Abstract
Aims/hypothesis
Elevated fasting non-esterified free fatty acids (fasting NEFA) are thought to promote type 2 diabetes. Three prospective studies support this concept, showing increased diabetes risk associated with fasting NEFA. However, these prospective associations may be confounded by strong cross-sectional correlations between fasting NEFA and metabolic predictors of diabetes. To examine this assumption, we used cohort data from the Insulin Resistance Atherosclerosis Study (IRAS).
Methods
Within the IRAS cohort (n=902, 145 incident cases), we examined nine metabolic variables for their confounding effect on the fasting NEFA-diabetes association: 2-hour glucose (2hG), fasting plasma glucose, body mass index (BMI), waist circumference, waist-to-hip ratio, weight, insulin sensitivity (SI), fasting insulin, and acute insulin response. We compared odds ratios for fasting NEFA (log-transformed and adjusted for age, gender, ethnicity, and clinic) before and after inclusion of each metabolic variable into a logistic regression model.
Results
Three variables (2hG, BMI, and SI) cross-sectionally correlated with fasting NEFA (r≥0.1, p<0.05). Unadjusted for metabolic predictors, fasting NEFA levels were positively associated with diabetes risk: OR=1.37 (0.87–2.15) per unit on a log-scale. All metabolic variables except AIR showed confounding. Inclusion of 2hG reversed the positive association [OR=0.50 (0.30–0.82)], whereas other predictors reduced the association to the null. The final model included the parameters correlated with baseline fasting NEFA (2hG, BMI, SI) and the demographic variables resulting in OR =0.47 (0.27–0.81).
Conclusions
Our results indicate that 2hG strongly confounds the prospective association between fasting NEFA and diabetes; carefully adjusted fasting NEFA levels are inversely associated with diabetes risk.
Keywords: Type 2 Diabetes, Nonesterified Fatty Acids, Prospective Study, Confounding
Introduction
Non-esterified fatty acids (NEFA) present the major fuel substrate for skeletal muscles during long periods between meals [1;2]. Because the fasting circulating levels of NEFAs are proportional to body fat storage [1;2], cross-sectional studies have consistently demonstrated positive correlations between fasting NEFA levels and obesity as well as two of the sequelae of obesity, insulin resistance and glucose intolerance [1;2]. It is unclear whether elevated NEFA levels during fasting are relevant to further deterioration of glucose homeostasis or are simply associated with metabolic type 2 diabetes predictors. Answering the question of how fasting NEFA levels relate to the development of type 2 diabetes is important, because it could clarify whether fasting NEFA are on the causal pathway of lipotoxicity – one of the important etiological bases for the development of type 2 diabetes.
To determine how fasting NEFA relates to the development of type 2 diabetes requires examining their prospective association. However, baseline cross-sectional correlations between fasting NEFA and type 2 diabetes metabolic predictors can have a profound effect on the prospective fasting NEFA-diabetes association, distorting this association. Extreme cases of distorted associations have been previously documented and are known as Simpson's paradox [3] or qualitative confounding [4]. The most common textbook example of this phenomenon is the effect of age-adjustment on mortality statistics. Age is a major correlate of mortality; therefore, direct comparison of mortality rates in the US versus Venezuela shows increased mortality risk in the US (mortality ratio=1.98), while age-adjustment reverses the association (mortality ratio=0.78) [4]. Similarly, strong correlates of fasting NEFA such as glucose intolerance, insulin sensitivity, and obesity may drive the unadjusted estimate for the prospective fasting NEFA-diabetes association.
Therefore, we hypothesized that the choice of adjustment variables may strongly influence the results of prospective analysis. Among the previously published prospective studies on this topic, three of the four found an increased risk of glucose intolerance and/or diabetes in individuals with elevated fasting NEFA [5–7]. The fourth study, in contrast, showed the expected cross-sectional correlation between fasting NEFA and glucose intolerance but did not confirm the positive prospective association [8]. All four studies used different metabolic parameters for adjustment and/or stratification to examine the prospective fasting NEFA-diabetes association. One study compared the means for NEFA in different sub-groups stratified by metabolic variables, but did not present the estimates of relative risk [8]. Three other studies presented the estimates of the relative risk from multiple models adjusting for different sets of metabolic variables, but the confounder adjustment approaches varied and may not have optimally controlled for the important associations between fasting NEFA and other baseline type 2 diabetes predictors; also these three studies did not present a sub-group analysis [5–7]. To examine carefully the influence of metabolic variables on the prospective association between fasting NEFAs and type 2 diabetes, we conducted a detailed analysis of the prospective fasting NEFA-diabetes association using cohort data from the well-documented Insulin Resistance Atherosclerosis Study (IRAS).
Methods
IRAS study population
The IRAS is a multicenter, population-based cohort study [9] that recruited a total of 1625 men and women, 40 to 69 years of age, from four U.S. communities from 1992 to 1993. The study recruited approximately equal numbers of persons with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes, as well as equal numbers of non-Hispanic whites, Hispanics, and African Americans. In the follow-up examination conducted 5 years after recruitment, 80% of the cohort participated. The IRAS protocol was approved by local institutional review committees, and all subjects gave informed consent.
