Abstract
The CD30-targeted agent brentuximab vedotin has shown impressive activity in relapsed/refractory Hodgkin lymphoma and anaplastic large cell lymphoma in phase II studies. We have treated 24 patients with relapsed/refratory disease enrolled onto a Named Patient Programme during 2010-11 at a single UK center. Overall response rate across all histologies was 67% (Hodgkin 72%; anaplastic large cell 60%), complete response rate 25% (Hodgkin 17%; anaplastic large cell 60%), median progression-free survival 5.1 months, and toxicity mild to moderate in the majority of cases. Six patients proceeded to allogeneic transplantation and one patient awaits this procedure. These results are similar to phase II data and show that brentuximab vedotin provides a bridge to allogeneic transplantation in approximately one quarter of patients refractory to conventional salvage therapies. Best response was seen after four doses, so consideration of allogeneic transplantation should be made early and scheduled following the first assessment indicating response.
Introduction
Modern chemotherapy regimens such as ABVD,1,2 Stanford V and BEACOPP4 have rendered Hodgkin lymphoma one of the most curable disseminated malignancies. However, 20-30% of patients will experience relapse, and 5-10%5 will not respond to primary chemotherapy at all. These patients usually receive salvage chemotherapy based upon either ifosfamide (ICE, IVE) or platinum (DHAP, ESHAP) and, if chemosensitive, are then consolidated with high-dose chemotherapy and autologous stem cell transplantation (autotransplant). Unfortunately, approximately 50%6 of these patients will relapse again, and at this point treatment options become limited to palliative chemotherapy, radiotherapy or experimental approaches, including new molecules and, in selected cases, allogeneic stem cell transplant (allotransplant).7,8
Brentuximab vedotin (BV, previously known as SGN-35, Seattle Genetics, WA, USA) is a novel antibody-drug conjugate targeting CD30 linked to a payload comprising a potent tubulin toxin, monomethyl auristatin E. The cell surface antigen CD30 is ubiquitous across Hodgkin Reed-Sternberg9 and ALCL10 cells. Two pivotal studies have demonstrated its efficacy in relapsed/refractory Hodgkin lymphoma (HL)11 and anaplastic large cell lymphoma (ALCL),12 with overall response rates (ORR) of 75% and 86%, respectively, when administered as monotherapy. During 2010-11, this agent was made available on a Named Patient Programme (NPP) by Millennium/Takeda. Here we describe objective response rates, adverse events, subsequent allogeneic stem cell transplantation (allotransplant) rate, progression-free and overall survival of patients enrolled onto this program at a single tertiary referral center in the UK.
Design and Methods
The brentuximab vedotin NPP was open at our institution between December 2010 and August 2011. All patients presenting between these times with either HL, ALCL or CD30+ T-cell lymphoma refractory to at least 2 lines of chemotherapy or autotransplant, a positive PET-CT scan and deemed suitable for systemic therapy were considered for the NPP. This included patients with poor performance status due to progressive disease. Exclusion criteria included previous allotransplant, severe myelosuppression, active infection and significant hepatic or renal dysfunction, but no patient was excluded on any of these grounds. Ultimately, all patients considered for the NPP received at least one dose of BV.
All patients underwent baseline assessments including physical examination, routine hematology and biochemistry, as well as FDG-PET/CT prior to treatment. BV was dosed at 1.8 mg/kg (capped at 100 kg of body weight) and administered in 250 mL of 0.9% saline by intravenous infusion over 30 min once every three weeks. No routine pre-medication was given, although any patient who experienced an infusion reaction received oral paracetamol, intravenous (iv) chlorpheniramine and iv hydrocortisone before all subsequent infusions of BV. Side effects such as nausea/vomiting were treated according to institutional guidelines. Granulocyte colony stimulating factor in the form of PEG-filgrastim was used as secondary prophylaxis of neutropenic complications. A single dose reduction to 1.2 mg/kg was allowed for ongoing toxicity despite these measures. Dose delays were also permitted to allow full recovery of any treatment-induced myelosuppression.
Toxicity was graded by Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Response was assessed by FDG-PET/CT after cycles 4 and 8 (PET4, PET8) using the International Working Group revised response criteria for malignant lymphoma.13 Additional scans were performed as clinically indicated such as prior to transplantation or on suspicion of progressive disease. Four patients did not undergo PET4 because they had either died or developed unequivocal clinical evidence of progressive disease.
Eligibility for allotransplant was assessed according to institutional guidelines (including age under 66 years). Allotransplants in HL were performed utilizing a reduced intensity conditioning (RIC) regimen as follows: fludarabine 30 mg/m2 Days -7 to -3, melphalan 140 mg/m2 Day -2, alemtuzumab 30-90 mg Day -1 (according to degree of donor mismatch). Allotransplants for ALCL utilized a myeloablative regimen of cyclophosphamide 4 g/m2, 12 Gy total body irradiation and alemtuzumab. Cyclosporin 3 mg/kg was used as prophylaxis against graft-versus-host disease and anti-infective prophylaxis was as per institutional guidelines.
