Abstract
Deletion of the Ikaros (IKZF1) gene is an oncogenic lesion frequently associated with BCR-ABL1-positive acute lymphoblastic leukemias. It is also found in a fraction of BCR-ABL1-negative B-cell precursor acute lymphoblastic leukemias, and early studies showed it was associated with a higher risk of relapse. Therefore, screening tools are needed for evaluation in treatment protocols and possible inclusion in risk stratification. Besides monosomy 7 and large 7p abnormalities encompassing IKZF1, most IKZF1 alterations are short, intragenic deletions. Based on cohorts of patients, we mapped the microdeletion breakpoints and developed a breakpoint-specific fluorescent multiplex polymerase chain reaction that allows detection of recurrent intragenic deletions. This sensitive test could also detect IKZF1 subclonal deletions, whose prognostic significance should be evaluated. Moreover, we show that consensus breakpoint sequences can be used as clonal markers to monitor minimal residual disease. This paper could be useful for translational studies and in clinical management of BCP-ALL.
Introduction
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is the most common malignancy in children. It is made up of genetically distinct subtypes defined by chromosomal rearrangements, such as t(9;22)/BCR-ABL1, t(12;21)/ETV6-RUNX1, t(1;19)/E2A-PBX1, rearrangements of MLL, hyperdiploid and hypodiploid karyotypes, most of which were found to be associated with a distinct prognosis and are currently used for risk-adapted treatment protocols.1 Although these oncogenic lesions are crucial in leukemia initiation, they are not sufficient to define the biology of leukemia. High-resolution, microarray-based techniques have recently been used to identify a myriad of co-operating oncogenic microdeletions and to describe their distribution within the known genetic subtypes.2 In particular, deletion of IKZF1 coding the lymphoid transcription factor IKAROS has been shown to be a hallmark of BCR-ABL1-positive ALL. IKZF1 deletions have also been found in a fraction of BCR-ABL1-negative BCP-ALL3 and early studies suggest it is associated with a poor prognosis.4-7 Therefore, testing for IKZF1 alterations is necessary for retrospective prognostic evaluation and future implementation in risk stratification in treatment protocols. Classical techniques allowing detection of microdeletions, include whole-genome hybridization-based technologies such as array-CGH and SNP-arrays, and targeted gene-dosage methods such as multiplex-ligation probe assay (MLPA) and quantitative polymerase chain reaction (PCR). Although effective, all these techniques estimate the copy-number and, therefore, have limited sensitivity. This may be an important caveat when assessing cooperating oncogenic lesions that may be subclonal at the time of diagnosis.
IKZF1 deletions are whole gene deletions that can be the result of monosomy 7 or del(7p), and intragenic deletions. Most IKZF1 intragenic deletions encompass exons 4 to 7 (thereafter named Δ4-7), driving the expression of a non-DNA binding Ikaros isoform (Ik6) with dominant negative activity.4 Other types of intragenic deletions have been reported whose functional consequences are less clear. These rearrangements are thought to be mediated by aberrant RAG recombination, with breakpoints highly clustered at the vicinity of recombination signal sequence (RSS)-like sequences.4,8 This offers the possibility of designing PCR assays with primers bridging recurrent breakpoints in order to amplify the fusion genomic sequence created by the deletion. Unlike methods based on copy-number measurement, this approach can detect genomic deletions with a sensitivity level similar to that obtained for detection of translocations.
Here we describe a breakpoint-specific multiplex PCR assay for sensitive detection of most IKZF1 intragenic deletions that can be easily included in the initial molecular screening of BCP-ALL. Importantly, this assay can detect minor subclones. Moreover, we developed a panel of Taqman-based quantitative PCR systems to use these rearrangements as leukemia-specific markers for minimal residual disease (MRD) monitoring.
