Abstract
Adverse cardiac remodeling following myocardial infarction (MI) remains a significant cause of congestive heart failure. Additional and novel strategies that improve our ability to predict, diagnose, or treat remodeling are needed. Numerous groups have explored single and multiple biomarker strategies to identify diagnostic prognosticators of remodeling progression, which will improve our ability to promptly and accurately identify high-risk individuals. The identification of better clinical indicators should further lead to more effective prediction and timely treatment.
Matrix metalloproteinase (MMP-9) is one potential biomarker for cardiac remodeling, as demonstrated by both animal models and clinical studies. In animal MI models, MMP-9 expression significantly increases and is linked with inflammation, diabetic microvascular complications, extracellular matrix degradation and synthesis, and cardiac dysfunction. Clinical studies have also established a relationship between MMP-9 and post-MI remodeling and mortality, making MMP-9 a viable candidate to add to the multiple biomarker list.
By definition, a proximal biomarker shows a close relationship with its target disease, whereas a distal biomarker exhibits non-targeted disease modifying outcomes. In this review, we explore the ability of MMP-9 to serve as a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. We summarize the current molecular basis and clinical platform that allow us to include MMP-9 as a biomarker in both categories.
Keywords: biomarker, cardiovascular, congestive heart failure, inflammation, MMP-9, myocardial infarction
1. Introduction
Despite significant advancements in risk prediction, cardiovascular disease remains a leading cause of death (Roger, et al., 2011). Myocardial infarction (MI) is one of the most highly prevalent cardiovascular diseases, with over 1.2 million Americans being diagnosed with MI annually. While short-term one month survival rates have dramatically improved over the last 30 years, post-MI remodeling progressing to heart failure remains a significant clinical issue. This issue is further fueled by increased incidences of obesity, metabolic syndrome, and diabetes, all of which exacerbate the cardiac remodeling response (Horwich & Fonarow, 2010; Roger, et al., 2011). Because heart failure is associated with substantial morbidity and mortality, as well as an impaired quality of life (Goldberg, Ciampa, Lessard, Meyer, & Spencer, 2007), improved methods to identify at risk patients before they develop heart failure is a primary goal. MI modulates several biological pathways that converge in the remodeling response, which is characterized by changes in left ventricle (LV) size, shape, and function (M. L. Lindsey & Zamilpa, 2010; Pfeffer & Braunwald, 1990).
Several plasma or serum proteins have been characterized in the context of heart failure, and these are broadly classified as markers of LV remodeling. Included in the list are extracellular matrix (ECM) markers- collagen, matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs); inflammatory markers- C-reactive protein (CRP), tumor necrosis factor α, and interleukins (IL)- 1, 6, and 18; oxidative stress markers- homocysteine and myeloperoxidase; neurohormonal activation markers- renin, angiotensin II, and aldosterone; myocyte injury markers- cardiac specific troponins and creatine kinase; and myocyte stress markers- brain natriuretic peptide (BNP) and N-terminal pro-BNP (Braunwald, 2008; Fertin, et al., 2012; Maisel, et al., 2002; Opdenakker, et al., 2001; Tang, et al., 2007; Velagaleti, et al., 2010). To date, a myriad of candidate circulating biomarkers have been examined as LV remodeling or heart failure predictors, but the use of one biomarker to accurately assess disease diagnosis, stage, and progression has not been successful and is not expected to be fruitful. To illustrate this point, while average BNP levels are higher in patients with heart failure, individual levels vary from 100–1400 ng/ml. BNP shows a wide spectrum of values and does not stratify with heart failure stage, and BNP responses to heart failure treatments are influenced by comorbidities such as renal failure (Lang & Mancini, 2007). This variation is so large that BNP cannot effectively separate patients with and without heart failure (Maisel, et al., 2002). A more successful approach will likely be to use a multi-marker panel profiling scheme to assess markers during each category (diagnosis, stage, progression) from the initial MI event to progressive remodeling to the development of heart failure. Of the analytes that have been examined, MMPs provide several candidate biomarkers.
MMPs are zinc-dependent endopeptidases that cleave several ECM proteins and as such modulate outcome of various physiological and pathological processes including MI, atherosclerosis and congestive heart failure. In addition to structural ECM components, MMP substrates also include a multitude of ligand and receptor substrates such as cytokines, chemokines, growth factors, and adhesion molecules that alter cellular migration, adhesion, and activation. MMPs, therefore, exert a strong influence on cardiac remodeling through multiple mechanisms (M. L. Lindsey, 2004; M. L. Lindsey & Zamilpa, 2010; Sternlicht & Werb, 2001). MMPs are endogenously inhibited by the tissue inhibitors of metalloproteinases (TIMPs), a family comprised of four members, TIMP-1, -2, -3, and -4. Pre-clinical and clinical studies in the post-MI setting indicate that MMP-1, -2, -3, -7, -8, -9, -12, -13, and -14 and TIMP-1, -2 -3, and -4 are relevant to MI and LV remodeling (Hansson, et al., 2011; M. L. Lindsey & Zamilpa, 2010; Rohde, et al., 1999; Yarbrough, et al., 2003; Zamilpa & Lindsey, 2010).
For the most part, MMPs are secreted from the cell as proMMPs and are activated extracellularly by tissue or plasma proteinases. The first step in activation involves cleavage of a part of the propeptide, and complete activation occurs with removal of the entire propeptide by the MMP intermediate or by other active MMPs (Nagase, Visse, & Murphy, 2006). MMPs can also be activated in vitro by treatment with organomercurial compounds, urea, SH reagents, and chaotropic agents, which chemically perturb the proMMP to alter its structure and permit activitiy without loss of the 10 kD pro-domain. Other exogenous MMP activators include oxidants such as HOCl and ONOO−, which activate proMMPs by reacting with the cysteine in the propeptide. This activation process can also take place in vivo, under inflammatory conditions (Gu, et al., 2002; Peppin & Weiss, 1986). On the other hand the major endogenous MMP inhibitor in serum is α2-macroglobulin and in tissue are the TIMPs (Sorokin, 2010).
In 2001, an NIH working group standardized the definition of a biomarker as any characteristic that can be objectively evaluated as an indicator of a normal biological process, a pathological process, or a pharmacological responses to therapeutic intervention (Biomarkers Definitions Working Group, 2001; Vasan, 2006). The American Heart Association released a scientific statement focused on the importance for developing biomarkers to enhance diagnostic methods and provide surrogate measures of treatment efficacy (Balagopal, et al., 2011; Fortmann, et al., 2004; Hlatky, et al., 2009; Richards, 2009; Smith, et al., 2004; Vasan, 2006). Because no single biomarker will likely provide sufficient information to predict disease progression, the next step is to identify the combination of markers that improve risk prediction beyond what is currently available. A combination biomarker strategy can also be used strategically to make go or no-go decisions that will accelerate drug discovery (Krishna & Wagner, 2010). An essential biomarker, by definition, would modulate both the target response as well as distal events related to disease outcome (Krishna, Herman, & Wagner, 2008; Krishna & Wagner, 2010).
In this review, we provide rationale for using MMP-9 as a biomarker. We will discuss its effectiveness as a proximal biomarker for cardiac remodeling (one that shows a close relationship with its target disease) and a distal biomarker for inflammation (one that exhibits non-targeted disease modifying outcomes). We provide a logic model by which to evaluate the inclusion of MMP-9 as a candidate marker for post-MI remodeling that may also serve as a template to evaluate other candidate markers.
2. Methods of review
We searched PubMed for all papers that included MMP-9, which was over 13,000 papers. We then focused the search by articles published in the past 1, 2, 3, 5, or 10 years. Subsequently, we added inflammation, cardiac remodeling, cardiovascular, myocyte, fibroblast, neutrophils, or leukocytes to the MMP-9 keyword search (each term was searched individually with MMP-9). We included all clinical reports, review articles, journal articles, clinical trial reports, meta-analysis studies, randomized controlled trials, and original research manuscripts that were published in English. The numbers of manuscripts with these key words are shown in Figure 1.