Definition of glucose tolerance
Glucose tolerance was measured precisely at each examination using an oral glucose tolerance test and the World Health Organization criteria. A 75-gram glucose load (Orange-dex; Custom Laboratories, Baltimore, MD) was administered over a period of <10 minutes. Blood was collected at 0 and 2 hours. Normal glucose tolerance was defined as fasting glucose and 2-hour glucose < 140 mg/dL. Impaired glucose tolerance was defined as fasting glucose < 140 mg/dL and 2-hour glucose ≥ 140 and < 200 mg/dl. Diabetes was defined as fasting glucose ≥ 140 mg/dL or 2-hour glucose ≥ 200 mg/dL or use of hypoglycemic medications.
Measurements of metabolic parameters
Clinical measures and procedures
All subjects fasted for 12 hours and refrained from heavy exercise, smoking, and alcohol consumption for 24 hours before the visit. Insulin sensitivity and insulin secretion were determined using the frequently sampled intravenous glucose tolerance test (FSIGTT), with two modifications to the original protocol [10]. First, an injection of regular insulin, rather than tolbutamide, was used to ensure adequate plasma insulin levels for the accurate computation of insulin sensitivity across a broad range of glucose tolerance [11]. Second, a reduced sampling protocol (with 12 rather than 30 samples) was employed for efficiency, given the large number of participants [12]. Both insulin sensitivity (SI) and acute insulin response (AIR) were calculated using mathematical modeling methods (MINMOD version 3.0 1994) [13]. The reliability of SI and AIR calculations was demonstrated in a sub-sample of the IRAS cohort. The estimate of SI produced by the modified protocol has been validated against the gold-standard measures of insulin resistance from the hyperinsulinemic euglycemic clamp technique (r=0.55) [14], and AIR has been validated by others using gold-standard measures of insulin secretion from the hyperglycemic clamp technique [15].
Anthropometric measurements included height, waist and hip circumferences (all three measured to the nearest 0.5 cm), and weight (measured to the nearest 0.1 kg). All measures were obtained in duplicate following a standardized protocol, and averages were used in the analysis. Body mass index (BMI), calculated as weight/height2 (kg/m2), was used as an estimate of overall adiposity. The average of duplicate measurements was used to calculate the waist-to-hip ratio.
Laboratory measurements
Glucose concentration was determined using standard methods [9]. Insulin levels were measured using the dextran-charcoal radioimmunoassay [16]. Plasma NEFA concentrations were measured colorimetrically as previously described [17].
Statistical analysis
The analytical cohort included participants (n = 902) who had normal or impaired glucose tolerance at baseline, had baseline measurements of fasting NEFA, and participated in the follow-up examination. During the 5-year study period, 145 IRAS participants in the analytical cohort developed type 2 diabetes while the remaining 757 participants showed normal or impaired glucose tolerance at the follow-up examination. Participant characteristics (mean (SD) for continuous parameters and percent observed for categorical characteristics) were estimated overall, by glucose tolerance status at baseline (NGT/IGT) and by type 2 diabetes converter status (yes/no). Further the analyses were performed using the following steps.
Step 1
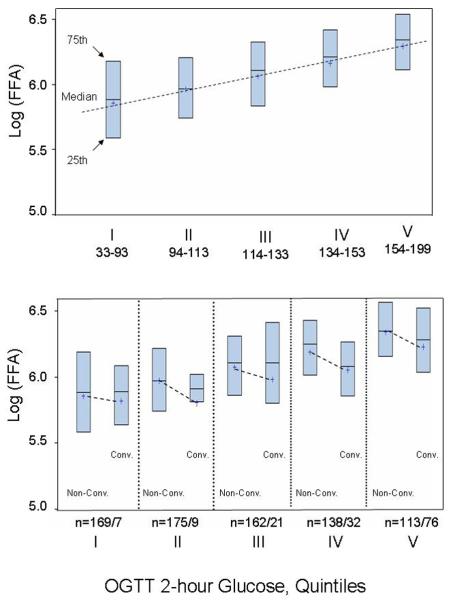
Using Spearman's rank correlation coefficients (r) and the corresponding p-values, we examined simple bivariate correlations between baseline fasting NEFA and eight metabolic variables: (1) 2-hour glucose (2hG), (2) fasting plasma glucose (FPG), (3) BMI, (4) waist-to-hip ratio (WHR), (5) weight, (6) insulin sensitivity (Si), (7) fasting insulin (FI), and (8) acute insulin response (AIR). These correlations were examined overall and stratified by baseline glucose tolerance status (NGT/IGT). To better visualize cross-sectional relationships between fasting NEFA and 2-hour glucose – the parameter that was most strongly correlated with baseline fasting NEFA, we graphically examined the levels of fasting NEFA (log-transformed) by the quintiles of 2-hour glucose (Fig 1, upper panel). Further, asked a question whether type 2 diabetes converters had higher levels of fasting NEFA at baseline; to answer this question, we graphically examined mean values of fasting NEFA (log-transformed) by converter status within each quintile of 2-hour glucose (Fig 1, lower panel).
Figure 1.
Distribution of fasting NEFA levels by quintiles of OGGT 2-hour glucose at baseline of the IRAS: boxes' indicate 25th and 75th percentiles; horizontal lines within boxes depict medians; “+” indicate mean values within subgroups; dashed lines emphasize the differences between the mean values.
