Abstract
The course of fatigue and quality of life in survivors of non-Hodgkin’s lymphoma is unknown. The aims of this study were, therefore, to assess fatigue and quality of life in patients with non-Hodgkin’s lymphoma following primary treatment, compare fatigue and quality of life in these patients with those of an age- and sex matched normative population to assess the severity of concerns and identify associations with fatigue of survivors who remained fatigued. The population-based Eindhoven Cancer Registry was used to select all patients diagnosed with non-Hodgkin’s lymphoma from 1999–2009. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire and the Fatigue Assessment Scale were completed once by 824 survivors of non-Hodgkin’s lymphoma (80% response rate); 434 survivors completed these questionnaires again 1 year later. Survivors of non-Hodgkin’s lymphoma reported more clinically relevant fatigue up till 10 years post-diagnosis compared to a normative population (P<0.001). Mean fatigue scores remained fairly stable over time (T1: =28, SD=26; T2:=30, SD=27, P=0.14): 22–28% of survivors reported deterioration, 19–23% reported improvement and 44–54% reported constant fatigue. Survivors who reported constant fatigue were more often diagnosed with stage IV disease and had more comorbid diseases. They were additionally more often female and divorced. Having comorbidities and being without a partner were also associated with constant fatigue in the normative population. In conclusion, six out of every ten responding non-Hodgkin’s lymphoma survivors reported a high level of fatigue up till 10 years after diagnosis. Mean fatigue scores remained stable over time and survivors reporting constant fatigue more often had stage IV disease at diagnosis and comorbidities.
Introduction
As a result of new therapies, the survival of patients with non-Hodgkin’s lymphoma (NHL) has improved considerably. Although the statistics vary, depending on the type of NHL, stage of disease at diagnosis, treatment, and age of the patient, the overall 5-year relative survival rate for all types of NHL (2001–2007) is 50–62%.1 A person diagnosed with cancer is defined as a survivor from the moment of diagnosis through the rest of his or her life.2 The number of NHL survivors in the USA increased from approximately 347,000 in 2001 to approximately 454,000 in 2008.1 In the Netherlands there were approximately 19,600 NHL survivors at the end of 2008.3,4
As many cancer survivors live longer, they are at risk of adverse physical and psychosocial long-term effects, secondary tumors, and recurrence as a result of their cancer and/or of their medical treatments.5–7 These long-term effects, such as fatigue, depression, marital disruption, and problems with infertility, can have a negative influence on survivors’ health-related quality of life (HRQOL).8–12
In the last decades, more attention is being paid to HRQOL after cancer diagnosis. Some studies have investigated HRQOL and fatigue in NHL survivors,13–21 but almost all used a cross-sectional approach (only one measurement at a defined time).13,17–21 However, the longitudinal course of fatigue and HRQOL in patients with NHL and their return to normal life remains largely unknown. The aims of the present study were, therefore, to: (i) assess fatigue and HRQOL twice following primary treatment, (ii) compare fatigue and HRQOL with an age- and sex matched normative population to assess the severity of the concerns, and (iii) identify associations with fatigue in survivors who remained fatigued.
Design and Methods
Setting and population
This study is part of a dynamic, longitudinal, population-based survey among NHL survivors registered with the Eindhoven Cancer Registry (ECR) of the Comprehensive Cancer Centre South (CCCS). The ECR records data on all patients who are newly diagnosed with cancer in the southern part of the Netherlands, an area with 2.3 million inhabitants, 18 hospital locations and two large radiotherapy institutes. The ECR was used to select all patients who were diagnosed with NHL between January 1st, 1999 and July 1st, 2009. We included all patients with indolent (including chronic lymphocytic leukemia) and aggressive B-cell NHL as defined by the International Classification of Diseases for Oncology-3 (ICD-O–3) codes.22
Participants aged ≥85 years at time of the first measurement were excluded, because they would likely have had difficulty in completing self-administered questionnaires without assistance. To exclude patients who had died, our database was linked on every measurement with the database of the Central Bureau for Genealogy, which collects data on all deaths of Dutch citizens through the civil municipal registries. Ethical approval for the study was obtained from a local, certified Medical Ethics Committee.
Study measures
We used the Dutch validated version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ-C30) to assess HRQOL and fatigue. Answer categories range from one (not at all) to four (very much). After linear transformation, all scales and single item measures range in score from 0 to 100. A higher score on function scales and global health and quality of life scales implies a better HRQOL, whereas for symptoms a higher score refers to more symptoms.23
Fatigue was also assessed with the Fatigue Assessment Scale (FAS), a questionnaire consisting of ten items: five questions exploring physical fatigue and five questions exploring mental fatigue. The response scale is a 5-point scale (1 never to 5 always) and scores can range from 10 to 50. A score >21 indicates substantial fatigue. The psychometric properties are good.24,25
Comorbidity at the time of the survey was categorized according to the adapted Self-administered Comorbidity Questionnaire (SCQ).26 Survivors’ marital status and educational level were also assessed in the questionnaire. Clinical information was available from the ECR which routinely collects data on tumor characteristics, including date of diagnosis, tumor grade, histology, Ann Arbor stage,27 primary treatment, and patients’ background characteristics, including gender and date of birth.
Data collection
Data were collected within PROFILES (Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship). PROFILES is a registry for the study of the physical and psychosocial impact of cancer and its treatment from a dynamic, growing population-based cohort of both short and long-term cancer survivors. PROFILES contains a large web-based component and is linked directly to clinical data from the ECR. Details of the data collection method have been described previ-ously.28
From May until November 2009, patients diagnosed between 6 months and 10 years previously received the baseline questionnaire (T1). A year later, patients who were willing to participate again received a 1-year follow-up questionnaire (T2).
EORTC QLQ-C30, SCQ, marital status and educational level data were also collected from an age-and sex-matched normative population29 for comparison with the NHL survivors.