Figure 1.
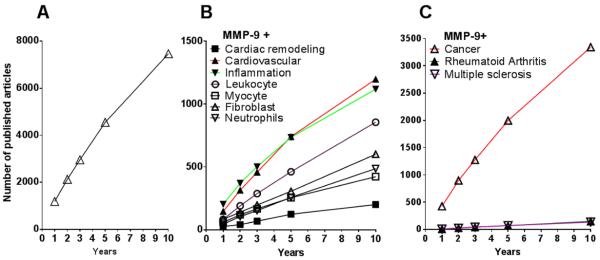
Research articles and reviews on MMP-9 published in the last decade (2001–2010). A. Total number of articles on MMP-9 published. B. Number of articles published on MMP-9 and cardiovascular diseases, including articles on the proximal effect on cardiac remodeling. C. Number of articles published on MMP-9 in other inflammatory diseases (cancer, arthritis, and multiple sclerosis).
3. Pre-clinical and clinical studies: MMP-9-mediated proximal effects (Figure 2)
Figure 2.
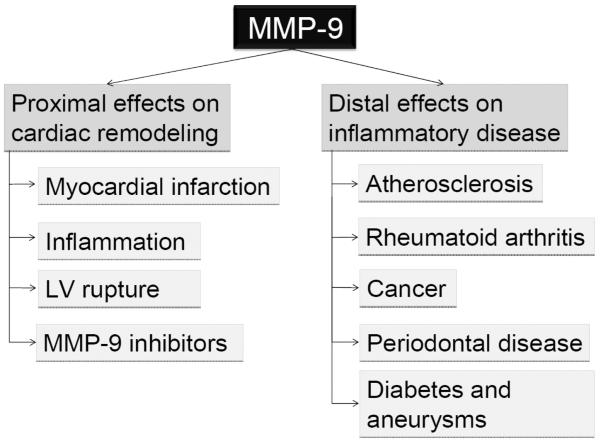
The effect of MMP-9 is broadly classified into effects proximal and distal to cardiac remodeling. 1. Proximal effects targeted on cardiac remodeling and 2. Distal effects (non-targeted) on inflammatory diseases.
3.1 Post-MI LV healing phases
Following MI, both the infarcted region as well as the remote non-infarcted zone undergo cardiac remodeling as a part of the wound healing response (Pfeffer & Braunwald, 1990). The LV healing response can be divided into two overlapping phases, the inflammatory and reparative phases. The first phase, the inflammatory phase, is characterized by the robust release of inflammatory mediators and degradation of ECM that occurs in the setting of myocyte necrosis. During the inflammatory phase, cardiomyocyte death triggers the rapid activation of the complement system, which induces free radical production and activates the toll-like receptor-mediated pathway. The second phase, the reparative phase, is characterized by fibroblast proliferation and release of fibrosis-promoting cytokines that contribute positively to scar formation, with the net result being increased ECM synthesis and deposition (Frantz, Bauersachs, & Ertl, 2009).
The inflammatory and fibrotic pathways share several components. For example, both pathways involve activation of nuclear factor kappa-B (NF-κB) in infiltrating and resident myocardial cells to stimulate the expression of cytokines, chemokines, growth factors, and adhesion molecules. Among these factors are IL-1β, tumor necrosis factor α, monocyte chemotactic protein (MCP)-1/(CCL2), and intercellular adhesion molecule 1, which stimulate and facilitate leukocyte intravasation into the infarct region. During permanent occlusion, neutrophil infiltration occurs primarily during days 1–3 post-MI, while macrophage infiltration occurs primarily during days 3–7 post-MI. During reperfusion, the kinetics and amplitude of the inflammatory response shifts, such that both neutrophils and macrophages enter the tissue simultaneously as soon as reperfusion is initiated. The transition from the inflammatory to reparative phase is associated with the activation of pathways that turns off inflammation and promotes ECM scar formation (Dobaczewski, Gonzalez-Quesada, & Frangogiannis, 2010; Frantz, et al., 2009). Targets involved in inflammatory events and reparative processes will be central components of a successful LV remodeling biomarker discovery and drug target development.
3.2 MMP-9 expression in post-MI cardiac remodeling
Table 1 highlights basic science studies evaluating LV MMP-9 levels in animal models, while Table 2 highlights clinical trials examining LV MMP-9 levels in humans. MMP-9 is expressed in cardiac myocytes, fibroblasts, vascular smooth muscle cells, endothelial cells, neutrophils, macrophages, and fibroblasts (Coker, et al., 2001; Hasty, et al., 1990; Heymans, et al., 1999; Kawakami, et al., 2004; M. Lindsey, et al., 2001; M. L. Lindsey, Escobar, Mukherjee, et al., 2006; Opdenakker, et al., 2001; Porter & Turner, 2009; van den Borne, et al., 2009). MMP-9 was first described as being able to process only collagen that was first denatured or already cleaved by collagenases such as MMP-1. Recent literature, however, has shown that MMP-9 can process full length interstitial collagens (Egeblad & Werb, 2002; Lauer-Fields, et al., 2008). Further, MMP-9 does not require an activation cleavage step to proteolyze substrates. Pro-MMP-9, in the presence of substrate, has enzymatic activity without the loss of the 10 kDa pro-domain (Bannikov, Karelina, Collier, Marmer, & Goldberg, 2002).
Table 1.
Pre-clinical studies: A selection of articles to summarize the role of MMP-9 in cardiac remodeling
| Reference and year | Animal model | Sex | Significant findings |
|---|---|---|---|
|
| |||
| Heymans, et al., 1999 | MMP-9 null mice post-MI | Male | ↓ cardiac rupture |
|
| |||
| Ducharme, et al., 2000 | MMP-9 null mice post-MI | Male | ↓ collagen accumulation and macrophage infiltration |
| ↓ LV dimension | |||
| ↑ MMP-2, MMP-13, and TIMP-1 | |||
|
| |||
| Romanic, et al., 2001 | Rabbit post-MI | Female | ↑ MMP-9 within 24 hours following MI |
|
| |||
| Lindsey, et al., 2005 | Aging CB6F1 mice | Both | ↑ LV end-diastolic dimensions and wall thickness in middle aged and old mice. |
| ↑ MMP-9 in old mice | |||
|
| |||
| Lindsey, et al., 2005 | MMP-9 null mice post-MI | Both | ↑ neovascularization post-MI |
|
| |||
| Mukherjee, et al., 2006 | Gelatinase B/ lacZ transgenic mice post-MI | Both | ↑ MMP-9 promoter induction at day 3, peaks at day 7 |
|
| |||
| Yang, et al., 2006 | C57BL/6J mice post-MI | Male | ↑ LV rupture in middle aged mice |
| ↑ LV remodeling and MMP-9 activity | |||
|
| |||
| Chiao, et al., 2011 | Aging C57/BL6J mice | Both | ↑ MMP-9 and MCP-1 levels in plasma and LV |
| ↑ macrophage density in LV with aging | |||
Table 2.