Step 2
Further, to evaluate the association between fasting NEFA and type 2 diabetes incidence, we fit a series of unconditional logistic regression models with the development of type 2 diabetes (yes/no) as the outcome and fasting NEFA (log-transformed) as the predictor of interest using different sets of risk factors included in each model. Fasting NEFA was log-transformed in these models, because its distribution was strongly right-skewed and a natural logarithmic transformation made its distribution appear more symmetric. For each model, we examined the odds ratio (OR) and the corresponding 95% confidence interval (95% CI) describing the relationship between fasting NEFA and the incidence of type 2 diabetes.
The first model (Model 1) included fasting NEFA and demographic/study site variables: age (years), gender, ethnicity (non-Hispanic White, African American, Hispanic), and study site. To assess whether metabolic predictors of type 2 diabetes confound the association between fasting NEFA and type 2 diabetes risk, we examined how the addition of metabolic predictors influenced the associations observed in Model 1. For each metabolic predictor, we fit two types of models. The first included all variables in Model 1 plus the specific metabolic predictor and was fit using all participants. The next set of models stratified the participants based on the baseline value of the metabolic predictor. For each metabolic predictor, the stratified models were based either upon using conventionally accepted categories or were based on a median split. Variables using conventionally accepted categories included 2-hour glucose (NGT/IGT), FPG (cut point at 110), and BMI [normal vs. (overweight+obese)]. Variables using categories based on the median included WHR, weight, waist circumference, AIR, Si, and FI.
Step 3
Based on the results from step 2, we fit a final model using the following principles. The final model included metabolic variables that changed the strength of the association between fasting NEFA and type 2 diabetes by at least 20%: [OR (Model 1) − OR (Model 1 + metabolic predictor)] / OR (Model 1) ≥ |0.2|. We based this criterion on the change-in-estimate principle suggested by Mickey and Greenland suggest [18]. The authors suggested the criterion of 10% change in the estimate; however, they noted that this criterion was arbitrary [18]. We increased the threshold of this criterion to a 20% change to focus on more robust confounding effects. When we used this algorithm for selecting the metabolic variables to be included in the final model, we noted that some of the metabolic variables reflected similar metabolic phenomena; for example FPG and 2 hour glucose are used to describe individual status of glucose homeostasis. If this occurred, that is, if two (or more) metabolic parameters showed important confounding (≥ 20%) and both related to the same metabolic phenomenon, we included in the final model the one that had the stronger correlation with fasting NEFA levels at baseline. Using this approach, the final model included log(NEFA), 2-hr glucose, BMI, SI, age, gender, ethnicity, and study site.
Statistical analysis has been performed using SAS 9.2 (SAS institute, Cary NC).
Results
The baseline characteristics show an ethnically diverse cohort (Table 1), with large proportions of African Americans (26.5%) and Hispanics (33.5%). One-third of the study population showed glucose intolerance at baseline and approximately 16% of the cohort converted to type 2 diabetes during the 5-year follow-up period.
Table 1.
Baseline characteristics of the study population
| Characteristicsa | All (N=902) | NGT (N=601) | IGT (N=301) | Non-converters (N=757) | Converters (N=145) |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 54.6 (8.4) | 53.7 (8.5) | 56.5 (8.0) | 54.4 (8.5) | 55.9 (7.8) |
|
| |||||
| Gender (Females) | 56.5% | 53.7% | 62.1% | 56.0% | 59.3% |
|
| |||||
| Ethnicity: Non-Hispanic White | 40.0% | 40.8% | 38.5% | 40.6% | 37.2% |
| African American | 26.5% | 25.8% | 27.9% | 26.2% | 28.3% |
| Hispanic | 33.5% | 33.4% | 33.6% | 33.3% | 34.5% |
|
| |||||
| NEFA–Fast (μmol/L) | 468.9 (186.4) | 429.2 (171.5) | 548.2 (190.0) | 464.6 (185.0) | 491.4 (192.8) |
|
| |||||
| 2-hour glucose (mg/dl) | 124.8 (33.8) | 105.5 (21.0) | 163.4 (17.2) | 119.4 (31.5) | 153.2 (30.8) |
|
| |||||
| Fasting plasma glucose, FPG (mg/dl) | 98.5 (11.2) | 95.4 (9.9) | 104.7 (11.0) | 96.9 (10.3) | 106.8 (12.0) |
|
| |||||
| BMI (kg/m2) | 28.4 (5.6) | 27.4 (4.8) | 30.5 (6.5) | 28.0 (5.3) | 31.2 (6.4) |
|
| |||||
| Waist-to-hip ratio | 0.86 (0.09) | 0.85 (0.08) | 0.87 (0.09) | 0.85 (0.09) | 0.88 (0.08) |
|
| |||||
| Waist (cm) | 90.3 (12.7) | 88.0 (11.5) | 94.9 (13.8) | 89.3 (12.4) | 95.7 (13.1) |
|
| |||||
| Weight (kg) | 79.8 (16.9) | 77.6 (15.5) | 84.2 (18.7) | 78.6 (16.3) | 85.8 (18.9) |
|
| |||||
| Fasting insulin (pmol/L) | 94.1 (89.1) | 82.7 (58.1) | 116.7 (127.7) | 86.2 (66.9) | 134.9 (155.5) |
|
| |||||
| Acute insulin response, AIR (pmol.ml−1. min−1 | 489.6 (489.4) | 555.0 (524.1) | 359.0 (380.0) | 529.5 (506.8) | 277.4 (308.0) |
|
| |||||
| Insulin sensitivity, SI (×10−4 min−1.μU−1.ml−1) | 2.2 (2.0) | 2.6 (2.2) | 1.3 (1.2) | 2.4 (2.1) | 1.3 (1.6) |
Mean (SD) presented for continuous parameters;
% presented for categorical characteristics
Correlations between baseline fasting NEFA and metabolic predictors of type 2 diabetes
Fasting NEFA correlated positively with three metabolic variables (2-hr glucose, BMI, waist circumference, and fasting insulin), and correlated negatively with insulin sensitivity (SI) (Table 2, all participants). Correlations with other variables were did not reach our statistical cut point (p<0.05) (Table 2, all participants).