Results and Discussion
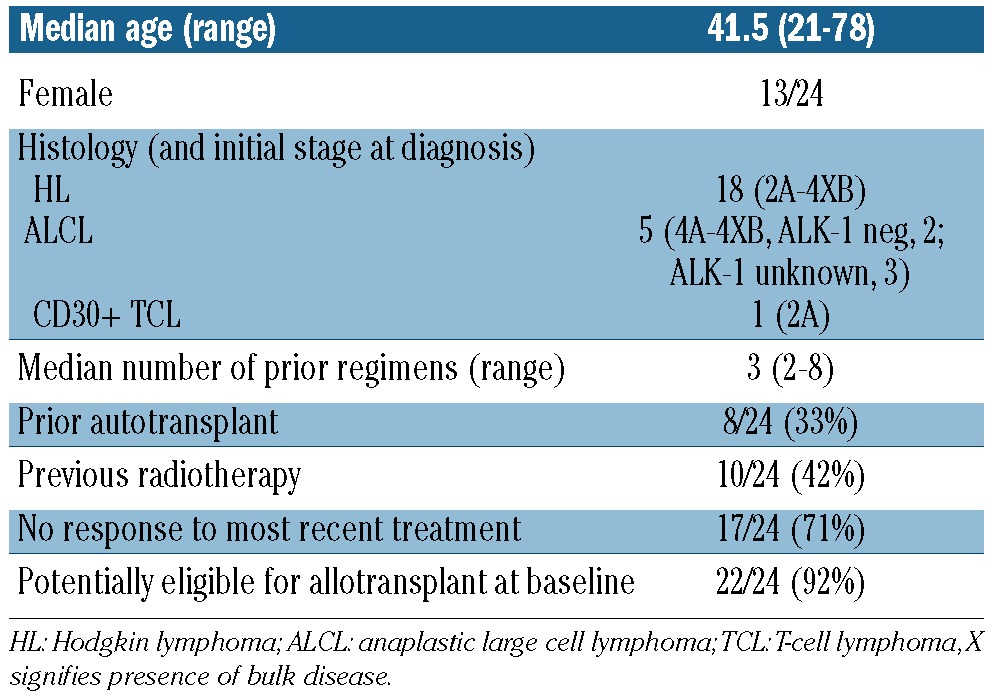
Demographics of the 24 patients are detailed in Table 1. In brief, 13 were female, 11 were male and median age was 41.5 years. Stage at initial diagnosis of lymphoma ranged from 2A to 4XB and all patients had widespread disease unsuitable for radiotherapy at the start of BV. They had received a median of 3 previous treatments: 8 (33%) had undergone auto-transplantation and 17 had not responded to the previous line of treatment. The 16 patients who had not received a previous auto-transplant had not done so due to lack of response to preceding salvage chemotherapy in 12 cases (75%), age over 70 years in 2 (13%), failure of stem cell harvest in one (6%), and patient choice in another (6%).
Table 1.
Demographics, histology and prior therapies of patients in the brentuximab vedotin Named Patient Programme.
Patients received a median of 5.5 cycles of BV (range 1-13). Most toxicity was mild to moderate. Nine patients experienced grade 3/4 events including sepsis (n=5), neuropathy (n=3) and sub-acute bowel obstruction (n=1). Three of the five septic episodes (including a case of Epstein Barr virus (EBV) reactivation) proved fatal and all occurred in patients with widespread aggressive disease and reduced performance status. One patient with grade 4, non-neutropenic sepsis at the time of CR was subsequently dose-reduced to 1.2 mg/kg, had no further episodes of sepsis, and successfully underwent allotransplant. Another survived neutropenic sepsis but developed progressive disease and died two months later. Three patients with neurotoxicity were dose-reduced to 1.2 mg/kg and experienced no further deterioration in symptoms but subsequently died of progressive disease. The patient with sub-acute bowel obstruction first experienced this after cycle 3 BV. She subsequently developed progressive disease in the abdomen, experienced further episodes of sub-acute obstruction and died nine months later. The relationship of the bowel obstruction to BV is, therefore, unclear.