Clinical studies: A selection of articles to summarize the role of MMP-9 in cardiac remodeling
| Reference and year | Patient population (n) | Location | Significant findings | Conclusion |
|---|---|---|---|---|
| Blankenberg, et al., 2003 | coronary artery disease (1127)* | Germany | MMP-9 higher at baseline in patients with a subsequent fatal CV event | MMP-9 as a novel predictor of future CV mortality |
| Squire, et al., 2004 | acute MI (60)* | UK | MMP-9 peaks at days 1–4 post-MI | MMP-9 present during LV remodeling |
| Sundström, et al., 2004 | previous MI but no heart failure (699)* | USA | Plasma MMP-9 linked to vascular risk factors and echocardiography measurements in males | MMP-9 levels associate with increased LV diastolic dimensions and increased wall thickness |
| Yan, et al., 2006 | symptomatic heart failure with reduced ejection fraction (184)* | Canada and USA | ↑ MMP-9 levels correlate with increased LV volumes and reduced LV ejection fraction in patients with heart failure | MMP-9 levels associate with cardiac dysfunction |
| Hlakty, et al., 2007 | acute MI or stable angina (199)* | USA | ↑ MMP-9 in acute MI but not stable angina patients | MMP-9 independently associates with development of an acute MI rather than stable angina |
| Martos, et al., 2007 | hypertensive with diastolic dysfunction (86)* | Ireland | ↑ MMP-9 in diastolic heart failure | ↑ MMP-9 levels associate with active fibrosis |
| Orn, S et al., 2007 | Long-term survivors after MI (52)* | UK and USA | ↑ MMP-9 in the acute phase after MI, protective effect during late LV remodeling | No relationship between MMP-9 levels and scar size at any time point after MI |
| Van den Borne, et al., 2009 | autopsy samples of post-MI ruptures (20)¥ | Netherlands | ↑ MMP-9 in ruptured LVs | ↑ MMP-9 in infarcted area associates with rupture |
| Hansson, et al., 2011 | Uppsala Longitudinal Study of Adult Men (ULSAM)(1082)* | Sweden | ↑ MMP-9 and TIMP-1 in men with CV mortality | MMP-9 and TIMP-1 are related to CV mortality risk |
| Kobayashi, et al., 2011 | compare ST elevated ACS and non-ST elevated ACS stable angina patients (266)* | Japan | ↑ MMP-9 early post-MI | MMP-9 has higher diagnostic accuracy for ACS than hstroponin |
Indicates that MMP-9 was analyzed by ELISA;
indicates MMP-9 was analyzed by zymography or immunocapture activity.
Abbreviations: CV; cardiovascular, LV; left ventricle, ESV; end systolic volume, HF; heart failure, MI; myocardial infarction
MMP-9 activates several chemokines, including CXCL5, CXCL6, and CXCL8, and contributes to the release of cell surface receptors (e.g., tumor necrosis factor-α receptor) to alter the local microenvironment (Van Den Steen, et al., 2003). MMP-9 also has several inflammatory response elements, including activator protein-1, specificity protein-1, and NF-κB sites that makes it highly responsive to inflammatory stimuli (Benbow & Brinckerhoff, 1997; M. L. Lindsey & Zamilpa, 2010). In the mouse, rat, pig, rabbit, and dog models of MI, MMP-9 levels are consistently increased in the infarct region (Ducharme, et al., 2000; Etoh, et al., 2001; Heymans, et al., 1999; M. Lindsey, et al., 2001; M. L. Lindsey, Escobar, Dobrucki, et al., 2006; M. L. Lindsey, et al., 2005; Romanic, Burns-Kurtis, Gout, Berrebi-Bertrand, & Ohlstein, 2001; Tao, Cavasin, Yang, Liu, & Yang, 2004). In mouse, rabbit, and pig MI models, pharmacological MMP inhibition reduces LV dilation and preserves cardiac function (Chancey, Brower, Peterson, & Janicki, 2002; Mukherjee, et al., 2003; Rohde, et al., 1999; Spinale, et al., 1999a, 1999b). Mice with targeted deletion of the MMP-9 gene show attenuated LV dilation after experimental MI accompanied by decreased collagen accumulation (Ducharme, et al., 2000; Heymans, et al., 1999). Interestingly, however, MMP-9 deletion also stimulates neovascularization in the post-MI infarct region (M. L. Lindsey, Escobar, Dobrucki, et al., 2006). This suggests that MMP-9 serves both beneficial and detrimental roles in the post-MI response.
A striking increase in MMP-9 activity is found at days 1 to 4 in the infarct region, and this increase corresponds with neutrophil and macrophage infiltration (Ducharme, et al., 2000; Heymans, et al., 1999; Hudson, et al., 2006; Ramani, et al., 2004; Tao, et al., 2004). Mukherjee et al demonstrated that MMP-9 promoter transcripts with a β-galactosidase reporter show MMP-9 promoter activity at day 3 post-MI that peaked at day 7 (Mukherjee, et al., 2010). The earlier initial increase in MMP-9 protein levels seen at day 1 post-MI is due to the release of pre-formed MMP-9 from infiltrating neutrophils, where it is stored in gelatinase granules (Mukherjee, et al., 2010).
Kelly and colleagues provided insight into the complexity of MMP-9 in terms of its having both beneficial and detrimental roles during post-MI remodeling (Kelly, et al., 2007). They found that increased early levels of MMP-9 associated with both neutrophil numbers and the extent of LV remodeling, indicating that MMP-9 from the neutrophil has an overall detrimental effect. In contrast, increased late levels of MMP-9 associated with preservation of LV function, indicating that MMP-9 after the initial wound healing phase may serve an overall beneficial effect. The temporal profile of MMP-9, in addition to its magnitude, is an important consideration. Post-MI, the establishment of new blood vessel networks is needed to supply oxygen to the highly metabolically active infarct area (Post, Laham, Sellke, & Simons, 2001; Sim, Zhang, Shim, Lim, & Ge, 2002). Of note, MMP-9 deletion enhanced neovascularization in the post-MI setting in mice, suggesting that targeted strategies to inhibit MMP-9 early post-MI might improve rather than impair angiogenesis (M. L. Lindsey, Escobar, Dobrucki, et al., 2006).
Recent advances in mass spectrometry–based proteomic approaches and new emerging technologies hold particular promise for unbiased discovery and subsequent validation of novel biomarkers of cardiovascular disease (Gerszten, Asnani, & Carr, 2011). As an example of such an approach, Zamilpa et al identified multiple proteins that are differentially expressed in the infarct region of MMP-9 null mice compared to wild type mice. Among previously known in vitro MMP-9 substrates, fibronectin was validated as an in vivo MMP-9 substrate in the post-MI setting (Zamilpa, et al., 2010).
3.3 MMP-9 effects on LV rupture
LV wall rupture is one of the more serious complications, accounting for 5 to 31% of all inhospital MI deaths (Figueras, Cortadellas, & Soler-Soler, 2000). While rupture rates in humans have fallen due to the success of reperfusion, the incidence of LV ruptures remains at 0.5%–1.4% (Lopez-Sendon, et al., 2010). LV ruptures are more frequent in patients with STEMI (0.9%) than patients with other acute coronary syndromes (NSTEMI, 0.17%; unstable angina, 0.25%) (Lopez-Sendon, et al., 2010). In C57BL/6J male mice, the 7 day post-MI survival rate is approximately 60%, and about one in three deaths will occur as a result of rupture (Gao, Xu, Kiriazis, Dart, & Du, 2005; Yang, et al., 2008; Zamilpa, et al., 2011). Survival in female mice is about 90% at day 7 post-MI, with about one in ten deaths occurring as a result of rupture. Gender studies in 129sv mice showed that males have higher MMP-9 activity in the infarct region associated with increased inflammatory cell infiltration, as well as increased MMP-9 expression in circulating peripheral blood mononuclear cells (Fang, Du, Gao, & Dart, 2010). MMP inhibition using the CP471, 474 inhibitor significantly reduced both rupture incidence and MMP-9 activity in mice, supporting a role of MMP-9 in the pathogenesis of rupture. In humans, increased MMP-9 levels have been detected in ruptured human ventricles (van den Borne, et al., 2009). LV rupture in human and mice share an association between rupture rates and the accumulation of inflammatory cells, as well as a common location at the border zone. LV rupture in human and mice are disparate in the influence of sex on rupture rates (Gao, et al., 2005). In the clinical setting, the risk of post-MI rupture is higher in females than males (Figueras, et al., 2000; Reardon, et al., 1997).