Table 2.
Baseline correlations between fasting NEFA and metabolic variablesa
| Spearman Correlation Coefficients (p-value for r) Number of observations |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 hr glucose | FPG | BMI | WHR* | Weight | Waist | Fasting Insulin | AIR (MM) | SI | |
| All participants | |||||||||
| Fasting NEFA | 0.371 (<0.0001) 902 |
0.056 (0.09) 902 |
0.156 (<0.0001) 900 |
−0.059 (0.08) 899 |
0.013 (0.7) 900 |
0.075 (0.02) 899 |
0.113 (0.001) 901 |
− 0.024 (0.5) 878 |
−0.228 (<0.0001) 838 |
| NGT | |||||||||
| Fasting NEFA | 0.244 (<0.0001) 601 |
−0.042 (0.3) 601 |
0.081 (0.05) 599 |
−0.080 (0.05) 599 |
−0.059 (0.15) 599 |
0.001 (1.0) 599 |
0.085 (0.04) 600 |
0.067 (0.1) 585 |
−0.139 (0.001) 560 |
| IGT | |||||||||
| Fasting NEFA | 0.170 (0.003) 301 |
−0.147 (0.01) 301 |
0.103 (0.08) 301 |
−0.142 (0.01) 300 |
0.013 (0.8) 301 |
−0.002 (1.0) 300 |
−0.013 (0.8) 301 |
0.044 (0.5) 293 |
−0.135 (0.03) 278 |
All the variables are in the original scale;
WHR- waist-to-hip ratio
Distribution of fasting NEFA stratified by 2-hr glucose levels
We selected 2-hour glucose levels, the strongest type 2 diabetes predictor, as a stratification factor to examine the distribution of fasting NEFA levels at baseline. Fasting NEFA levels steadily but not strongly increased by quintiles of 2-hour glucose (Fig 1, upper panel), confirming their cross-sectional association. However, when we stratified the distributions by converter- status within each quintile of 2-hour glucose, converters did not have higher levels of fasting NEFA at baseline (Fig 1, lower panel).
Prospective association between fasting NEFA and type 2 diabetes risk
Exploratory analysis
After adjustment for demographic variables The odds ratio of the association between NEFA and type 2 diabetes risk was 1.37, although it was not statistically significant. In fact, this adjustment did not meaningfully change the univariate association between fasting NEFA and type 2 diabetes risk: the OR for univariate association was 1.32 (95% CI: 0.86–2.02). However, simple stratification by glucose-tolerance status reversed the direction of odds ratio: the overall OR was 1.37, but the subgroup-specific OR for the NGT and IGT subgroups were 0.83 and 0.66, respectively. This demonstrates the confounding effect of glucose-tolerance status, a major metabolic predictor of type 2 diabetes. Substitution of a continuous 2-hour glucose variable for dichotomous glucose-tolerance status revealed an obvious inverse association between fasting NEFA and type 2 diabetes risk (OR=0.50, p<0.05), which persists in the NGT and IGT subgroups (OR=0.57 and 0.50, respectively). Adjustment for other metabolic predictors (except AIR) brought the OR for the association between fasting NEFA and type 2 diabetes risk from OR=1.37 closer to the null. Adjustment for AIR did not noticeably change the association from Model 1. Stratification by the baseline levels of metabolic predictors did not reveal any strong effect modification: the p-values for interaction between fasting NEFA and BMI, waist circumference, and weight were 0.1, 0.3, and 0.3, respectively.
Final model
The results of the correlation and exploratory analyses show that 2-hour glucose exerts the strongest confounding effect on the association between fasting NEFA and type 2 diabetes risk. Besides 2-hour glucose, FPG, BMI, waist circumference, and SI reduced the OR from Model 1 by 30%, 21%, 21%, and 26%, respectively. Out of the five metabolic parameters that exerted a confounding effect above the selected threshold, we retained three variables (2-hour glucose, BMI, and SI) in the final model (Table 4). Because FPG is analogous to the 2-hour glucose variable (reflecting glucose-tolerance status) but has a lesser confounding effect and did not correlated with fasting NEFA levels at baseline, it was not included in the model. Similarly, BMI showed a stronger cross-sectional correlation with fasting NEFA at baseline than waist circumference: correlation coefficient for NEFA-BMI was approximately twice greater that for NEFA-waist (Table 2). For this reason we chose BMI to represent adiposity in the final model.
