D.H.: collection and/or assembly of data, data analysis and interpretation; M.K.H. and L.B.: collection and/or assembly of data; L.J.N.C. and D.A.L.: provision of study materials; D.S.K.: conception and design, financial support, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Ljunggren H-G, Malmberg K-J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 2.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galic Z, Kitchen SG, Kacena A, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:11742–11747. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmermans F, Velghe I, Vanwalleghem L, et al. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 2009;182:6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- 6.Woll PS, Martin CH, Miller JS, et al. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- 7.Ni Z, Knorr DA, Clouser CL, et al. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. J Virol. 2011;85:43–50. doi: 10.1128/JVI.01774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woll PS, Grzywacz B, Tian X, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill KL, Obrtlikova P, Alvarez DF, et al. Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function. Exp Hematol. 2010;38:246–257. doi: 10.1016/j.exphem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian X, Hexum MK, Penchev VR, et al. Bioluminescent imaging demonstrates that transplanted human embryonic stem cell-derived CD34+ cells preferentially develop into endothelial cells. Stem Cells. 2009;27:2675–2685. doi: 10.1002/stem.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng ES, Davis R, Stanley EG, et al. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc. 2008;3:768–776. doi: 10.1038/nprot.2008.42. [DOI] [PubMed] [Google Scholar]
- 15.Ng ES, Davis RP, Azzola L, et al. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 16.Kopher RA, Penchev VR, Islam MS, et al. Human embryonic stem cell-derived CD34+ cells function as MSC progenitor cells. Bone. 2010;47:718–728. doi: 10.1016/j.bone.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mrózek E, Anderson P, Caligiuri M. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- 18.Carson WE, Fehniger TA, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 20.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman DS. Toward clinical therapies utilizing hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. New Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torikai H, Reik A, Liu PQ, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119:5697–5705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knorr DA, Kaufman DS. Pluripotent stem cell-derived natural killer cells for cancer therapy. Transl Res. 2010;156:147–154. doi: 10.1016/j.trsl.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadelain M, Brentjens R, Rivie I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 28.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]