3.4 Clinical studies: MMP-9 is a biomarker for cardiac remodeling
Blankenberg and colleagues performed the first comprehensive clinical study that implicated MMP-9 as a novel prognostic biomarker for individuals at increased risk for CV mortality (Blankenberg, et al., 2003). MMP-9 correlated with the acute-phase reactant proteins IL-6, hs-CRP, and fibrinogen, indicating that MMP-9 could have its own pathophysiological significance in cardiovascular mortality. Squire and colleagues extended these studies to demonstrate that, in humans, higher MMP-9 correlated with larger LV volumes and greater dysfunction following MI (Squire, Evans, Ng, Loftus, & Thompson, 2004). The Vasan team examined patients from the Framingham Heart Study and found that plasma MMP-9 levels associated with increased LV diastolic dimensions and increased wall thickness (Sundstrom, et al., 2004). Hlakty and colleagues showed that circulating MMP-9 levels independently associated with acute MI but not stable angina (Hlatky, et al., 2007). MMP-9 levels correlated with LV enlargement, lower ventricular ejection fraction, and persistent adverse LV remodeling in chronic systolic heart failure patients (Yan, et al., 2006). Fertin et al examined 112 correlations among 52 different biomarkers and LV remodeling indices. The most consistent biomarkers associated with LV remodeling were related to ECM turnover or neurohormonal activation. Among the biomarkers, MMP-9, collagen peptides, and B-type natriuretic peptide were prominent biomarkers that predicted adverse LV remodeling after MI (Fertin, et al., 2012). Of note, several polymorphisms have been evaluated within the MMP-9 gene and have been shown to influence gene expression (B. Zhang, et al., 1999). Specifically, the C1562T allele associates with increased MMP-9 plasma concentrations, whereas the R279Q polymorphism had no effect on plasma levels but associated with future CV events. The 279 amino acid where these polymorphisms occur resides in the catalytic domain of the MMP-9 enzyme, suggesting that MMP-9 activity levels may be higher in patients with the R279Q polymorphism (Shipley, et al., 1996; Tanner, et al., 2011). Combined, these studies offer strong evidence for a proximal role of MMP-9 in LV remodeling. In heart failure patients, serum carboxy-terminal telopeptide of procollagen type I, carboxy-terminal telopeptide of procollagen type I, and amino-terminal propeptide of procollagen type III are all elevated and serve as indicators of diastolic dysfunction. In these same patients, serum MMP-9 levels are elevated, suggesting increased degradation of myocardial collagen (Martos, et al., 2007). This particular study elegantly demonstrated the role of MMP-9 in stimulating LV remodeling in hypertensive and diastolic heart failure patients.
3.5 MMP-9 roles in inflammation: neutrophils, macrophages, and lymphocytes are cell sources of MMP-9
MMP-9 is secreted by neutrophils early post-MI, and by macrophages, lymphocytes, and fibroblasts at later phases post-MI (Figure 3). In neutrophils, MMP-9 is synthesized during bone marrow granulocyte differentiation and is released following neutrophil activation (Jonsson, et al., 2011). Fang et al quantified MMP-9 levels in peripheral blood mononuclear cells that were differentiated into macrophages in vitro (Fang, et al., 2007). Circulating cells isolated at day 4 after MI in 129sv mice showed increased MMP-9 levels compared to cells isolated from the sham mice. Peripheral blood mononuclear cells isolated from patients with acute MI and differentiated to macrophages also produced a higher amount of MMP-9 compared to cells isolated from patients with stable angina or healthy controls, indicating that macrophages are an important cellular source of plasma MMP-9 (Fang, et al., 2010).
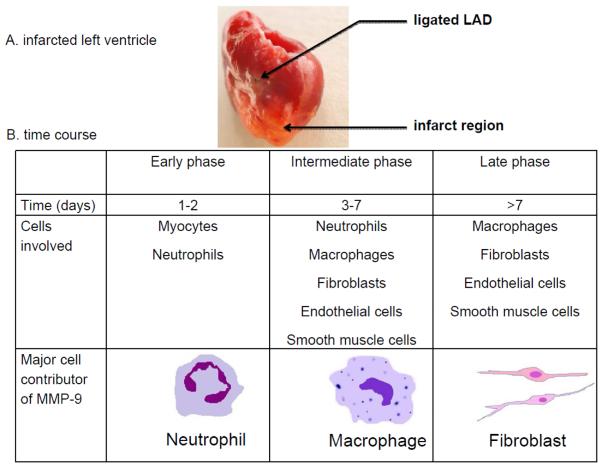
Figure 3.
Schematic diagram presenting infarcted left ventricle and cell sources during the different stages of MMP-9 release as result of post-MI cardiac remodeling. A. Infarcted LV at 7 days post-MI. B. Following MI, MMP-9 is released by neutrophils in the early inflammatory phase (days 0–3), macrophages during the late inflammatory phase (days 3–7), and fibroblasts during the remodeling phase. Within the myocardium, cardiac myocytes, endothelial cells, and vascular smooth muscle cells are additional MMP-9 sources.
3.6 MMP-9 effects on inflammatory chemokines and cytokines
MMP-9 modulates leukocyte function through a number of cytokine-mediated mechanisms. MMP-9 can process pro-IL-1β into active IL-1β and can truncate IL-8 into a more active form. As both IL-1β and IL-8 can stimulate MMP-9 degranulation from neutrophils, providing an important positive feedback loop for neutrophil activation and chemotaxis (Opdenakker, et al., 2001).
In the post-MI setting, the overexpression of human CRP in mice results in more severe LV remodeling with increased LV dilation, a greater extent of LV dysfunction, and more prominent cardiomyocyte hypertrophy and fibrosis than their littermate controls (Mano, et al., 2011). The CRP transgenic mice also display enhanced macrophage infiltration into the infarct region, at rates that are directly proportional to increased MCP-1 expression and MMP-9 activity (Takahashi, et al., 2010). Increased CRP, therefore, leads to increased macrophage accumulation through a direct MMP-9 role.
3.7 MMP-9 inhibitors
It is well established that an increased expression of MMP-9 associates with the pathological status in a wide range of inflammatory diseases, including MI, rheumatoid arthritis, liver fibrosis, and periodontal disease. A pathogenic role of MMP-9 in tissue breakdown and remodeling during aggressive tumor growth and angiogenesis is also established. Because of past failures with global non-specific MMP inhibitors, the current focus in the MMP inhibitor drug discovery arena is to develop inhibitors specific for particular MMPs.
The main structural requirement of an MMP inhibitor is the zinc binding group (ZBG) that chelates the active-site zinc ion. Tandon and Sinha applied a docking and molecular dynamics approach to study the binding of inhibitors to the active site of MMP-9. Three categories of zinc binding groups were chosen: 1) sulfonamide hydroxamate, 2) thioester, and 3) carboxylic moieties. Out of these three categories, the thioester based zinc binding moiety provided the most promising docking scores compared to the other two groups (Tandon & Sinha, 2011).
Gutierrez and colleages demonstrated that the MMP-9 inhibitor doxycline attenuated Trypanosoma cruzi infection induced cardiac injury (Gutierrez, et al., 2008). These results indicate that MMP-9 inhibition in myocarditis mollifies inflammation to increase survival in mice (Gutierrez, et al., 2008). Of interest, doxycycline is the only FDA-approved MMP inhibitor currently on the market (Lee, et al., 2004; Y. Zhang, et al., 2012).
Pharmacological inhibition of MMPs has been effective in limiting tissue damage after MI in animal models. Villarreal et al observed that short-term treatment of doxycline reduced adverse LV remodeling and improved LV function in male Male Sprague-Dawley rats (Villarreal, et al., 2003). MMP inhibition in humans, however, has not been as successful, as broad-spectrum MMP inhibitors showed adverse secondary effects on the musculoskeletal system that were linked to the non-selective nature of these inhibitors (Creemers, Cleutjens, Smits, & Daemen, 2001; Spinale, 2002). .