Design and Methods
Patients and samples
BCP-ALL cases analyzed in this study included 2 series of pediatric patients aged 1-17 years: a series of 60 BCR-ABL1-positive ALL (sex ratio 1.9, median age 9 years) referred to our laboratory between 1995 and 2012, and a validation cohort of 512 BCR-ABL1-negative BCP-ALL (sex ratio 1.2, median age 5 years) enrolled in the EORTC-CLG 58 951 trial. In addition, 3 other BCR-ABL1-negative IKZF1-deleted BCP-ALL cases identified by high-resolution array-CGH were used for breakpoint mapping of Δ4-8. Informed consent was obtained from the patients and/or their parents or guardians according to the Declaration of Helsinki. This study has been approved by the Protocol Review Committee of the EORTC Children Leukemia Group. Leukemia cells were obtained at diagnosis and/or relapse from bone marrow or blood samples. All diagnostic samples had more than 50% of blasts after Ficoll separation. Samples were processed according to standard procedures as previously described.9BCR-ABL1 fusion transcript assessment and karyotype analysis were performed at diagnosis according to standard routine procedures. Ig/TCR-based MRD monitoring was performed and analyzed following the EuroMRD guidelines.10
Array-CGH and cloning of deletion breakpoints
Array-CGH was performed using the 1×1M Microarray SurePrint G3 Catalog (www.agilent.com) and analyses were performed using the Agilent Genomic Workbench software with the help of the ADM-2 algorithm, as previously described.11
Genomic deletion breakpoints centromeric to exon 8 and variant breakpoints in intron 1 were mapped by tiling primers outward from the genomic locations of array-CGH probes defining the minimal regions of deletion at approximately 500-bp intervals to beyond the next non-deleted probes telomeric and centromeric to the deletion. Long-distance PCR was performed using the Expand Long Template PCR system (Roche Applied Science) according to the manufacturer's instructions. Breakpoint sequences were determined by direct sequencing of PCR products using the Big Dye Terminator Sequencing Kit v1.1 (Applied Biosystems, Foster City, CA, USA). Reaction products were run on an automated capillary sequencer (ABI 3130XL Genetic Analyzer, Applied Biosystems). Reference genome sequence data were obtained from the University of Santa Cruz browser (www.genome.ucsc.edu/, hg19 assembly).
Breakpoint-specific multiplex fluorescent PCR
Primers were designed using Primer3Plus software (www.bioinformatics.nl/cgi-bin/primer3plus) in such a way that they could be combined in a single multiplex PCR and that the amplicon length and fluorescent labeling allowed direct identification of each type of deletion. A reverse primer in intron 3 was added to generate an amplicon of the non-rearranged genomic sequence (731 bp length) as an optional PCR control. Fifty ng DNA were amplified in a final volume of 25 μL containing 1 × buffer (Gotaq flexi, colorless, Promega), 1.5 mM MgCl2, 0.2 mM dNTP, 0.4 μM of each primer, and 0.25 U GoTaq® DNA Polymerase (Promega). The cycling protocol was a denaturation step at 95°C for 4 min followed by 30 s at 95°C, 30 s at 60°C, 1 min at 72°C for 30 cycles, and a final extension phase of 4 min at 72°C. PCR products were run on an ABI 3130 analyzer using a fragment size analysis program, and analyses were performed using GeneMapper software (Applied Biosystems). PCR products were sequenced as described earlier. Assay sensitivity was tested by analyzing serial 10-fold dilutions of deleted DNA into normal DNA.
Multiplex-ligation probe assay (MLPA)
MLPA was performed using the SALSA MLPA kits P335 ALLIKZF1-A3, and P202-A1 IKZF1 for control (MRC-Holland), according to the manufacturer's instructions. Samples were run on an ABI 3130 analyzer (Applied Biosystems) and results were analyzed using the Tumor Analysis (LS) method of the Coffalyser version 8 software (www.mlpa.com/coffalyser, MRC Holland). The ratio results obtained for each probe were then adjusted for the percentage of blasts using the following formula: (ratio-1+blast percentage)/blast percentage.12 Isolated reduced signal for exon 1 probes was not considered because it could be an artifact due to the presence of GC-rich sequences. MLPA sensitivity was evaluated by analysis of serial dilutions of IKZF1 deleted cases into non-tumoral DNA.
MRD analysis by real-time quantitative PCR for IKZF1 intragenic deletions
Primers and Taqman probes were designed using Primer3Plus software. MRD analyses were performed and interpreted according to the EuroMRD guidelines designed for Ig/TCR based MRD analyses.10 500 ng DNA were amplified in triplicate in a final volume of 25 μL, containing 1 × Platinum® Quantitative PCR SuperMix-UDG (Invitrogen), 0.5 μL ROX Reference Dye (Invitrogen), 1 mM MgCl2, 0.04% BSA, 0.2 μM of each forward and reverse primer, and 0.1 μM of TaqMan® probe. Amplification was performed using an ABI Prism 7700 thermocycler (Applied Biosystems) as follows: incubation at 50°C for 2 min, denaturation at 95°C for 10 min, followed by 50 cycles of 15 s at 95°C and 1 min 20 s at 62°C.