Table 4.
Final model for the association of fasting NEFA and type 2 diabetes risk
| Variables included in the modela | OR (95%CI) |
|---|---|
|
| |
| Log (NEFA) (1 unit on a log-scale) | 0.47 (0.27,0.81) |
|
| |
| 2-hour glucose (per SD)b | 3.01 (2.31,3.97) |
|
| |
| SI (per SD) | 0.70 (0.47,1.00) |
|
| |
| BMI (per SD) | 1.28 (1.03, 1.60) |
|
| |
| Age (per SD) | 1.13 (0.91, 1.40) |
|
| |
| Gender (Female vs. Male) | 1.06 (0.68, 1.65) |
|
| |
| Ethnicity: Non-Hispanic White | Ref. |
| African American | 0.81 (0.43, 1.51) |
| Hispanic | 1.13 (0.61, 2.15) |
Study site was included in the model; there was no association with the study site.
OR shows the change in the risk of type 2 diabetes associated with increase equal to 1 standard deviation
With careful adjustment for confounders, the final model showed an inverse association between fasting NEFA and type 2 diabetes risk (OR=0.47). This association did not change meaningfully when stratified by IGT status: OR= 0.50 and 0.51 for participants with NGT and IGT at baseline, respectively. Because AIR is a known independent predictor of type 2 diabetes, we added this parameter into the final model to examine whether the association between fasting NEFA and type 2 diabetes would be sensitive to such an addition. The OR for fasting NEFA did not change (OR= 0.48, 95% CI 0.27–0.85). As expected, however, the AIR predicted type 2 diabetes risk independently of other risk factors (OR= 0.38, 95% CI 0.26–0.55).
Discussion
Our analysis of the IRAS cohort data documents a classic case of confounding. To be a confounder, a suspected parameter should be associated with both the main exposure at interest and the outcome. In our study, several metabolic parameters are known to be independently associated with the risk of diabetes [19] and also correlated with the main effect variable – baseline fasting NEFA levels (Table 2). Adjusted for the confounders (2-hour glucose, BMI, Si) and the demographic/study variables, fasting NEFA levels were inversely and independently associated with the risk of diabetes (final model, Table 4). The expected associations with known type 2 diabetes risk factors – positive associations with 2-hour glucose and BMI and an inverse associations with SI and AIR – demonstrated that the analytical cohort is comparable to other study populations and that our results are generalizable. Further, data presented in Figure 1 suggest that this is not cause but a consequence of deterioration og glucose tolerance.
In our analysis, 2-hour glucose operated as the key confounder. This parameter showed the strongest correlation with baseline fasting NEFA levels and most strongly predicted type 2 diabetes incidence. Adjustment for baseline 2-hour glucose represents the crucial distinction between our study and two earlier studies that reported a positive fasting NEFA-diabetes association. The first, a prospective study of Pima Indians conducted by Paolisso and colleagues [7], measured glucose tolerance by the OGGT but did not adjust for baseline 2-hour glucose; the relative risk associated with the comparison of the 90th versus 10th percentiles of fasting NEFA was 2.3 (95% CI 1.1–4.7).
In the second study, a prospective case-control study nested in the Atherosclerosis Risk in Communities (ARIC) cohort [5], Pankow and co-investigators used FPG (instead of 2-hour glucose) for diagnosis of type 2 diabetes and for adjustment of the fasting NEFA-diabetes association. The study found a positive association between fasting NEFA and type 2 diabetes risk after adjustment for baseline FPG; the hazard ratio for comparing the fourth versus first fasting NEFA quartiles was 1.68 (95% CI 1.20–2.34). Based on our findings, adjustment for FPG is not equivalent to the adjustment for 2-hour glucose (Table 3), which can explain the discrepancy in the results. Besides, there was no baseline correlation between FPG and fasting NEFA in the ARIC study, indicating that in the ARIC population FPG did not confound the fasting NEFA-diabetes association. Although substitution of 2-hour glucose by FPG is a conventional choice in many epidemiological studies and probably is well justified, some associations can be highly sensitive to such substitution.
Table 3.