4. MMP-9 distal effects on other inflammatory diseases (Figure 2)
4.1 Atherosclerosis
Atherosclerosis is an inflammatory disease characterized by plaque formation and artery wall thickening as a result of the accumulation of lipids. Atherosclerosis mainly affects vein grafts, arterial blood vessels, and also includes the accumulation of macrophages, low-density lipoproteins, plasma proteins that transport cholesterol, and triglycerides (Ross, 1999).
Konstantino et al have reviewed the role of MMP-9 in the pathophysiology of atherosclerosis and plaque rupture (Konstantino, et al., 2009). While other MMPs (including MMPs -1,-2,-3,-7,-8,-10,-11,-12, and -13) have been evaluated, MMP-9 has been the most studied MMP in atherosclerosis pathology (Konstantino, et al., 2009). Despite the number of studies that demonstrate increased MMP-9 levels in the atherosclerotic lesion, few studies have been designed to determine the causal roles of MMP-9 or to explore the clinical applicability of MMP-9 inhibition. Mechanistic studies in apolipoprotein E (Apo E)-null mice model provide conflicting insight on MMP-9 roles in plaque formation. Lutton et al showed that after 25 weeks of a cholesterol-rich diet, Apo E / MMP-9 double-null mice had 70% smaller sized plaques with less collagen and macrophage content compared with Apo E null / MMP-9+/+ mice, suggesting that MMP-9 deficiency protects from plaque development (Luttun, et al., 2004).
Conversely, Johnson et al demonstrated a larger lesion area and increased macrophage content in Apo E / MMP-9 double-null mice compared with Apo E null / MMP-9+/+ mice after 8 weeks of a high-fat diet, indicating that MMP-9 deficiency promoted rather than impaired atherosclerosis progression (Johnson, George, Newby, & Jackson, 2005). The inconsistent results could be ascribed to a variation in timing of lesion measurements, as Johnson et al determined the lesion size after 8 weeks of a high-fat diet, whereas Lutton et al assessed the lesion size after 25 weeks of a cholesterol-rich diet. The concept that MMP function can switch from deleterious to beneficial can be explained by a shift in substrate availability, since net MMP activity is determined by what substrates are processed. To support this idea, Nooijer et al. demonstrated that negative effects of MMP-9 overexpression on plaque stability appear to be more prominent in advanced atherosclerotic plaques (de Nooijer, et al., 2006). Advanced plaques showed more significant features of vulnerable plaque with a high incidence of intraplaque hemorrhage (de Nooijer, et al., 2006). The overexpression of activated MMP-9 in macrophages induced substantial plaque disruption in advanced atherosclerotic lesions of Apo E null mice, revealing that enhanced macrophage MMP-9 proteolytic activity can induce acute plaque disruption. MMP-9, therefore, is a therapeutic target for stabilizing rupture-prone plaques that are in the advanced stage. The fact that MMP-9 and macrophages co-exist in vulnerable plaques highlights the role for MMP-9 in this process. Future studies examining the temporo-spatial dynamics of MMP-9 expression during plaque development and destabilization are required to fully understand the significance of MMP-9 activity in atherosclerosis. (Gough, Gomez, Wille, & Raines, 2006). The cholesterol lowering drug statins (e.g. simvastatin, atorvastatin, and pravastatin) downregulate 3-hydroxy-3-methylglutaryl coenzyme A to improve plaque quality in atherosclerotic patients. Statins reduce macrophage accumulation and collagen degradation by reducing CD40 ligand/CD40 and expression of the adhesion molecule VCAM-1 (Libby & Aikawa, 2003).
4.2 Rheumatoid arthritis
Rheumatoid arthritis is a systemic inflammatory disorder that affects synovial joints. Patients with rheumatoid arthritis are more prone to atherosclerosis and have increased risks for MI and stroke (Symmons & Gabriel, 2011). The synovial fluid from patients of rheumatoid arthritis contains increased levels of MMP-9 (Ahrens, Koch, Pope, Stein-Picarella, & Niedbala, 1996; Yoshihara, et al., 2000). Proteolytic degradation of articular cartilage is one of the early features of the disease and is mediated by an increased activity of MMP-3, -8, and -9 (Tchetverikov, et al., 2003). In particular, MMP-9 cleavage of aggrecan releases multiple neo-epitopes that stimulate an immune response to both initiate the pathogenesis and aggravate the progression (Ram, Sherer, & Shoenfeld, 2006). MMP-9 increases in various autoimmune diseases such as systemic lupus erythematosus, Sjogren's syndrome, systemic sclerosis, multiple sclerosis, and polymyositis (Ram, et al., 2006). Therefore, MMP-9 is considered an important target for therapy in autoimmune diseases.
4.3 Cancer
Cancer is a disease of dysregulated tissue growth. As cancer progresses, the uncontrolled growth often metastasizes and becomes invasive, wherein the tumor cells spread to other locations in the body via the lymphatic system or the bloodstream. MMPs are involved in many cancer-related processes including invasion, metastasis, angiogenesis, and cell proliferation (Egeblad & Werb, 2002). Although a number of MMPs (MMPs -2, -7, -9, -11, and -14) are readily detected in most tumor types at all stages, the pattern of expression of other MMPs (MMP-1,-3 -8,-13) varies considerably by tumor type and stage.
Roy et al reviewed the role of specific MMPs as novel biomarkers in different types of cancer such as breast (MMP-1,-9,-13), pancreas (MMP-2,-7,-9), lung (MMP-1,-7,-9), bladder (MMP-2,-9), colorectal (MMP-1,-2,-7,-9,-13), ovarian (2,-9,-14), prostate (MMP-2,-9) and brain (MMP-2,-9) (Roy, Yang, & Moses, 2009). MMP-9 is in common to each of the above-mentioned cancers and has been proposed as an overarching biomarker of cancer. Further, MMP-9 has been shown to have epigenetic regulation that may provide additional biomarker candidates.
Cancer and cardiovascular disease intersect, as treatment strategies to fight cancer often induce cardiac dysfunction. One example of this is the use of anthracyclines (e.g., doxorubicin) as an anti-oncogenic therapy. Anthracyclines have known cardiotoxic side effects, including a significant activation of MMP-9 (Goetzenich, et al., 2009). The inflammatory component is also a strong connection between these two diseases.
4.4 Periodontal disease
Periodontal disease is broadly classified into two subgroups: periodontitis and gingivitis. Periodontitis is an inflammatory disease that mainly affects the supporting tissues of the teeth leading to the progressive destruction of connective tissue attachments to alveolar bone. Gingivitis is a non-destructive inflammatory disease characterized by an increased build-up of plaque on tooth surfaces. Longtime untreated gingivitis progressing to periodontitis is the most destructive form of periodontal disease. MMP-8 and MMP-9 are major diagnostic markers that have been well described in periodontal disease (Ramseier, et al., 2009). Periodontal disease shows a multifaceted pattern and progresses as a feed forward continuum of infection and inflammatory dysregulation with subsequent bone loss. Specific biomarkers, including MMP-8, MMP-9, IL-1β IL-6, and type I collagen pyridinoline cross-linked telopeptide (ICTP), have been used for periodontal disease identification (Ramseier, et al., 2009).
4.5 Diabetes mellitus and vascular complications
Diabetes mellitus stimulates a strong the immune system response by upregulating specific cytokines, chemokines, and leukocyte populations to contribute to increased vascular cell apoptosis and tissue fibrosis during plaque formation (Donath & Shoelson, 2011). The increase in macrophage numbers associates with reduced collagen content and MMP-9 overexpression in human diabetic plaques (Cipollone, et al., 2003). Furthermore, advanced glycation end products (the product of non-enzymatic glycation reactions stimulated by increased circulating glucose levels) stimulate COX-2/PGES-1 expression and induce MMP-9 synthesis in macrophages (Cipollone, et al., 2003; Kadoglou & Liapis, 2004). Diabetes, therefore, exacerbates the inflammatory response in atherosclerosis.