Results and Discussion
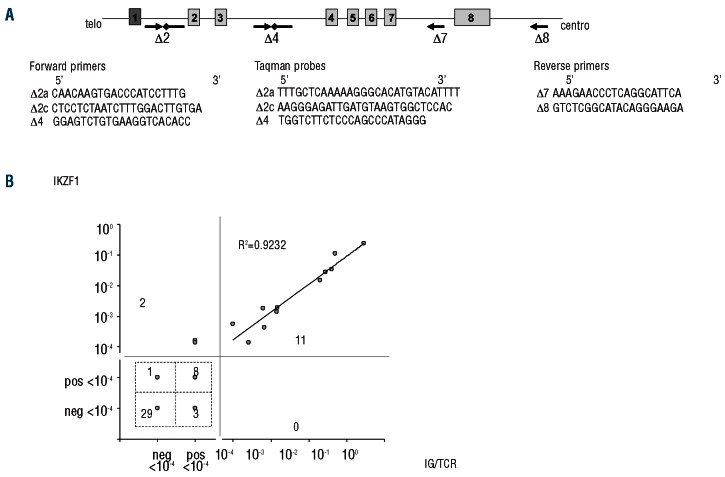
We first set up a test to detect intragenic deletions of IKZF1. Some deletion breakpoint sequences have been previously reported4,8 that allowed us to delineate the breakpoint cluster regions of Δ4-7 and Δ2-7 rearrangements. Less frequent deletions, such as Δ4-8 and Δ2-8, were also reported but breakpoint cluster regions were not characterized. We retrospectively looked for the presence of such deletions in a series of BCP-ALL with available high-resolution array-CGH data. Three cases with Δ4-8 rearrangements were identified (Figure 1A) and breakpoint sequences were further characterized by long-distance PCR (Online Supplementary Table S1). The proximal breakpoints were located in intron 3 in the same region as Δ4-7 rearrangements while the distal breakpoints clustered at approximately 12 kb centromeric from the 3'UTR of the IKZF1 gene. Non-template nucleotides and RSS-like sequences were observed at the breakpoint junctions, as previously described for other types of IKZF1 intragenic rearrangements.4,8 Then we designed a PCR assay with appropriate forward and reverse primers to amplify the junction regions of all types of characterized intragenic rearrangements. Multiplexing and fluorochrome-labeled primers allowed detection of all rearrangements in a single PCR reaction (Figure 1B-C). In addition, a reverse primer in intron 3 designed to amplify the germline sequence was added to provide a control for DNA amplification.
Figure 1.
(A) Array-CGH plots showing an IKZF1 intragenic Δ4-8 deletion (case CGH #1). (B) Schematic representation of IKZF1 gene and recurrent intragenic deletions with primer sequences for breakpoint-specific PCR. Boxes represent exons, with the non-coding exon 1 in dark gray. Blue and green arrows represent forward fluorescent-labeled primers, black arrows represent non-labeled reverse primers. GL, germline, designates the primer for control amplification of a non-rearranged IKZF1 sequence. (C) Representative Genescan pattern of each type of IKZF1 rearrangement amplified by breakpoint-specific fluorescent multiplex PCR. Amplicon size ranges are indicated. Dotted lines indicate amplicons size ranges for variant breakpoints. GL, germline, indicates the 760 bp control amplicon.