Statistical analyses
All statistical analyses were performed using SAS (version 9.1 for Windows; SAS Institute Inc., Cary, NC, USA). P values <0.05 were considered statistically significant. Clinically relevant differences were determined using evidence-based guidelines for the interpretation of EORTC QLQ-C30 scores between groups30 and changes in scores31 and Norman’s ‘rule of thumb’ was used for the FAS whereby a ± 0.5 SD difference indicates a threshold of discriminating change in HRQOL scores.32
Differences in socio-demographic and clinical characteristics between respondents and non-respondents or patients with unverifiable addresses and patients who completed one or two questionnaires were compared with a chi-square or t-tests, where appropriate. The mean EORTC QLQ-C30 scores among the NHL survivors were compared with those from an age- and sex-matched Dutch normative population using independent sample t-tests. Paired sample t-tests were performed to compare the mean EORTC QLQ-C30 (both NHL survivors and the normative population) and FAS (only NHL survivors) Fatigue scale scores on T1 and T2.
Multivariate logistic regression analyses were carried out to investigate the independent association between the socio-demographic and clinical variables and constant fatigue (versus not constant fatigue). The “constant fatigue group” was defined by survivors/respondents of the normative population who had a Fatigue score >22 on both T1 and T2 for the EORTC QLQ-C30 (i.e. at least a small, clinically relevant higher score than that of the normative population30) versus the group who did not have a fatigue score >22 on both T1 and T2. With respect to the FAS, the “constant fatigue group” was defined by survivors who had a Fatigue score >21 on both T1 and T2 (i.e. indication of substantial fatigue25) versus the group who did not have a fatigue score >21 on both T1 and T2.
Results
Characteristics of the patients and normative population
Eight hundred and twenty-four NHL survivors completed the first questionnaire (80% response rate). Subsequently, 434 (53%) survivors completed this questionnaire again 1 year later, which represents 36% of the total group of NHL survivors. Of the 1731 respondents of the normative population who completed the EORTC QLQ-C30, 602 could be age- and sex-matched with the NHL survivors. Of those 602, 515 (86%) respondents completed the questionnaire again 1 year later.
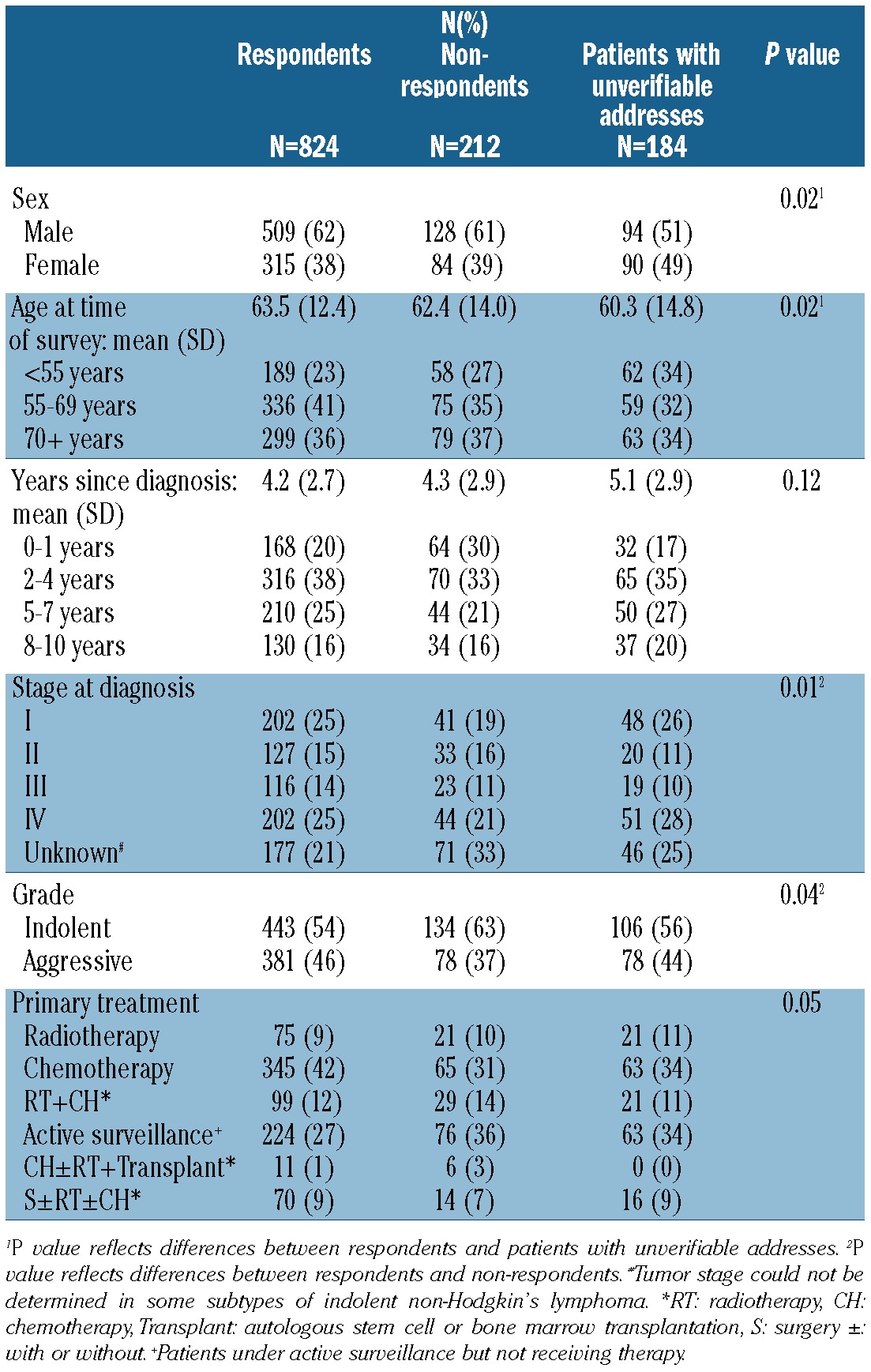
Survivors with unverifiable addresses were more often female and younger compared to respondents, and non-respondents were more often diagnosed with indolent NHL and less often diagnosed with stage I disease (Table 1).