Association of the fasting NEFA levels with the risk of diabetes – adjustment for and stratification by metabolic risk factors
| Variables included in the modela | Stratification by baseline characteristics OR (95% CI) (n) |
||
|---|---|---|---|
| All | Sub-groups | ||
| Log (NEFA) (Model1) | 1.37 (0.87, 2.15) (902) |
NGT 0.83 (0.40, 1.73) (601) |
IGT 0.66 (0.34, 1.27) (301) |
| Log (NEFA), 2 hr glucose | 0.50 (0.30, 0.82) (902) |
NGT 0.57 (0.27, 1.20) (601) |
IGT 0.50 (0.25, 1.01) (301) |
| Log (NEFA), FPG | 0.97 (0.60, 1.55) (902) |
FPG < 110 0.99 (0.57, 1.75) (754) |
FPG ≥ 110 0.95 (0.37, 2.45) (148) |
| Log (NEFA), BMI | 1.08 (0.69, 1.71) (900) |
Normal (BMI <25.0) 0.62 (0.21, 1.78) (234) |
Overweight/Obese (BMI ≥25.0) 1.21 (0.72, 2.02) (666) |
| Log (NEFA), waist-to-hip ratio (WHR) | 1.22 (0.77, 1.93) (899) |
WHR < 0.857 (median) 1.21 (0.58, 2.54) (448) |
WHR ≥ 0.857 (median) 1.18 (0.65, 2.15) (451) |
| Log (NEFA), waist | 1.08 (0.68, 1.71) (899) |
Waist < 89.5 (median) 0.86 (0.41, 1.80) (449) |
Waist ≥ 89.5 (median) 1.22 (0.68, 2.22) (450) |
| Log (NEFA), weight | 1.15 (0.73, 1.81) (900) |
Weight < 77.84 (median) 0.73 (0.34, 1.57) (449) |
Weight ≥ 77.84 (median) 1.41 (0.77, 2.57) (451) |
| Log (NEFA), SI | 1.01 (0.60, 1.70) (838) |
SI < 2.65 (median) 1.01 (0.51, 1.98) (421) |
SI ≥ 2.65 (median) 0.82 (0.36, 1.87) (417) |
| Log (NEFA), fasting insulin | 1.17 (0.74, 1.85) (901) |
Fasting insulin < 13 (median) 1.10 (0.48, 2.51) (433) |
Fasting insulin ≥ 13 (median) 1.13 (0.65, 1.98) (468) |
| Log (NEFA), AIR (MM) | 1.40 (0.88, 2.23) (878) |
AIR < 374.42 (median) 1.42 (0.82, 2.47) (438) |
AIR ≥ 374.42 (median) 1.17 (0.48, 2.86) (440) |
All models also included the following demographic and study variables: age, ethnicity, gender, study site
In a two-year study among male police employees in Paris [6], Charles and colleagues included 2-hour glucose in their analysis. Even after adjustment for this key confounder (among several other variables), ORs associated with the increase in fasting NEFA by one standard deviation (0.12 mmol/L) were 1.3 (95% CI 1.1–1.4) for converting from NGT to either IGT or type 2 diabetes [6]. However, the Paris study population seems to differ from the general population in at least two important ways: by a high rate of reversal from IGT to NGT (65%) and no independent association between BMI and diabetes risk. Such deviations from other study populations preclude generalization of these results to other populations.
The discrepancy between the inverse association found in our study and the positive associations reported by the Pima Indian [7] and ARIC [5] cohort studies can be explained by incomplete adjustment for confounding, while the discrepancy with the Paris study [6] is probably due to the specifics of the study population. In contrast, a fourth population-based cohort Ely study [8] stratified the analysis by baseline IGT-status as measured by the OGTT. The analysis compared means of fasting NEFAs in different sub-groups without modeling relative risk with sophisticated adjustment for confounders. In the absence of multivariable modeling but stratifying by IGT-status, this analysis clearly demonstrated trends similar to our findings. The study found a cross-sectional correlation between fasting NEFA and glucose intolerance with higher fasting NEFA levels in IGT participants. At the same time, those who developed diabetes or IGT did not have higher fasting NEFA levels at baseline. Taken together, our analysis and the Ely study confirm the importance of adjusting for 2-hour glucose. Even crude adjustment by baseline IGT status disproves the hypothesis that increased fasting NEFA levels predict diabetes risk.
The striking finding that the cross-sectional correlation between fasting NEFA and type 2 diabetes risk factors is positive while the prospective association is negative can be explained by two factors: the paradoxical relationship between fat oxidation and diabetes on the one hand and on the other hand, the relationships between circulating NEFA levels and intensity of fat oxidation. In frank diabetes and obesity, fat oxidation on average is increased [20]. However, prospective metabolic studies demonstrate that high respiratory quotient (RQ), an indicator of low fat oxidation, predicts weight gain [21–26], implying that slow fat oxidation predisposes to obesity. Moreover, a number of cross-sectional studies support the concept that low ability to oxidize fat is involved in metabolic deterioration. Lower levels of fat utilization have been demonstrated in formerly-obese individuals as compared to their never obese counterparts [27–30], and reduced NEFA oxidation in skeletal muscles has been demonstrated in obese patients with type 2 diabetes compared to obese controls without diabetes [31–33]. Insulin resistance also has been shown to be inversely associated with whole-body fat oxidation in the non-diabetic offspring of type 2 diabetes patients, suggesting that genetic predisposition to type 2 diabetes involves low individual ability to oxidize fat [34]. Finally, the opposite effect of exercise training on fat oxidation (increase) [35–37] and type 2 diabetes risk (decrease) suggest the protective role of intensive fat oxidation against diabetes [38;39]. Thus, despite the positive cross-sectional association with type 2 diabetes and its correlates, intensity of fat oxidation plays a protective role in type 2 diabetes etiology and should be inversely associated with type 2 diabetes risk.
The intensity of fat oxidation is proportional to circulating NEFA [20;27;40–44]. Therefore, we would expect to find similar relationships between fasting NEFA levels and type 2 diabetes risk, i.e. positive cross-sectional and inverse prospective. A recently published study supports this idea. The study compared fasting NEFA levels in normal controls, metabolically healthy obese individuals without reduced insulin sensitivity, and obese individuals with insulin resistance [45]. Fasting NEFA levels were lower in normal controls compared to the obese participants, confirming the well-established cross-sectional correlation between fasting NEFA levels and obesity. However, metabolically healthy obese individuals had higher fasting NEFA levels versus their insulin-resistant counterparts, suggesting that obese individuals with high fasting NEFA levels are less likely to develop type 2 diabetes.