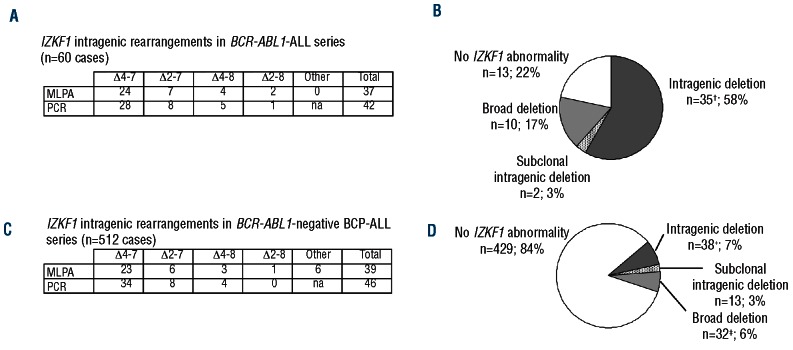
To test this breakpoint-specific PCR assay, we analyzed a series of 60 BCR-ABL1-positive ALL pediatric cases. MLPA was used as a reference method for IKZF1 gene dosage. Using MLPA, copy-number alterations of IKZF1 gene were observed in 45 of 60 patients (75%). This was consistent with previous data based on SNP-array analyses that reported frequencies of 63-84% in series of adult or mixed adult/pediatric BCR-ABL1-positive ALL.4,13 Complete deletions of IKZF1 were observed in 10 cases, including 7 cases with monosomy 7 or large 7p chromosomal aberrations detected on karyotype, consistent with the previously known association between BCR-ABL1-positive ALL and 7p abnormalities.14 Thirty-seven IKZF1 intragenic deletions were detected in 35 patients (2 patients having deletion of both alleles), all of which were Δ4-7, Δ2-7, Δ4-8 or Δ2-8 (Figure 2A). When tested by breakpoint-specific PCR, all but 4 cases with IKZF1 intragenic deletions as detected by MLPA showed a PCR product that was sequenced to confirm the rearrangement and map the breakpoint regions. The 4 intragenic deletions detected by MLPA but not by PCR were further characterized by high-resolution array-CGH and long-distance PCR (Online Supplementary Figure 1S). They included two Δ2-7 and two Δ2-8 rearrangements, two of them having clustered breakpoints in intron 1 indicating a variant, more telomeric breakpoint region. All the sequences of the rare breakpoints characterized (i.e. in intron 1 and centromeric to exon 8) are shown in the Online Supplementary Table S1. The combination of primers used in the PCR assay was then implemented so that all the recurrent rearrangements could be detected.
Figure 2.
(A) and (C) Numbers of IKZF1 intragenic deletions detected by either MLPA or PCR, in BCR-ABL1-positive ALL and BCR-ABL1-negative BCP-ALL cases, respectively. (B) and (D) Pie charts representing the frequency of IKZF1 deletions in patients with BCR-ABL1-positive ALL and BCR-ABL1-negative BCP-ALL, respectively. *include 2 cases with biallelic intragenic deletions (one case with Δ4-7 + Δ4-8 and one case with Δ4-7 + Δ2-8) and 3 cases with a clonal rearrangement associated to a subclonal rearrangement (one case with Δ4-7 + minor Δ2-7, one case with Δ2-7 + minor Δ4-7 and one case with 2 Δ4-7). †include one case with biallelic intragenic deletions and 2 cases with a clonal rearrangement associated to a subclonal rearrangement. ‡include one case with biallellic broad deletion and 2 cases with broad deletion and a subclonal intragenic deletion.
Using this breakpoint-specific PCR assay, a total of 42 IKZF1 intragenic rearrangements were detected in 37 BCR-ABL1-positive ALL. Six rearrangements were detected by PCR but not by MLPA (Figure 2A). The sensitivity of MLPA for detecting IKZF1 deletions is limited to 50%, which is the average sensitivity of all gene dosage-based methods for genomic deletions. By contrast, the PCR assay was able to detect IKZF1 rearrangements with a reproducible sensitivity of at least 10-2. Therefore, the PCR assay might have detected intragenic deletions restricted to minor subclones, which is consistent with the observation that some of the MLPA-negative rearrangements yielded a weak PCR signal (Online Supplementary Figure S2). MRD-based quantification showed that the percentage of leukemic cells bearing IKZF1 rearrangement ranged from 1% to 20% in these cases (data not shown). Three of these subclonal deletions were associated with another IKZF1 rearrangement presumably present in the major clone, whereas they were the sole IKZF1 abnormality in the 2 other cases (Figure 3B). The detection of IKZF1 deletion in a leukemia subclone is in line with the model of oncogenesis in which IKZF1 genomic lesion is a secondary, optional, genetic event that can appear later in BCR-ABL1-positive ALL.15 Interestingly, in one case, an oligoclonal pattern of Δ4-7 rearrangement was observed, similar to what can sometimes be observed with Ig/TCR rearrangements, suggesting an active, ongoing recombination at IKZF1 deletion junction during the leukemogenic process (Online Supplementary Figure S2, lower panel).
Figure 3.