Table 1.
Socio-demographic and clinical characteristics of questionnaire respondents, non-respondents, and patients with unverifiable addresses.
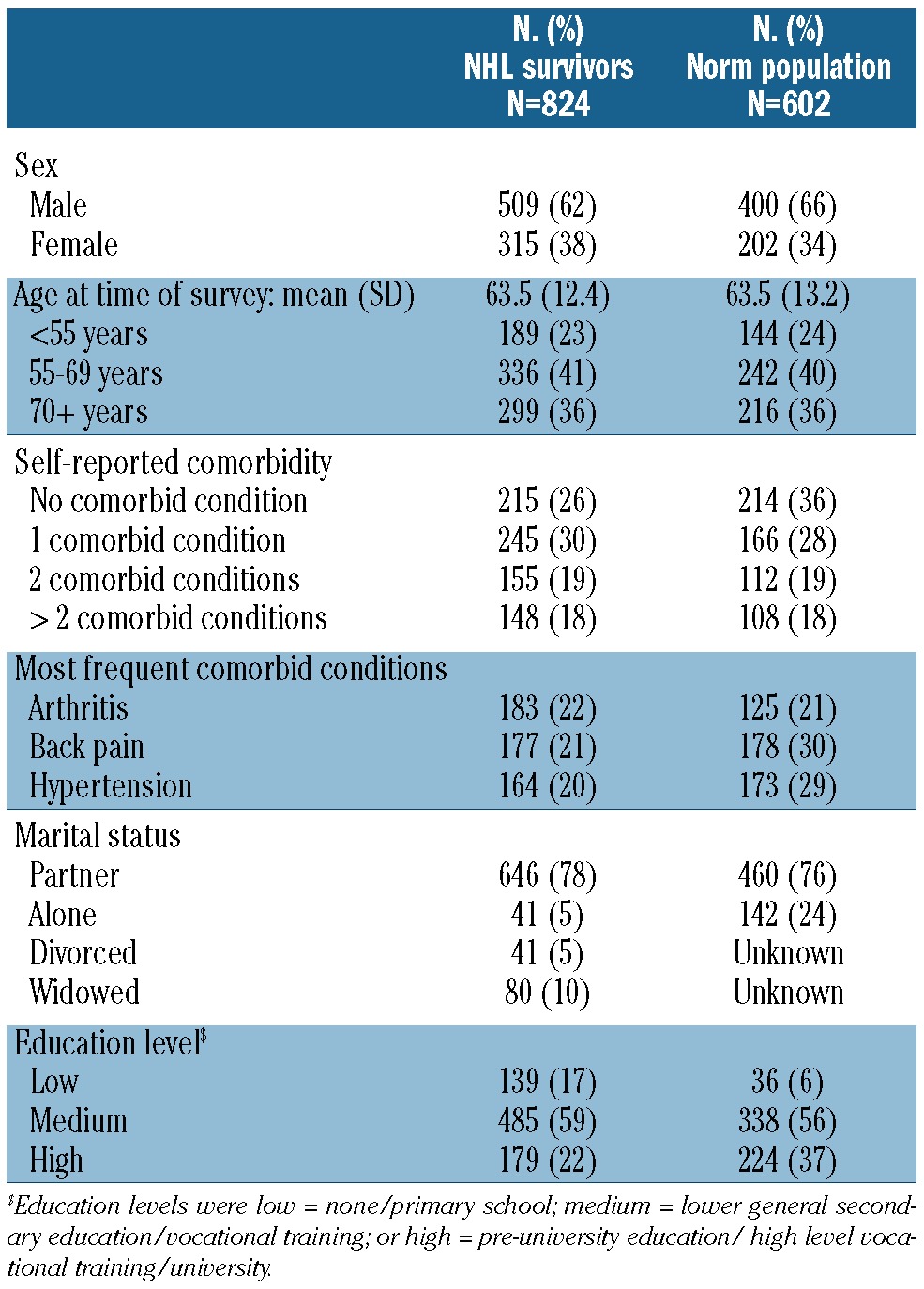
The mean age at completion of the baseline survey was 63.5 years with a mean time since diagnosis of 4.2 years. Chemotherapy was the most frequent primary treatment (42%; Table 1). Two-thirds of survivors reported one or more comorbid conditions, the most common being arthritis, back pain and hypertension (Table 2). In the age-and sex-matched normative population, the mean age at completion of the baseline survey was 63.5 years. Almost two thirds (65%) of respondents reported one or more comorbid conditions, the most common again being hypertension, back pain and arthritis (Table 2).
Table 2.
Socio-demographic characteristics of NHL survivors (n=824), and respondents of an age- and sex-matched normative population (n=602).
A comparison between survivors who completed one or both questionnaires indicated that those who completed both questionnaires had a significantly longer mean time since diagnosis at time of first enrollment (4.2 versus 5.1 years, P<0.001) and more often had a high educational level (19% versus 25%, P=0.013). No differences were observed between these groups for EORTC QLQ-C30 –– Fatigue ( =28.6 versus =28.3, P=0.88) or FAS Fatigue –– scores ( =21.9 versus =21.4, P=0.33).