In summary, our results indicate that (a) 2-hour glucose strongly confounds the prospective association between fastinf NEFA and type 2 diabetes and (b) properly adjusted fasting NEFA levels are inversely associated with T2DM risk. The mechanistic explanation of this finding awaits further confirmation.
Acknowledgements
We thank Dr. Christopher Byrne (University of Southampton, Southampton, U.K.) for the interesting discussions and insights into the physiology of fasting FFA. This research is supported by National Institutes of Health grant 1R01DK081028.
Abbreviations
- AIR
acute insulin response
- BMI
body mass index
- CI
confidence interval
- FI
fasting insulin
- FPG
fasting plasma glucose
- NEFA
non-esterified fatty acids
- FSIGTT
frequently sampled intravenous glucose tolerance test
- IGT
impaired glucose tolerance
- NGT
normal glucose tolerance
- OR
odds ratio
- SI
insulin sensitivity
- WC
waist circumference
- WHR
waist-to-hip ratio
- 2hG
postload 2-hour glucose
Footnotes
Duality of interest statement: The authors declare that there is no duality of interest associated with this manuscript.
Reference List
- 1.Boden G. Obesity and free fatty acids. Endocrinol.Metab Clin North Am. 2008;37:635–6ix. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arner P. Free fatty acids--do they play a central role in type 2 diabetes? Diabetes Obes.Metab. 2001;3(Suppl 1):S11–S19. [PubMed] [Google Scholar]
- 3.Simpson E. The interpretation of interaction in contingency tables. J R Stat Soc. 1951;13:238–241. [Google Scholar]
- 4.Szklo M, Nieto FJ. 2nd ed edn. Jones and Bartlett Publishers; Sudbury, Mass: 2007. Identifying noncausal associations: Confounding. In: AnonymousEpidemiology: beyond the basics; pp. 151–182. [Google Scholar]
- 5.Pankow JS, Duncan BB, Schmidt MI, et al. Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2004;27:77–82. doi: 10.2337/diacare.27.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Charles MA, Eschwege E, Thibult N, et al. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40:1101–1106. doi: 10.1007/s001250050793. [DOI] [PubMed] [Google Scholar]
- 7.Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- 8.Byrne CD, Maison P, Halsall D, Martensz N, HALES CN, Wareham NJ. Cross-sectional but not longitudinal associations between non-esterified fatty acid levels and glucose intolerance and other features of the metabolic syndrome. Diabet.Med. 1999;16:1007–1015. doi: 10.1046/j.1464-5491.1999.00184.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagenknecht LE, Mayer EJ, Rewers M, et al. The Insulin Resistance Atherosclerosis Study (IRAS), : Objectives, design, and recruitment results. Annals of Epidemiology. 1995;5:464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 10.Bergman RN, Finegood DT, Ader M. Assessment of Insulin Sensitivity in Vivo. Endocr Rev. 1985;6:45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 11.Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol.Metab. 1990;71:1508–1518. doi: 10.1210/jcem-71-6-1508. [DOI] [PubMed] [Google Scholar]
- 12.Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes. 1993;42:250–256. doi: 10.2337/diab.42.2.250. [DOI] [PubMed] [Google Scholar]
- 13.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput.Methods Programs Biomed. 1986;23:113–122. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 14.Saad MF, Anderson RL, Laws A, et al. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Insulin Resistance Atherosclerosis Study. Diabetes. 1994;43:1114–1121. doi: 10.2337/diab.43.9.1114. [DOI] [PubMed] [Google Scholar]
- 15.Korytkowski MT, Berga SL, Horwitz MJ. Comparison of the minimal model and the hyperglycemic clamp for measuring insulin sensitivity and acute insulin response to glucose. Metabolism. 1995;44:1121–1125. doi: 10.1016/0026-0495(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 16.Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol.Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 17.Noma A, Okabe H, Kita M. A new colorimetric micro-determination of free fatty acids in serum. Clin Chim.Acta. 1973;43:317–320. doi: 10.1016/0009-8981(73)90468-3. [DOI] [PubMed] [Google Scholar]
- 18.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 19.Hanley AJG, DΓÇÖAgostino R, Wagenknecht LE, et al. Increased Proinsulin Levels and Decreased Acute Insulin Response Independently Predict the Incidence of Type 2 Diabetes in the Insulin Resistance Atherosclerosis Study. Diabetes. 2002;51:1263–1270. doi: 10.2337/diabetes.51.4.1263. [DOI] [PubMed] [Google Scholar]
- 20.Tataranni PA, Ravussin E. Effect of fat intake on energy balance. Ann.N Y.Acad.Sci. 1997;819:37–43. doi: 10.1111/j.1749-6632.1997.tb51797.x. [DOI] [PubMed] [Google Scholar]