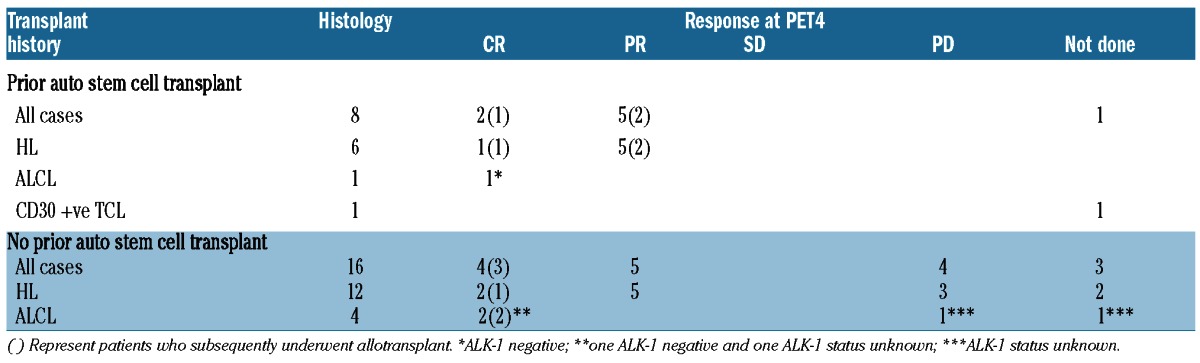
Response in all patients, by histology and prior auto-transplant are shown in Table 2. Best responses were seen at PET4 with 6 CR (n=3 HL, n=3 ALCL), 10 PR (all HL) and 4 PD (n=3 HL, n=1 ALCL) to give an overall response rate of 67% at this time. Four (n=3 HL, n=1 T-cell lymphoma) patients experienced clinical progression or death before PET4. Despite tolerating the drug without difficulty, one patient in PR discontinued treatment with BV after three cycles to explore alternative ‘natural’ remedies. Although still alive, this patient has progressed at an unknown time point. There were no significant differences in response between patients who had previously received auto-transplant (n=8) and those who had not (n=16), with a CR rate of 25% in both groups. In terms of response by histology, 13 of 18 (72%) patients with HL and 3 of 5 (60%) patients with ALCL achieved objective response but more patients with ALCL achieved CR (3 of 5, 60%) than patients with HL (3 of 18, 17%).
Table 2.
Responses at PET4 by histology and transplant history of patients in the brentuximab vedotin Named Patient Programme.
At a median follow up of 12.9 months (as of April 2012), 16 of 24 patients are alive (67%) and median PFS for all patients is 5.1 months (Figure 1). Six of twenty-two (27%) eligible patients, 4 with HL and 2 with ALCL, have undergone allotransplant and another with HL is awaiting the procedure. One HL patient in probable PD with a single new FDG avid lymph node site following BV proceeded to allotransplant but subsequently died of adenovirus and cytomegalovirus (CMV) reactivation at Day 81; all other patients were in CR (n=4: n=2 ALCL, n=2 HL) or PR (n=1 HL) at time of admission for allotransplant. Three of these patients (n=1 HL, n=2 ALCL) had not received prior auto-transplant due to inadequate peripheral blood stem cell harvest (n=1) or inadequate response to salvage chemotherapy (n=2), respectively. Twelve patients potentially eligible for allotransplant at baseline did not proceed due to PD at PET4 (n=2) or PET8 (n=10). So far in early post-transplant follow up, one patient with HL has converted from PR to CR and another patient with HL has received treatment for grade 3 colitis and genital papillomatosis. There have been no other unexpected toxicity events.
Figure 1.
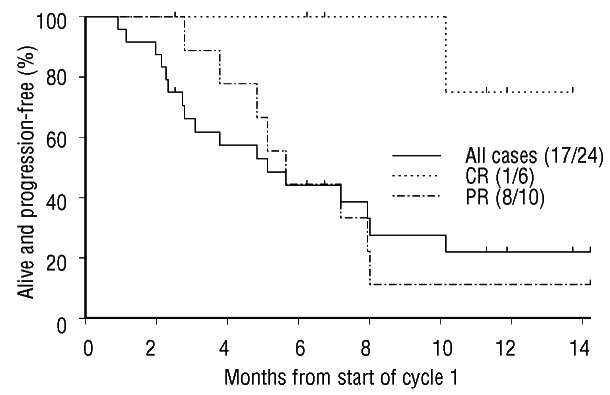
Progression-free survival of patients treated in the brentuximab vedotin Named Patient Programme. CR: complete remission; PR: partial remission.
Our data demonstrate that BV was effective with an overall response rate (ORR) of 67% and median PFS of 5.1 months in a population of heavily pre-treated patients with CD30+ lymphoma managed in a non-trial setting at a single UK center. Most of the patients in this series had HL and for this group the ORR was 72% and CR rate 17%, very similar to the 75% and 34% reported in the pivotal phase II trial in HL where all patients had received a prior autotransplant.11 The ORR and CR rate for the 5 patients in our series with ALCL was 60% compared with an ORR of 86% and CR rate of 57% in patients taking part in the phase II trial in ALCL.