Health-related quality of life and fatigue among survivors of non-Hodgkin’s lymphoma and the normative population
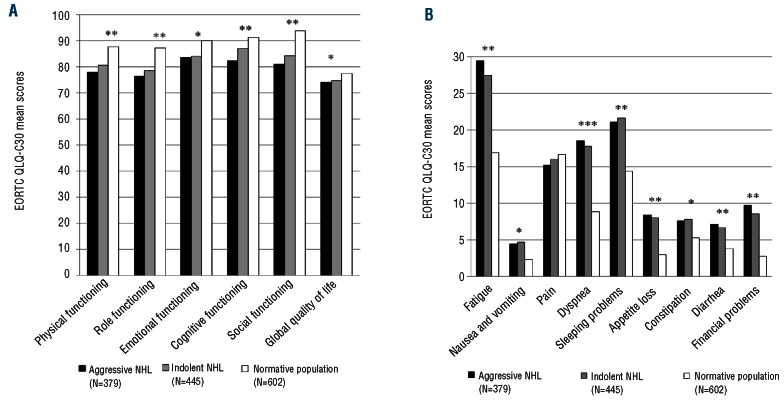
Compared to an age- and sex-matched normative population, responding NHL survivors had, on average, worse scores for the EORTC QLQ-C30 Physical, Role, Cognitive and Social Functioning domains. NHL survivors also reported more Fatigue, Dyspnea, Sleeping Problems, Appetite Loss, Diarrhea and Financial Problems (all P≤0.001 and clinically relevant; Figure 1A and 1B). Scores between survivors of indolent and aggressive NHL were not significantly different. No clinically significant differences were found in EORTC QLQ-C30 mean fatigue scores depending on years since diagnosis (Figure 2).
Figure 1.
(A) Differences in EORTC QLQ-C30 mean functioning and global quality of life scores between survivors of aggressive NHL (n=379), indolent NHL survivors (n=445) and an age- and sex-matched normative population (n=602) *P<0.001**P<0.001 and small clinically important differences.30 A higher score implies a better HRQOL. (B) Differences in EORTC QLQ-C30 mean symptom scores between survivors of aggressive NHL (n=379), indolent NHL (n=445) and an age- and sex-matched normative population (n=602)*P<0.001; **P<0.001 and small clinically important differences;30 ***P<0.001 and medium clinically important differences.30 A higher score indicates to more symptoms.
Figure 2.
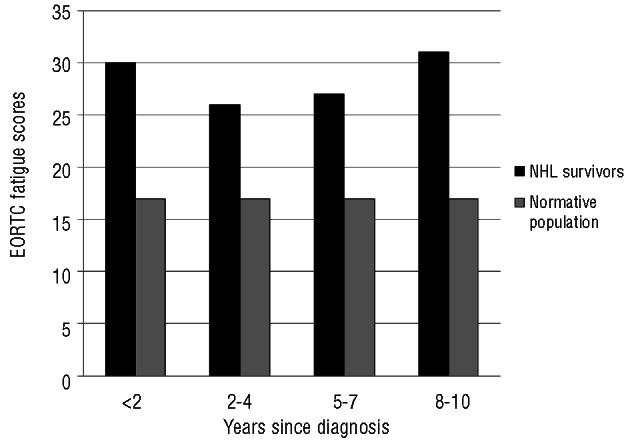
Differences between EORTC QLQ-C30 fatigue scores of all NHL survivors (n=824) according to years survived since diagnosis and an age- and sex-matched normative population (N=602). All P<0.001 and small or medium clinically important differences.30 A higher score indicates more fatigue.
Thirty-nine percent (n=321) of the NHL survivors did not have clinically relevant worse scores, i.e. they had a ≤5 point difference, for the EORTC QLQ-C30 Fatigue scale than the normative population. The other 61% did have clinically relevant worse scores for Fatigue, with the difference being small (>5 to 13 point difference) in 17% (n=140) of survivors; medium (>13 to 19 point difference) in 15% (n=124) and large (>13 point difference) in 29% (n=239).
Fatigue over time
The 1-year follow-up questionnaire was completed by 434 NHL survivors and 514 respondents of the normative population. With respect to FAS Fatigue (NHL survivors only), mean scores remained significantly stable over time –– (T1: =21; T2:=22, Table 3). However, 22% reported deteriorated fatigue scores with a mean difference of 6.4 and 19% reported improved scores with a mean difference of 5.9. With respect to the EORTC QLQ-C30 Fatigue, mean scores also remained significantly stable over time –– (T1: =28; T2: =29, Table 3), 32% reported deteriorated scores with a mean difference of 21 points, and 31% showed improved scores with a mean difference of 19 points. Similar mean scores and percentages of deterioration and improvement were observed when focusing on diffuse large B-cell lymphoma or follicular lymphoma only (Table 3). Mean scores of the normative population –– changed slightly over time (T1:=17; T2: =18, P<0.04; Table 2) with 31% reporting deteriorated and 24% reporting improved EORTC QLQ-C30 Fatigue scores.
Table 3.
Mean fatigue scores (SD) at baseline (T1) and follow-up (T2) among NHL survivors and respondents of the normative population who completed two questionnaires (NHL survivors; n=434; normative population, n=515), and percentages of patients/respondents who deteriorated/improved between these time points (mean difference and SD).