- 21.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol Endocrinol Metab. 1990;259:E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 22.Seidell JC, Muller DC, Sorkin JD, ANDRES R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int.J Obes Relat Metab Disord. 1992;16:667–674. [PubMed] [Google Scholar]
- 23.Valtuena S, Salas-Salvado J, Lorda PG. The respiratory quotient as a prognostic factor in weight-loss rebound. Int.J Obes Relat Metab Disord. 1997;21:811–817. doi: 10.1038/sj.ijo.0800480. [DOI] [PubMed] [Google Scholar]
- 24.Marra M, Scalfi L, Contaldo F, Pasanisi F. Fasting respiratory quotient as a predictor of long-term weight changes in non-obese women. Ann Nutr Metab. 2004;48:189–192. doi: 10.1159/000079556. [DOI] [PubMed] [Google Scholar]
- 25.Marra M, Scalfi L, Covino A, Esposito-Del PA, Contaldo F. Fasting respiratory quotient as a predictor of weight changes in non-obese women. Int.J Obes Relat Metab Disord. 1998;22:601–603. doi: 10.1038/sj.ijo.0800612. [DOI] [PubMed] [Google Scholar]
- 26.Filozof CM, Murua C, Sanchez MP, et al. Low plasma leptin concentration and low rates of fat oxidation in weight-stable post-obese subjects. Obes Res. 2000;8:205–210. doi: 10.1038/oby.2000.23. [DOI] [PubMed] [Google Scholar]
- 27.Lean ME, James WP. Metabolic effects of isoenergetic nutrient exchange over 24 hours in relation to obesity in women. Int.J Obes. 1988;12:15–27. [PubMed] [Google Scholar]
- 28.Buemann B, Astrup A, Madsen J, Christensen NJ. A 24-h energy expenditure study on reduced-obese and nonobese women: effect of beta-blockade. Am J Clin Nutr. 1992;56:662–670. doi: 10.1093/ajcn/56.4.662. [DOI] [PubMed] [Google Scholar]
- 29.Larson DE, Ferraro RT, Robertson DS, Ravussin E. Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr. 1995;62:735–739. doi: 10.1093/ajcn/62.4.735. [DOI] [PubMed] [Google Scholar]
- 30.Ranneries C, Bulow J, Buemann B, Christensen NJ, Madsen J, Astrup A. Fat metabolism in formerly obese women. Am J Physiol. 1998;274:E155–E161. doi: 10.1152/ajpendo.1998.274.1.E155. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–2356. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes. 2000;49:2102–2107. doi: 10.2337/diabetes.49.12.2102. [DOI] [PubMed] [Google Scholar]
- 33.Blaak EE, Wagenmakers AJM, Glatz JFC, et al. Plasma FFA utilization and fatty acid-binding protein content are diminished in type 2 diabetic muscle. Am J Physiol Endocrinol Metab. 2000;279:E146–E154. doi: 10.1152/ajpendo.2000.279.1.E146. [DOI] [PubMed] [Google Scholar]
- 34.Lattuada G, Costantino F, Caumo A, et al. Reduced whole-body lipid oxidation is associated with insulin resistance, but not with intramyocellular lipid content in offspring of type 2 diabetic patients. Diabetologia. 2005;48:741–747. doi: 10.1007/s00125-005-1686-6. [DOI] [PubMed] [Google Scholar]
- 35.Hurley BF, Nemeth PM, Martin WH, III, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol. 1986;60:562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- 36.Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992;262:E791–E799. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]
- 37.Poehlman ET, Melby C. Resistance training and energy balance. Int.J Sport Nutr. 1998;8:143–159. doi: 10.1123/ijsn.8.2.143. [DOI] [PubMed] [Google Scholar]
- 38.Kelley DE, Goodpaster BH. Effects of physical activity on insulin action and glucose tolerance in obesity. Med Sci Sports Exerc. 1999;31:S619–S623. doi: 10.1097/00005768-199911001-00021. [DOI] [PubMed] [Google Scholar]
- 39.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of Exercise on Glycemic Control and Body Mass in Type 2 Diabetes Mellitus: A Meta-analysis of Controlled Clinical Trials. JAMA. 2001;286:1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 40.Ravussin E, Gautier JF. Metabolic predictors of weight gain. Int.J Obes.Relat Metab Disord. 1999;23(Suppl 1):37–41. doi: 10.1038/sj.ijo.0800793. [DOI] [PubMed] [Google Scholar]
- 41.Schutz Y, Tremblay A, Weinsier RL, Nelson KM. Role of fat oxidation in the long-term stabilization of body weight in obese women. Am J Clin Nutr. 1992;55:670–674. doi: 10.1093/ajcn/55.3.670. [DOI] [PubMed] [Google Scholar]
- 42.Groop LC, Bonadonna RC, Shank M, Petrides AS, DeFronzo RA. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest. 1991;87:83–89. doi: 10.1172/JCI115005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groop LC, Bonadonna RC, Simonson DC, Petrides AS, Shank M, DeFronzo RA. Effect of insulin on oxidative and nonoxidative pathways of free fatty acid metabolism in human obesity. Am J Physiol. 1992;263:E79–E84. doi: 10.1152/ajpendo.1992.263.1.E79. [DOI] [PubMed] [Google Scholar]
- 44.Astrup A, Buemann B, Western P, Toubro S, Raben A, Christensen NJ. Obesity as an adaptation to a high-fat diet: evidence from a cross-sectional study. Am J Clin Nutr. 1994;59:350–355. doi: 10.1093/ajcn/59.2.350. [DOI] [PubMed] [Google Scholar]
- 45.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch.Intern.Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]