Abdominal aortic aneurysms (AAAs) are a chronic degenerative condition associated with a risk of vessel wall rupture. AAAs develop due to the progressive degradation of aortic wall elastin and collagen, and an increase in the local production of MMP-9 has been implicated in this process. The FDA approved MMP-9 inhibitor doxycycline reduces MMP-9 expression in human vascular wall cell types and in AAA tissue explants in vitro. Patients administered with doxycycline also show suppressed MMP-9 expression in the AAA tissue (Kadoglou & Liapis, 2004; Thompson & Baxter, 1999).
5 Future directions
Based on the many proximal effects of MMP-9 on cardiac remodeling and the many distal effects of MMP-9 on inflammatory diseases demonstrated in both pre-clinical and clinical studies, the further exploration of MMP-9 inhibitors is justified for the development of novel of cardiovascular agents that may benefit additional inflammatory diseases. Most currently used medications for heart failure (e.g., aldosterone antagonists, diuretics, ACE inhibitors, and beta-blockers) all decrease MMP-9 levels, indicating that screening for MMP-9 targets at early stages may help in the decision making process for cardiovascular drug discovery. While non-specific MMP inhibitor strategies have not proven useful,(Peterson, 2004) a specific inhibitor strategy that targets MMP-9 may prove effective. Several groups, however, are making headway in the MMP specific inhibitor arena (Johnson, et al., 2011; Robichaud, Steffensen, & Fields, 2011).
There are several investigation streams that remain to be explored, before the potential of MMP-9 to serve as a diagnostic marker can be fully realized. These include:
MMP-9 specificity and selectivity as a biomarker and comparative advantages over current gold standard biomarkers such as BNP, N-terminal pro-BNP, troponin, or CRP need to be determined.
The associations between MMP-9 levels and common risk factors for cardiovascular disease, including obesity, hypertension, smoking, diabetes, and dyslipidemia need to be dissected.
Spatial and temporal MMP-9 patterns during the cardiac remodeling continuum and during other inflammatory disease (e.g. cancer, arthritis, and periodontal disease) are needed.
The spatial and temporal patterns of MMP-9, compared with the patterns of other MMPs in cardiac remodeling and inflammatory diseases, need to be determined.
Standardized procedures and practices are needed for the pre-analytical, analytical, and post-analytical platforms to evaluate MMP-9 performance.
6 Conclusions
Current pre-clinical and clinical documentation strongly support MMP-9 as a panel member in the biomarker list to diagnose or treat the pathophysiology of post-MI ventricular remodeling and congestive heart failure. Immune cells such as neutrophils or macrophages modify many processes in the MI response, and future research focused on biochemical and structural approaches to examine the ECM will llikely provide new information on the remodeling process. Based on the evidence provided, further prospective studies are required to assess the prognostic value of MMP-9 for post-MI remodeling, particularly in comparison with traditional markers.
Acknowledgements
We acknowledge support from NIH-NCCAM 1K99AT006704-01 to GVH; from NSF 0649172, NIH EB009496, and NIH 1SC2 HL101430 to Y-FJ and from NHLBI HHSN 268201000036C (N01-HV-00244) for the UTHSCSA Cardiovascular Proteomics Center and R01 HL-075360, the Max and Minnie Tomerlin Voelcker Fund, and the Veteran's Administration (Merit) to MLL.
Abbreviations
- AAA
Abdominal aortic aneurysms
- ACS
acute coronary syndrome
- BNP
brain natriuretic peptide
- CRP
C-reactive protein
- ECM
extracellular matrix
- IL
Interleukin
- LV
left ventricle
- MI
myocardial infarction
- MMP
Matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement ML Lindsey and GV Halade have current grant funding from Amylin Pharmaceuticals. This article is unrelated to that project.
References
- Ahrens D, Koch AE, Pope RM, Stein-Picarella M, Niedbala MJ. Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis and rheumatism. 1996;39:1576–1587. doi: 10.1002/art.1780390919. [DOI] [PubMed] [Google Scholar]
- Balagopal PB, de Ferranti SD, Cook S, Daniels SR, Gidding SS, Hayman LL, McCrindle BW, Mietus-Snyder ML, Steinberger J. Nontraditional Risk Factors and Biomarkers for Cardiovascular Disease: Mechanistic, Research, and Clinical Considerations for Youth: A Scientific Statement From the American Heart Association. Circulation. 2011;123:2749–2769. doi: 10.1161/CIR.0b013e31821c7c64. [DOI] [PubMed] [Google Scholar]
- Bannikov GA, Karelina TV, Collier IE, Marmer BL, Goldberg GI. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J Biol Chem. 2002:M110931200. doi: 10.1074/jbc.M110931200. [DOI] [PubMed] [Google Scholar]
- Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix biology : journal of the International Society for Matrix Biology. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical pharmacology and therapeutics. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L, the AtheroGene Investigators Plasma Concentrations and Genetic Variation of Matrix Metalloproteinase 9 and Prognosis of Patients With Cardiovascular Disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Biomarkers in heart failure. The New England journal of medicine. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- Chancey AL, Brower GL, Peterson JT, Janicki JS. Effects of matrix metalloproteinase inhibition on ventricular remodeling due to volume overload. Circulation. 2002;105:1983–1988. doi: 10.1161/01.cir.0000014686.73212.da. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- Coker ML, Jolly JR, Joffs C, Etoh T, Holder JR, Bond BR, Spinale FG. Matrix metalloproteinase expression and activity in isolated myocytes after neurohormonal stimulation. American journal of physiology. Heart and circulatory physiology. 2001;281:H543–551. doi: 10.1152/ajpheart.2001.281.2.H543. [DOI] [PubMed] [Google Scholar]
- Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circulation research. 2001;89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- de Nooijer R, Verkleij CJ, von der Thusen JH, Jukema JW, van der Wall EE, van Berkel TJ, Baker AH, Biessen EA. Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:340–346. doi: 10.1161/01.ATV.0000197795.56960.64. [DOI] [PubMed] [Google Scholar]
- Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. Journal of molecular and cellular cardiology. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature reviews. Immunology. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature reviews. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Etoh T, Joffs C, Deschamps AM, Davis J, Dowdy K, Hendrick J, Baicu S, Mukherjee R, Manhaini M, Spinale FG. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. American journal of physiology. Heart and circulatory physiology. 2001;281:H987–994. doi: 10.1152/ajpheart.2001.281.3.H987. [DOI] [PubMed] [Google Scholar]
- Fang L, Du XJ, Gao XM, Dart AM. Activation of peripheral blood mononuclear cells and extracellular matrix and inflammatory gene profile in acute myocardial infarction. Clinical science. 2010;119:175–183. doi: 10.1042/CS20100011. [DOI] [PubMed] [Google Scholar]
- Fang L, Gao XM, Moore XL, Kiriazis H, Su Y, Ming Z, Lim YL, Dart AM, Du XJ. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. Journal of molecular and cellular cardiology. 2007;43:535–544. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Fertin M, Dubois E, Belliard A, Amouyel P, Pinet F, Bauters C. Usefulness of circulating biomarkers for the prediction of left ventricular remodeling after myocardial infarction. The American journal of cardiology. 2012;110:277–283. doi: 10.1016/j.amjcard.2012.02.069. [DOI] [PubMed] [Google Scholar]
- Figueras J, Cortadellas J, Soler-Soler J. Left ventricular free wall rupture: clinical presentation and management. Heart. 2000;83:499–504. doi: 10.1136/heart.83.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortmann SP, Ford E, Criqui MH, Folsom AR, Harris TB, Hong Y, Pearson TA, Siscovick D, Vinicor F, Wilson PF. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the population science discussion group. Circulation. 2004;110:e554–559. doi: 10.1161/01.CIR.0000148982.95775.BF. [DOI] [PubMed] [Google Scholar]
- Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovascular research. 2009;81:474–481. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 2005;65:469–477. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Gerszten RE, Asnani A, Carr SA. Status and prospects for discovery and verification of new biomarkers of cardiovascular disease by proteomics. Circulation research. 2011;109:463–474. doi: 10.1161/CIRCRESAHA.110.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzenich A, Hatam N, Zernecke A, Weber C, Czarnotta T, Autschbach R, Christiansen S. Alteration of matrix metalloproteinases in selective left ventricular adriamycin-induced cardiomyopathy in the pig. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2009;28:1087–1093. doi: 10.1016/j.healun.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Ciampa J, Lessard D, Meyer TE, Spencer FA. Long-term survival after heart failure: a contemporary population-based perspective. Archives of internal medicine. 2007;167:490–496. doi: 10.1001/archinte.167.5.490. [DOI] [PubMed] [Google Scholar]
- Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. The Journal of clinical investigation. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Gutierrez FR, Lalu MM, Mariano FS, Milanezi CM, Cena J, Gerlach RF, Santos JE, Torres-Duenas D, Cunha FQ, Schulz R, Silva JS. Increased activities of cardiac matrix metalloproteinases matrix metalloproteinase (MMP)-2 and MMP-9 are associated with mortality during the acute phase of experimental Trypanosoma cruzi infection. The Journal of infectious diseases. 2008;197:1468–1476. doi: 10.1086/587487. [DOI] [PubMed] [Google Scholar]
- Hansson J, Vasan RS, Arnlov J, Ingelsson E, Lind L, Larsson A, Michaelsson K, Sundstrom J. Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: cohort study. PloS one. 2011;6:e16185. doi: 10.1371/journal.pone.0016185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty KA, Pourmotabbed TF, Goldberg GI, Thompson JP, Spinella DG, Stevens RM, Mainardi CL. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem. 1990;265:11421–11424. [PubMed] [Google Scholar]
- Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- Hlatky MA, Ashley E, Quertermous T, Boothroyd DB, Ridker P, Southwick A, Myers RM, Iribarren C, Fortmann SP, Go AS. Matrix metalloproteinase circulating levels, genetic polymorphisms, and susceptibility to acute myocardial infarction among patients with coronary artery disease. Am Heart J. 2007;154:1043–1051. doi: 10.1016/j.ahj.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr., Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O'Donnell CJ, Smith SC, Jr., Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–293. doi: 10.1016/j.jacc.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Devel L, Czarny B, George SJ, Jackson CL, Rogakos V, Beau F, Yiotakis A, Newby AC, Dive V. A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2011;31:528–535. doi: 10.1161/ATVBAHA.110.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Lundberg A, Kalvegren H, Bergstrom I, Szymanowski A, Jonasson L. Increased levels of leukocyte-derived MMP-9 in patients with stable angina pectoris. PloS one. 2011;6:e19340. doi: 10.1371/journal.pone.0019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoglou NP, Liapis CD. Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Current medical research and opinion. 2004;20:419–432. doi: 10.1185/030079904125003143. [DOI] [PubMed] [Google Scholar]
- Kawakami R, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N, Nakagawa Y, Nakanishi M, Tanimoto K, Usami S, Yasuno S, Kinoshita H, Chusho H, Tamura N, Ogawa Y, Nakao K. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation. 2004;110:3306–3312. doi: 10.1161/01.CIR.0000147829.78357.C5. [DOI] [PubMed] [Google Scholar]
- Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, Squire IB. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. European heart journal. 2007;28:711–718. doi: 10.1093/eurheartj/ehm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantino Y, Nguyen TT, Wolk R, Aiello RJ, Terra SG, Fryburg DA. Potential implications of matrix metalloproteinase-9 in assessment and treatment of coronary artery disease. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2009;14:118–129. doi: 10.1080/13547500902765140. [DOI] [PubMed] [Google Scholar]
- Krishna R, Herman G, Wagner JA. Accelerating drug development using biomarkers: a case study with sitagliptin, a novel DPP4 inhibitor for type 2 diabetes. AAPS J. 2008;10:401–409. doi: 10.1208/s12248-008-9041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna R, Wagner JA. Applications of 'decisionable' biomarkers in cardiovascular drug development. Biomarkers in medicine. 2010;4:815–827. doi: 10.2217/bmm.10.107. [DOI] [PubMed] [Google Scholar]
- Lang CC, Mancini DM. Non-cardiac comorbidities in chronic heart failure. Heart. 2007;93:665–671. doi: 10.1136/hrt.2005.068296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer-Fields JL, Whitehead JK, Li S, Hammer RP, Brew K, Fields GB. Selective modulation of matrix metalloproteinase 9 (MMP-9) functions via exosite inhibition. The Journal of biological chemistry. 2008;283:20087–20095. doi: 10.1074/jbc.M801438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Ciancio SG, Tuter G, Ryan ME, Komaroff E, Golub LM. Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal anti-inflammatory drug. Journal of periodontology. 2004;75:453–463. doi: 10.1902/jop.2004.75.3.453. [DOI] [PubMed] [Google Scholar]
- Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. The American journal of cardiology. 2003;91:4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. 2001;103:2181–2187. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart failure reviews. 2004;9:7–19. doi: 10.1023/B:HREV.0000011390.44039.b7. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr., Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. American journal of physiology. Heart and circulatory physiology. 2006;290:H232–239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovascular research. 2005;66:410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Zamilpa R. Temporal and Spatial Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases Following Myocardial Infarction. Cardiovasc Ther. 2010 doi: 10.1111/j.1755-5922.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sendon J, Gurfinkel EP, Lopez de Sa E, Agnelli G, Gore JM, Steg PG, Eagle KA, Cantador JR, Fitzgerald G, Granger CB. Factors related to heart rupture in acute coronary syndromes in the Global Registry of Acute Coronary Events. European heart journal. 2010;31:1449–1456. doi: 10.1093/eurheartj/ehq061. [DOI] [PubMed] [Google Scholar]
- Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, Moons L. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004;109:1408–1414. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- Mano Y, Anzai T, Kaneko H, Nagatomo Y, Nagai T, Anzai A, Maekawa Y, Takahashi T, Meguro T, Yoshikawa T, Fukuda K. Overexpression of human C-reactive protein exacerbates left ventricular remodeling in diabetic cardiomyopathy. Circulation journal : official journal of the Japanese Circulation Society. 2011;75:1717–1727. doi: 10.1253/circj.cj-10-1199. [DOI] [PubMed] [Google Scholar]
- Martos R, Baugh J, Ledwidge M, O'Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–625. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Colbath GP, Justus CD, Bruce JA, Allen CM, Hewett KW, Saul JP, Gourdie RG, Spinale FG. Spatiotemporal induction of matrix metalloproteinase-9 transcription after discrete myocardial injury. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:3819–3828. doi: 10.1096/fj.10-155531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular research. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- Peppin GJ, Weiss SJ. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4322–4326. doi: 10.1073/pnas.83.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JT. Matrix Metalloproteinase Inhibitor Development and the Remodeling of Drug Discovery. Heart Fail Rev. 2004;9:63–79. doi: 10.1023/B:HREV.0000011395.11179.af. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. Journal of clinical immunology. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- Ramani R, Mathier M, Wang P, Gibson G, Togel S, Dawson J, Bauer A, Alber S, Watkins SC, McTiernan CF, Feldman AM. Inhibition of tumor necrosis factor receptor-1-mediated pathways has beneficial effects in a murine model of postischemic remodeling. American journal of physiology. Heart and circulatory physiology. 2004;287:H1369–1377. doi: 10.1152/ajpheart.00641.2003. [DOI] [PubMed] [Google Scholar]
- Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, Rayburn LA, Tran HM, Singh AK, Giannobile WV. Identification of pathogen and host-response markers correlated with periodontal disease. Journal of periodontology. 2009;80:436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon MJ, Carr CL, Diamond A, Letsou GV, Safi HJ, Espada R, Baldwin JC. Ischemic left ventricular free wall rupture: prediction, diagnosis, and treatment. Ann Thorac Surg. 1997;64:1509–1513. doi: 10.1016/S0003-4975(97)00776-5. [DOI] [PubMed] [Google Scholar]
- Richards AM. What we may expect from biomarkers in heart failure. Heart Fail Clin. 2009;5:463–470. doi: 10.1016/j.hfc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Robichaud TK, Steffensen B, Fields GB. Exosite interactions impact matrix metalloproteinase collagen specificities. J Biol Chem. 2011;286:37535–37542. doi: 10.1074/jbc.M111.273391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life sciences. 2001;68:799–814. doi: 10.1016/s0024-3205(00)00982-6. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis is an inflammatory disease. American heart journal. 1999;138:S419–420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009 doi: 10.1200/JCO.2009.23.5556. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley JM, Doyle GA, Fliszar CJ, Ye QZ, Johnson LL, Shapiro SD, Welgus HG, Senior RM. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. The Journal of biological chemistry. 1996;271:4335–4341. doi: 10.1074/jbc.271.8.4335. [DOI] [PubMed] [Google Scholar]
- Smith SC, Jr., Anderson JL, Cannon RO, 3rd, Fadl YY, Koenig W, Libby P, Lipshultz SE, Mensah GA, Ridker PM, Rosenson R. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the clinical practice discussion group. Circulation. 2004;110:e550–553. doi: 10.1161/01.CIR.0000148981.71644.C7. [DOI] [PubMed] [Google Scholar]
- Sorokin L. The impact of the extracellular matrix on inflammation. Nature reviews. Immunology. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circulation research. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Coker ML, Krombach SR, Mukherjee R, Hallak H, Houck WV, Clair MJ, Kribbs SB, Johnson LL, Peterson JT, Zile MR. Matrix metalloproteinase inhibition during the development of congestive heart failure : effects on left ventricular dimensions and function. Circulation research. 1999a;85:364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Coker ML, Krombach SR, Mukherjee R, Hallak H, Houck WV, Clair MJ, Kribbs SB, Johnson LL, Peterson JT, Zile MR. Matrix metalloproteinase inhibition during the development of congestive heart failure : effects on left ventricular dimensions and function. Circulation research. 1999b;85:364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- Squire IB, Evans J, Ng LL, Loftus IM, Thompson MM. Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. Journal of cardiac failure. 2004;10:328–333. doi: 10.1016/j.cardfail.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annual review of cell and developmental biology. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Sutherland P, Wilson PW, Vasan RS. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–2856. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nature reviews. Rheumatology. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Anzai T, Kaneko H, Mano Y, Anzai A, Nagai T, Kohno T, Maekawa Y, Yoshikawa T, Fukuda K, Ogawa S. Increased C-reactive protein expression exacerbates left ventricular dysfunction and remodeling after myocardial infarction. American journal of physiology. Heart and circulatory physiology. 2010;299:H1795–1804. doi: 10.1152/ajpheart.00001.2010. [DOI] [PubMed] [Google Scholar]
- Tandon A, Sinha S. Structural insights into the binding of MMP9 inhibitors. Bioinformation. 2011;5:310–314. doi: 10.6026/97320630005310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, Storrow AB, Christenson RH, Apple FS, Ravkilde J, Wu AH. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: Clinical utilization of cardiac biomarker testing in heart failure. Circulation. 2007;116:e99–109. doi: 10.1161/CIRCULATIONAHA.107.185267. [DOI] [PubMed] [Google Scholar]
- Tanner RM, Lynch AI, Brophy VH, Eckfeldt JH, Davis BR, Ford CE, Boerwinkle E, Arnett DK. Pharmacogenetic associations of MMP9 and MMP12 variants with cardiovascular disease in patients with hypertension. PloS one. 2011;6:e23609. doi: 10.1371/journal.pone.0023609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao ZY, Cavasin MA, Yang F, Liu YH, Yang XP. Temporal changes in matrix metalloproteinase expression and inflammatory response associated with cardiac rupture after myocardial infarction in mice. Life sciences. 2004;74:1561–1572. doi: 10.1016/j.lfs.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Tchetverikov I, Lard LR, DeGroot J, Verzijl N, TeKoppele JM, Breedveld FC, Huizinga TW, Hanemaaijer R. Matrix metalloproteinases-3, -8, -9 as markers of disease activity and joint damage progression in early rheumatoid arthritis. Annals of the rheumatic diseases. 2003;62:1094–1099. doi: 10.1136/ard.62.11.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RW, Baxter BT. MMP inhibition in abdominal aortic aneurysms. Rationale for a prospective randomized clinical trial. Annals of the New York Academy of Sciences. 1999;878:159–178. doi: 10.1111/j.1749-6632.1999.tb07682.x. [DOI] [PubMed] [Google Scholar]
- van den Borne SW, Cleutjens JP, Hanemaaijer R, Creemers EE, Smits JF, Daemen MJ, Blankesteijn WM. Increased matrix metalloproteinase-8 and -9 activity in patients with infarct rupture after myocardial infarction. Cardiovasc Pathol. 2009;18:37–43. doi: 10.1016/j.carpath.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. European journal of biochemistry / FEBS. 2003;270:3739–3749. doi: 10.1046/j.1432-1033.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- Velagaleti RS, Gona P, Larson MG, Wang TJ, Levy D, Benjamin EJ, Selhub J, Jacques PF, Meigs JB, Tofler GH, Vasan RS. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010;122:1700–1706. doi: 10.1161/CIRCULATIONAHA.109.929661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003;108:1487–1492. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- Yan AT, Yan RT, Spinale FG, Afzal R, Gunasinghe HR, Arnold M, Demers C, McKelvie RS, Liu PP. Plasma matrix metalloproteinase-9 level is correlated with left ventricular volumes and ejection fraction in patients with heart failure. Journal of cardiac failure. 2006;12:514–519. doi: 10.1016/j.cardfail.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ma Y, Han W, Li J, Xiang Y, Liu F, Ma X, Zhang J, Fu Z, Su YD, Du XJ, Gao XM. Age-related differences in postinfarct left ventricular rupture and remodeling. American journal of physiology. Heart and circulatory physiology. 2008;294:H1815–1822. doi: 10.1152/ajpheart.00831.2007. [DOI] [PubMed] [Google Scholar]
- Yarbrough WM, Mukherjee R, Escobar GP, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Lowry AS, O'Neill TP, Spinale FG. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation. 2003;108:1753–1759. doi: 10.1161/01.CIR.0000091087.78630.79. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Annals of the rheumatic diseases. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamilpa R, Kanakia R, Cigarroa J. t., Dai Q, Escobar GP, Martinez HM, Jimenez F, Ahuja S, Lindsey ML. CC Chemokine Receptor 5 Deletion Impairs Macrophage Activation and Induces Adverse Remodeling Following Myocardial Infarction. American journal of physiology. Heart and circulatory physiology. 2011 doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following MI: causes and consequences. Journal of molecular and cellular cardiology. 2010;48:558–563. doi: 10.1016/j.yjmcc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, Weintraub ST, Lindsey ML. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics. 2010;10:2214–2223. doi: 10.1002/pmic.200900587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Ye S, Herrmann SM, Eriksson P, de Maat M, Evans A, Arveiler D, Luc G, Cambien F, Hamsten A, Watkins H, Henney AM. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation. 1999;99:1788–1794. doi: 10.1161/01.cir.99.14.1788. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu Y, Lee HM, Hambardjieva E, Vrankova K, Golub LM, Johnson F. Design, synthesis and biological activity of new polyenolic inhibitors of matrix metalloproteinases: a focus on chemically-modified curcumins. Current medicinal chemistry. 2012;19:4348–4358. doi: 10.2174/092986712802884295. [DOI] [PubMed] [Google Scholar]