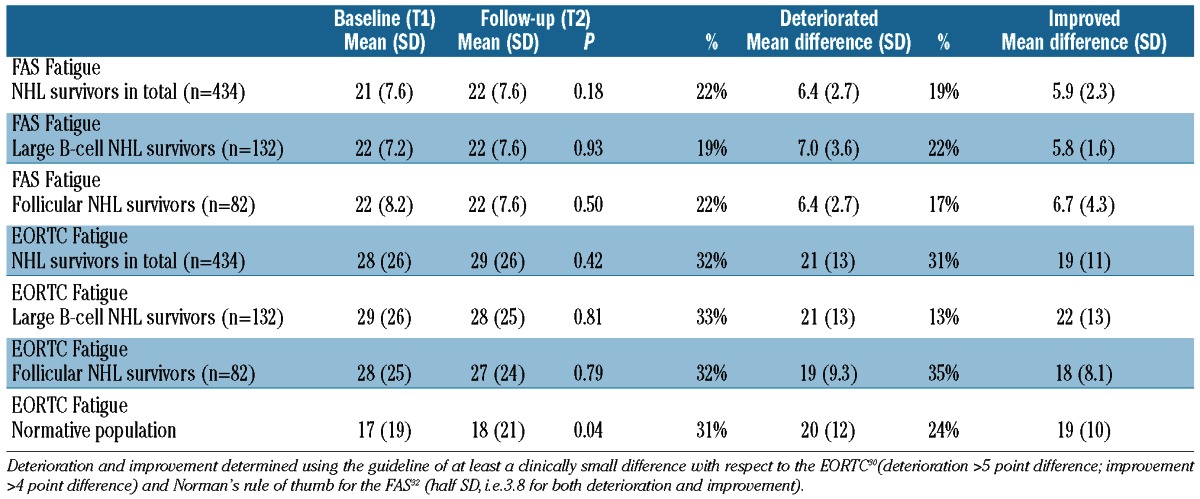
Of NHL survivors, 54% reported constant EORTC QLQ-C30 Fatigue, i.e. had a Fatigue score above 22 for both T1 and T2. Of respondents of the normative population, 30% reported constant EORTC QLQ-C30 Fatigue. With respect to FAS Fatigue, 40% of NHL survivors reported constant fatigue i.e. had a Fatigue score above 21 on both T1 and T2.
Associations with fatigue
Multivariate logistic regression analyses showed that NHL survivors who reported constant fatigue (on both EORTC QLQ-C30 and FAS) were more often diagnosed with stage IV disease and more often reported comorbid diseases. They were additionally more often female and divorced (Table 4). Survivors who remained fatigued (however only on FAS fatigue) were also more often diagnosed longer ago, were under active surveillance and had a lower educational level.
Table 4.
Odds ratios with confidence intervals (CI) of the multivariate logistic regression model evaluating independent variables for EORTC QLQ-C30 and FAS Fatigue scores for patients (n=434) and respondents of the normative population (n=515) who completed two questionnaires and remained fatigued.
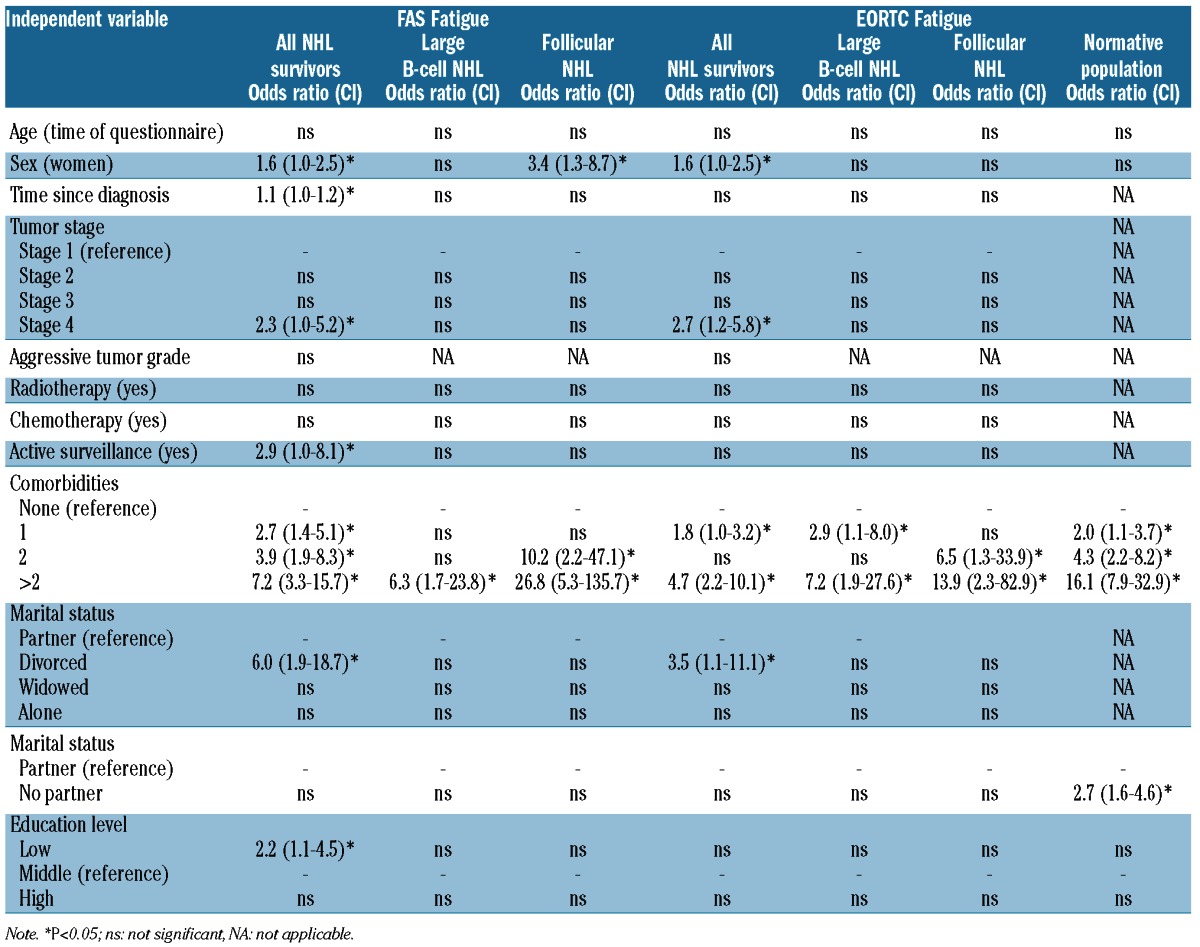
With respect to survivors of diffuse large B-cell and follic-ular lymphoma, survivors who reported constant fatigue (on both the EORTC QLQ-C30 and FAS) reported comorbid diseases more often. Survivors of follicular lymphoma who reported constant fatigue were also more often females; however, this was only found on the FAS (Table 4).