(A) Schematic representation of IKZF1 gene with primers and probes used for Q-PCR for MRD monitoring. Δ2c primer and probes are used for variant breakpoints. Arrows represent primers; solid lines with lozenges represent Taqman probes. (B) Concordance of 54 paired MRD results obtained by Ig/TCR and IKZF1 Q-PCR. Detectable but not quantifiable MRD under the 10-4 threshold is termed “pos <10-4” and the opposite “neg <10-4” for undetectable MRD measurement. R2 is the Pearson's correlation coefficient of the 11 MRD values positive ≥ 10-4 measured by both methods. As Ig/TCR MRD analysis was performed with 2 markers for most patients, the marker with the best sensitivity and quantitative range was retained for non-quantifiable results, and the mean result of the 2 markers for quantifiable results.
In order to validate our breakpoint-specific multiplex PCR assay in an independent cohort, we analyzed a cohort of 512 BCR-ABL1-negative pediatric BCP-ALL cases (Figure 2). Using MLPA, copy-number alterations of IKZF1 were detected in 69 of 512 patients (13.5%), including 39 intragenic rearrangements in 38 patients (7.4%). Using PCR assay, 46 IKZF1 rearrangements were detected in 42 patients (8.2%) (Figure 2C). Strikingly, 17 intragenic rearrangements were detected by PCR only, suggesting here again that PCR can detect minor subclones. In 4 cases, these subclonal rearrangements were associated with another IKZF1 deletion, either intragenic (2 cases) or complete (2 cases), while in 13 other cases they were present as sole IKZF1 abnormality (Figure 2D). Altogether, our results show that, using gene-dosage methods only, 2 of 60 (3.3%) of BCR-ABL1-positive ALL and 13 of 512 (2.5%) BCR-ABL1-negative BCP-ALL would have been classified germline for IKZF1 status, while having a subclonal IKZF1 rearrangement.
Finally, we set up an MRD assay based on IKZF1 recurrent genomic alterations as molecular oncogenic markers. Although present in only a fraction of patients, IKZF1 rearrangements provide molecular targets that do not require sequencing nor allele-specific primers. We, therefore, designed consensus primers and probes for quantification of the different types of intragenic rearrangements (Figure 3A). A sensitivity range of 10-4 or below was obtained for all the 18 IKZF1-rearranged cases analyzed and a quantitative range of 10-4 and 5.10-4 was obtained for 11 and 7 cases, respectively. Performances of the assay were, therefore, similar to those obtained with Ig/TCR-based QPCR. MRD analysis was performed on 54 follow-up samples and compared with MRD results obtained using Ig/TCR markers (Figure 3B). A very good correlation (r2 = 0.9232) was observed for the quantifiable follow-up samples. Random discrepancies were sometimes observed for low MRD levels that were only detected either with IKZF1 (n=3) or Ig/TCR (n=3) markers. These results, as previously shown by others for IKZF1 Δ4-7,16 confirm the accuracy of MRD quantification using IKZF1 markers. MRD monitoring in BCP-ALL is currently based on Immunoglobulin (Ig) and/or T-cell receptor (TCR) somatic clonal rearrangements. Since they are not directly involved in the oncogenic process, but are merely the consequence of active V(D)J recombination in lymphoid precursors, clonal Ig/TCR markers are not always stable during the course of the disease. The use of oncogenic lesions for MRD monitoring may overcome this caveat but has been limited by the fact that recurrent chromosomal translocations are not found in all ALL and that they are usually studied at the RNA level. Only a few genomic breakpoint sequences are currently used as MRD markers: TAL1 microdeletion in a subtype of T-cell ALL, and MLL rearrangements in infant ALL. Unlike them, IKZF1 lesion is not considered an initiating event, raising the question of the stability of this alteration during the course of the disease. We analyzed 9 relapse samples from BCR-ABL1-positive ALL cases with IKZF1 rearrangement at diagnosis and observed that the rearrangement was preserved in all cases. This strengthens previous data on relapse cases showing that IKZF1 lesions are preserved or even selected for after treatment.17
In conclusion, we describe a simple assay designed to detect most intragenic IKZF1 deletions with a higher sensitivity than classical techniques and allowing further MRD monitoring. In contrast to current gene-dosage methods, this assay can detect IKZF1 lesions in cases of low blast infiltration or hemodiluted samples. This assay has also highlighted IKZF1 deleted subclones in a significant number of patients in both BCR-ABL1-positive and BCR-ABL1-negative ALL at diagnosis. Analysis of larger cohorts will be necessary to refine the prevalence and the prognostic impact of subclonal IKZF1 lesions. The clinical relevance of detecting subclones with IKZF1 deletions is emphasized by observations showing that such clones can be selected for during tumor progression and drive relapse, in accordance with the poor prognosis conferred by IKZF1 deletions in BCP-ALL.17,18 Similarly, this makes IKZF1 rearrangement a very attractive marker for MRD monitoring in ALL, although its interpretation and significance, especially in cases of subclonal rearrangement, must be carefully evaluated.