In our series, 71% patients across all histologies were refractory to their previous line of therapy and 67% had not received an autotransplant, suggesting that ours may have been a worse prognosis group. Interestingly, across all histological types the same CR rate of 25% was seen in the 8 patients who had received a prior autotransplant and the 16 who had not. Best response was observed at PET4 in all patients with 10 patients in response at PET4 progressing by PET8.
In addition, we have shown that for patients with HL and ALCL refractory to conventional salvage chemotherapy yet deemed potentially eligible for allotransplant, BV monotherapy provides a bridge to this consolidation step in 27% of cases. By comparison, Chen et al. allotransplanted 16 of 46 (35%) patients treated in a variety of phase I/II studies,14 although in that series 5 patients received additional multiagent chemotherapy between BV and allotransplant, including one case of autotransplant. If these 5 patients are excluded from the Chen analysis, 11 of 46 (24%) had BV alone before allotransplant. This is almost identical to the 27% allotransplant rate seen in our series. In terms of timing, our observations of best response at PET4 and a median PFS of 5.1 months support the view that consideration of allogeneic transplantation should take place early in therapy and, if appropriate, scheduled following the first assessment indicating response to BV.
With respect to toxicity, BV was well tolerated by the majority (62.5%) of patients. Of the 9 grade 3/4 events, 3 (12.5% of the NPP cohort) were neurotoxicity and stabilized following dose reduction. This is very similar to the grade 3/4 neurotoxicity rate in the phase II studies of 8% in HL11 and 12% in ALCL.12 There were 3 septic deaths occurring in patients with very advanced disease and associated poor performance status. We believe these factors were critical in these cases. The three other grade 3/4 events either recovered (sepsis, n=2) or may have been due to causes other than treatment with BV (sub-acute bowel obstruction, n=1)
To conclude, this report describes the results of treatment with BV in a group of patients with relapsed/refractory CD30 positive lymphoma at a single tertiary cancer center in the UK. In this ‘real world’ non-trial setting, results are very similar to those reported in phase II trials and confirm that the drug is generally well tolerated. Crucially, 27% proceeded to allotransplant suggesting that BV can overcome resistance to prior conventional treatment including autotransplant and provide an additional therapeutic route for patients in whom palliation has previously been the only option. Whether these interventions lead to long-term survival will, of course, require follow up over several years.
Supplementary Material
Footnotes
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Duggan DB, Petroni GR, Johnson JL, Glick JH, Fisher RI, Connors JM, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: report of an intergroup trial. J Clin Oncol. 2003; 21(4):607-14 [DOI] [PubMed] [Google Scholar]
- 2.Canellos G, Anderson J, Propert K, Nissen N, Cooper R, Henderson E, et al. Chemotherapy of Advanced Hodgkin's Disease with MOPP, ABVD, or MOPP Alternating with ABVD. N Engl J Med. 1992;327:1478-84 [DOI] [PubMed] [Google Scholar]
- 3.Horning SJ, Hoppe RT, Breslin S, Bartlett NL, Brown BW, Rosenberg SA. Stanford V and radiotherapy for locally extensive and advanced Hodgkin's disease: mature results of a prospective clinical trial. J Clin Oncol. 2002;20(3):630-7 [DOI] [PubMed] [Google Scholar]
- 4.Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348(24):2386-95 [DOI] [PubMed] [Google Scholar]
- 5.Ansell SM, Armitage JO. Management of Hodgkin lymphoma. Mayo Clin Proc. 2006;81(3):419-26 [DOI] [PubMed] [Google Scholar]
- 6.Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625-33 [DOI] [PubMed] [Google Scholar]
- 7.Peggs KS, Hunter A, Chopra R, Parker A, Mahendra P, Milligan D, et al. Clinical evidence of a graft-versus-Hodgkin's-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005; 365:1934-41 [DOI] [PubMed] [Google Scholar]
- 8.Sureda A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin's lymphoma. Results of the HDR-ALLO study – a prospective clinical trial by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97(2):310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. The classical Hodgkin's lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol. 2010;221(3):248-63 [DOI] [PubMed] [Google Scholar]
- 10.Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12):3681-95 [PubMed] [Google Scholar]
- 11.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a Pivotal Phase 2 Study of Brentuximab Vedotin for Patients with Relapsed or Refractory Hodgkin's Lymphoma. J Clin Oncol. 2012;30:2183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large cell lymphoma: Results of a phase II study. J Clin Oncol. 2012;30:2190-6 [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-86 [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Palmer JM, Thomas SH, Tsai N, Farol L, Nademanee A, et al. Brentuximab Vedotin (SGN-35) enables successful reduced intensity allogeneic hematopoietic cell transplantation in relapsed/refractory Hodgkin lymphoma. Blood. 2012;119: 6379-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