Respondents of the normative population who reported constant fatigue also reported comorbid diseases more often and more often had no partner (Table 4).
Discussion
The majority of NHL survivors showed a constant, high level of fatigue in this population-based study up to 10 years after diagnosis. Six out of 10 survivors reported clinically relevant worse fatigue scores compared to the normative population. HRQOL was also worse to a clinically relevant degree among survivors. Mean fatigue scores remained significantly stable over time; 22–28% reported clinically relevant deterioration, whereas 19–23% reported clinically relevant improvement; 44–54% reported constant fatigue. No clinically significant differences in EORTC QLQ-C30 mean fatigue scores were observed in relation to years since diagnosis.
Changes over time in NHL survivors have so far been investigated in three small studies, only including short-term survivors for a maximum of 18 months after primary treatment. One prospective study found no clinically significant change in mean EORTC QLQ-C30 Fatigue scores16. One Dutch study and another Norwegian study showed mean deteriorations in EORTC QLQ-C30 Fatigue scores of 14 and 10 points when comparing start of treatment scores with those at 18 months and 1 year of follow up, respectively.14,15 A limitation of these studies is that they all focused on mean differences. Mean scores do not reflect individual changes. Given the large standard deviations, there must be high degrees of variations within these groups. A better way is, therefore, to make a distinction between patients who improved and patients who deteriorated.
The present study showed that survivors with stage IV disease and comorbid conditions more often reported constant fatigue. Females and divorced survivors were also more likely to remain fatigued. In the normative population, we also observed a relation between comorbidity and having a partner and fatigue. This relation is not, therefore, specific to NHL survivors but is probably applicable to people in general. Type of NHL (aggressive or indolent), treatment, and survival time since diagnosis were not associated, or only associated with one measure of fatigue in NHL survivors. The ECR collects data on primary treatment only. More detailed treatment information, longitudinally assessed, will enable us to study the relation between initial treatment and HRQOL and fatigue in more detail. Furthermore, detailed information about disease progression could also contribute to unraveling the course of HRQOL and fatigue and will help health care providers to give their patients better information about their expected HRQOL. As our HRQOL study is embedded in PHAROS (Population based HAematological Registry for Observational Studies) in which more detailed disease and treatment information is collected, as well as long-term side effects, we will be able to determine this relation better in the near future.
NHL survivors reported worse HRQOL compared to that of an age- and sex-matched normative population. Clinically relevant worse scores for survivors were observed for fatigue, appetite loss, diarrhea, dyspnea and all function scales including financial problems. One prospective and three cross-sectional studies also observed clinically worse scores for HRQOL domains for NHL survivors compared with those of a normative popula-tion.13,15,17,20
Numerous patients in our study showed large improvements (19–23%) or deteriorations (22–28%) within 1 year, which both indicate a clinically relevant change.31 However, it is too soon to determine whether this can be defined as an actual change, due to regression to the mean. A longer follow-up time is needed to identify whether these differences can be considered as real changes or fluctuations over time.
Significant differences were not observed between patients with indolent or aggressive NHL, recapitulating findings in an American cross-sectional study,33 nor between short- or long-term survivors, confirming results of a cross-sectional study among 761 NHL survivors.20 This suggests that there is no improvement in time, which is also shown by our 1-year follow-up results.
Prevalence rates for cancer-related fatigue vary widely. Percentages between 32% and 60% have been reported34–36 and in a recently published study an overall prevalence of 48% was found.37 The observed percentage of 61% in this study is somewhat higher. In our study, 29% of survivors reported large, clinically important fatigue, whereas 15% reported medium clinically important fatigue, making a total of 44%. Adding the survivors with small, clinically important fatigue produced the observed total of 61% of patients with cancer-related fatigue. Besides differences between types of cancer, the use of different cut-off scores and fatigue assessment instruments contribute to the differences in reported prevalences.38–40
The underlying mechanisms that cause constant cancer-related fatigue are not yet clear.41 Many factors are associated with the development of fatigue, such as type of treatment, the disease itself, medication-related adverse events, biological modifiers (such as interferon), depression, physical inactivity, anxiety, pain and sleep disturbances.42–46 Although the cause of fatigue is not completely clear, results of a recently published review47 show that patients with fatigue may benefit from pharmacological and/or non-pharmacological treatments, such as cognitive-behavioral interventions and exercise.48 Further research is necessary to determine whether an early intervention for fatigue can reduce this long-term complication and whether patients can benefit from late intervention.
The present study had the following limitations: although information was available concerning sociodemographic and clinical characteristics of the non-respondents and patients with unverifiable addresses, it remains unknown whether non-respondents declined to participate in the study because of poor health or the absence of symptoms. Comparing patients who completed one questionnaire with patients who completed two questionnaires only indicated differences in mean time since diagnosis and educational level. This perhaps resulted in a small selection bias. In addition, there is always an uncertainty with the reproducibility of self-reported questionnaires. Some of the changes might be ascribed to that arbitrariness.
The strengths of our study are the population-based sampling frame instead of a hospital-based sampling frame. Furthermore, the large range in elapsed time since diagnosis facilitates extrapolation of the results to a broad range of NHL survivors in the population. In addition, the longitudinal design provides important information about development over time.