Supplementary Material
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Moorman AV. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev. 2012;26(3):123-35 [DOI] [PubMed] [Google Scholar]
- 2.Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758-64 [DOI] [PubMed] [Google Scholar]
- 3.Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110-4 [DOI] [PubMed] [Google Scholar]
- 4.Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waanders E, van der Velden VH, van der Schoot CE, van Leeuwen FN, van Reijmersdal SV, de Haas V, et al. Integrated use of minimal residual disease classification and IKZF1 alteration status accurately predicts 79% of relapses in pediatric acute lymphoblastic leukemia. Leukemia. 2011;25(2):254-8 [DOI] [PubMed] [Google Scholar]
- 6.Beldjord K, Macintyre E, Lheritier V, Boulland ML, Leguay T, Thomas X, et al. Minimal residual disease at 3 months, combined to the presence of IKZF1 deletion in B-lineage or absence of NOTCH1 pathway mutation in T-lineage, recapitulates the disease risk assessment in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia - A GRAALL Study. Blood (ASH Annual Meeting Abstracts). 2011;118:572 [Google Scholar]
- 7.Chen IM, Harvey RC, Mullighan CG, Gastier-Foster J, Wharton W, Kang H, et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2012;119(15):3512-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacobucci I, Storlazzi CT, Cilloni D, Lonetti A, Ottaviani E, Soverini S, et al. Identification and molecular characterization of recurrent genomic deletions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: on behalf of Gruppo Italiano Malattie Ematologiche dell'Adulto Acute Leukemia Working Party (GIMEMA AL WP). Blood. 2009;114(10):2159-67 [DOI] [PubMed] [Google Scholar]
- 9.Clappier E, Collette S, Grardel N, Girard S, Suarez L, Brunie G, et al. NOTCH1 and FBXW7 mutations have a favorable impact on early response to treatment, but not on outcome, in children with T-cell acute lymphoblastic leukemia (T-ALL) treated on EORTC trials 58881 and 58951. Leukemia. 2010;24(12):2023-31 [DOI] [PubMed] [Google Scholar]
- 10.van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21(4):604-11 [DOI] [PubMed] [Google Scholar]
- 11.Clappier E, Gerby B, Sigaux F, Delord M, Touzri F, Hernandez L, et al. Clonal selection in xenografted human T cell acute lymphoblastic leukemia recapitulates gain of malignancy at relapse. J Exp Med. 2011;208(4):653-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertin R, Acquaviva C, Mirebeau D, Guidal-Giroux C, Vilmer E, Cave H. CDKN2A, CDKN2B, and MTAP gene dosage permits precise characterization of mono- and bi-allelic 9p21 deletions in childhood acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2003;37(1):44-57 [DOI] [PubMed] [Google Scholar]
- 13.Martinelli G, Iacobucci I, Storlazzi CT, Vignetti M, Paoloni F, Cilloni D, et al. IKZF1 (Ikaros) deletions in BCR-ABL1-positive acute lymphoblastic leukemia are associated with short disease-free survival and high rate of cumulative incidence of relapse: a GIMEMA AL WP report. J Clin Oncol. 2009;27(31):5202-7 [DOI] [PubMed] [Google Scholar]
- 14.Heerema NA, Harbott J, Galimberti S, Camitta BM, Gaynon PS, Janka-Schaub G, et al. Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are non-random and may be associated with outcome. Leukemia. 2004;18(4):693-702 [DOI] [PubMed] [Google Scholar]
- 15.Cazzaniga G, van Delft FW, Lo Nigro L, Ford AM, Score J, Iacobucci I, et al. Developmental origins and impact of BCR-ABL1 fusion and IKZF1 deletions in monozygotic twins with Ph+ acute lymphoblastic leukemia. Blood. 2011;118(20): 5559-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venn NC, van der Velden VH, de Bie M, Waanders E, Giles JE, Law T, et al. Highly sensitive MRD tests for ALL based on the IKZF1 Delta3-6 microdeletion. Leukemia. 2012;26(6):1414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuiper RP, Waanders E, van der Velden VH, van Reijmersdal SV, Venkatachalam R, Scheijen B, et al. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24(7):1258-64 [DOI] [PubMed] [Google Scholar]
- 18.Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.