In conclusion, six out of every ten NHL survivors reported a high level of fatigue up until 10 years after diagnosis. HRQOL and fatigue scores of survivors were clinically relevant and worse than those of an age- and sex-matched normative population. Fatigue mean scores remained significantly stable over time and 44–54% of survivors reported constant fatigue. Survivors with stage IV disease, comorbid conditions as well as females and divorced survivors were more likely to remain fatigued. Having comorbidities and being without a partner were also associated with continuous fatigue in the normative population. As research on the underlying determinants of fatigue proceeds, health care providers should continue to screen patients on their level of fatigue and inform them about possible rehabilitation programs.
Supplementary Material
Acknowledgments
We thank all patients and their doctors for their participation in the study. Special thanks go to Nicole Horevoorts for assistance with data collection and Dr. M. van Bommel for independent advice and answering questions of patients, invited to participate. Specialists in the following hospitals provided cooperation: Catharina-Hospital, Eindhoven; Jeroen Bosch Hospital, ‘s Hertogenbosch; Maxima Medical Centre, Eindhoven and Veldhoven; Sint Anna Hospital, Geldrop; St. Elisabeth Hospital, Tilburg; Twee Steden Hospital, Tilburg; VieCurie Hospital, Venlo and Venray and Hospital Bernhoven, Oss.
Footnotes
Funding
This study was financially supported by the Jonker-Driessen Foundation and ZonMW: the Netherlands organization for health research and development, and through PHAROS: Population-based HAematological Registry for Observational Studies (#80-82500-98-01007).
Dr. Floortje Mols is supported by a VENI grant (#451-10-041) from the Netherlands Organization for Scientific Research (The Hague, the Netherlands),
Dr. Lonneke van de Poll-Franse is supported by a Cancer Research Award from the Dutch Cancer Society (#UVT-2009-4349).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011, accessed on May 10, 2011 [Google Scholar]
- 2.US National Coalition for Cancer Survivorship. http://www.canceradvoca-cy.org/, accessed on December 28, 2009
- 3.Kankerbestrijding SvK. Kanker in Nederland tot 2020, Trends en prognoses. http://www.kankerbestrijding.nl 2011
- 4.van de Schans SA, Issa DE, Visser O, Nooijen P, Huijgens PC, Karim-Kos HE, et al. Diverging trends in incidence and mortality, and improved survival of non-Hodgkin’s lymphoma, in the Netherlands, 1989–2007. Ann Oncol. 2012;23(1):171–82 [DOI] [PubMed] [Google Scholar]
- 5.Geffen DB, Blaustein A, Amir MC, Cohen Y. Post-traumatic stress disorder and quality of life in long-term survivors of Hodgkin’s disease and non-Hodgkin’s lymphoma in Israel. Leuk Lymphoma. 2003;44(11):1925–9 [DOI] [PubMed] [Google Scholar]
- 6.Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol. 2011;29(14):1885–92 [DOI] [PubMed] [Google Scholar]
- 7.Seshadri T, Pintilie M, Kuruvilla J, Keating A, Tsang R, Zadeh S, Crump M. Incidence and risk factors for second cancers after autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Leuk Lymphoma. 2009;50(3):380–6 [DOI] [PubMed] [Google Scholar]
- 8.Fobair P, Hoppe RT, Bloom J, Cox R, Varghese A, Spiegel D. Psychosocial problems among survivors of Hodgkin’s disease. J Clin Oncol. 1986;4(5):805–14 [DOI] [PubMed] [Google Scholar]
- 9.Hjermstad MJ, Fossa SD, Oldervoll L, Holte H, Jacobsen AB, Loge JH. Fatigue in long-term Hodgkin’s disease survivors: a follow-up study. J Clin Oncol. 2005;23(27): 6587–95 [DOI] [PubMed] [Google Scholar]
- 10.Hjermstad MJ, Oldervoll L, Fossa SD, Holte H, Jacobsen AB, Loge JH. Quality of life in long-term Hodgkin’s disease survivors with chronic fatigue. Eur J Cancer. 2006;42(3):327–33 [DOI] [PubMed] [Google Scholar]
- 11.Knobel H, Loge J, Brit Lund M, Forfang K, Nome O, Kaasa S. Late medical complications and fatigue in Hodgkin’s disease survivors. J Clin Oncol. 2001;19(13):3226–33 [DOI] [PubMed] [Google Scholar]
- 12.Loge JH, Abrahamsen AF, Ekeberg O, Kaasa S. Reduced health-related quality of life among Hodgkin’s disease survivors: a comparative study with general population norms. Ann Oncol. 1999;10(1):71–7 [DOI] [PubMed] [Google Scholar]
- 13.Bellizzi KM, Rowland JH, Arora NK, Hamilton AS, Miller MF, Aziz NM. Physical activity and quality of life in adult survivors of non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(6):960–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doorduijn J, Buijt I, Holt B, Steijaert M, Uyl-de Groot C, Sonneveld P. Self-reported quality of life in elderly patients with aggressive non-Hodgkin’s lymphoma treated with CHOP chemotherapy. Eur J Haematol. 2005;75(2):116–23 [DOI] [PubMed] [Google Scholar]
- 15.Jerkeman M, Kaasa S, Hjermstad M, Kvaloy S, Cavallin-Stahl E. Health-related quality of life and its potential prognostic implications in patients with aggressive lymphoma: a Nordic Lymphoma Group Trial. Med Oncol. 2001;18(1):85–94 [DOI] [PubMed] [Google Scholar]
- 16.Merli F, Bertini M, Luminari S, Mozzana R, Berte R, Trottini M, et al. Quality of life assessment in elderly patients with aggressive non-Hodgkin’s lymphoma treated with anthracycline-containing regimens. Report of a prospective study by the Intergruppo Italiano Linfomi. Haematologica. 2004;89(8):973–8 [PubMed] [Google Scholar]
- 17.Mols F, Aaronson NK, Vingerhoets AJ, Coebergh JW, Vreugdenhil G, Lybeert ML, van de Poll-Franse LV. Quality of life among long-term non-Hodgkin lymphoma survivors: a population-based study. Cancer. 2007;109(8):1659–67 [DOI] [PubMed] [Google Scholar]
- 18.Pettengell R, Donatti C, Hoskin P, Poynton C, Kettle PJ, Hancock B, et al. The impact of follicular lymphoma on health-related quality of life. Ann Oncol. 2008;19(3):570–6 [DOI] [PubMed] [Google Scholar]
- 19.Smith SK, Crespi CM, Petersen L, Zimmerman S, Ganz PA. The impact of cancer and quality of life for post-treatment non-Hodgkin lymphoma survivors. Psychooncology. 2010;19(12):1259–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SK, Zimmerman S, Williams CS, Zebrack BJ. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer. 2009;115(14):3312–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallance JK, Courneya KS, Jones LW, Reiman T. Differences in quality of life between non-Hodgkin’s lymphoma survivors meeting and not meeting public health exercise guidelines. Psychooncology. 2005;14(11):979–91 [DOI] [PubMed] [Google Scholar]
- 22.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin , et al. International Classification of Diseases for Oncology (3rd ed). Geneva, World Health Organisation; 2000 [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76 [DOI] [PubMed] [Google Scholar]
- 24.Michielsen HJ, De Vries J, Drent M, Peros-Golubicic T. Psychometric qualities of the Fatigue Assessment Scale in Croatian sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(2):133–8 [PubMed] [Google Scholar]
- 25.Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J Psychosom Res. 2003;54(4):345–52 [DOI] [PubMed] [Google Scholar]
- 26.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–63 [DOI] [PubMed] [Google Scholar]
- 27.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31(11): 1860–1 [PubMed] [Google Scholar]
- 28.van de Poll-Franse LV, Horevoorts N, Eenbergen MV, Denollet J, Roukema JA, Aaronson NK, et al. The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47(14): 2188–94 [DOI] [PubMed] [Google Scholar]
- 29.van de Poll-Franse LV, Mols F, Gundy CM, Creutzberg CL, Nout RA, Verdonck-de Leeuw IM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2010;47(5):667–75 [DOI] [PubMed] [Google Scholar]
- 30.Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011; 29(1):89–96 [DOI] [PubMed] [Google Scholar]
- 31.Cocks K, King MT, Velikova G, de Castro G, Jr, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012; 48(11):1713–21 [DOI] [PubMed] [Google Scholar]
- 32.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92 [DOI] [PubMed] [Google Scholar]
- 33.Blaes AH, Ma L, Zhang Y, Peterson BA. Quality of life appears similar between survivors of indolent and aggressive non-Hodgkin lymphoma. Leuk Lymphoma. 2011;52(11):2105–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horneber M, Fischer I, Dimeo F, Ruffer JU, Weis J. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109(9):161–71; quiz 172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhnt S, Ernst J, Singer S, Ruffer JU, Kortmann RD, Stolzenburg JU, Schwarz R. Fatigue in cancer survivors--prevalence and correlates. Onkologie. 2009;32(6):312–7 [DOI] [PubMed] [Google Scholar]
- 36.Singer S, Kuhnt S, Zwerenz R, Eckert K, Hofmeister D, Dietz A, et al. Age- and sex-standardised prevalence rates of fatigue in a large hospital-based sample of cancer patients. Br J Cancer. 2011;105(3):445–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2011;118(8 Suppl): 2261–9 [DOI] [PubMed] [Google Scholar]
- 38.Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007;12 (Suppl 1):11–21 [DOI] [PubMed] [Google Scholar]
- 39.Piper BF, Cella D. Cancer-related fatigue: definitions and clinical subtypes. J Natl Compr Canc Netw. 2010;8(8):958–66 [DOI] [PubMed] [Google Scholar]
- 40.Seyidova-Khoshknabi D, Davis MP, Walsh D. Review article: a systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care. 2011; 28(2):119–29 [DOI] [PubMed] [Google Scholar]
- 41.Ray M, Rogers LQ, Trammell RA, Toth LA. Fatigue and sleep during cancer and chemotherapy: translational rodent models. Comp Med. 2008;58(3):234–45 [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage. 2003;26(1):604–14 [DOI] [PubMed] [Google Scholar]
- 43.Malik UR, Makower DF, Wadler S. Interferon-mediated fatigue. Cancer 2001;92(6 Suppl):1664–8 [DOI] [PubMed] [Google Scholar]
- 44.Mustian KM, Morrow GR, Carroll JK, Figueroa-Moseley CD, Jean-Pierre P, Williams GC. Integrative nonpharmacologic behavioral interventions for the management of cancer-related fatigue. Oncologist. 2007;12 (Suppl 1):52–67 [DOI] [PubMed] [Google Scholar]
- 45.Portenoy RK. Cancer-related fatigue: An immense problem. Oncologist. 2000;5(5): 350–2 [DOI] [PubMed] [Google Scholar]
- 46.Tsai LY, Li IF, Lai YH, Liu CP, Chang TY, Tu CT. Fatigue and its associated factors in hospice cancer patients in Taiwan. Cancer Nurs. 2007;30(1):24–30 [DOI] [PubMed] [Google Scholar]
- 47.Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22(6):1273–9 [DOI] [PubMed] [Google Scholar]
- 48.Goedendorp MM, Peters ME, Gielissen MF, Witjes JA, Leer JW, Verhagen CA, Bleijenberg G. Is increasing physical activity necessary to diminish fatigue during cancer treatment¿ Comparing cognitive behavior therapy and a brief nursing intervention with usual care in a multicenter randomized controlled trial. Oncologist. 2010;15 (10):1122–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.