Abstract
Curcuma comosa Roxb. is ginger-family plant used to relieve menopausal symptoms. Previous work showed that C. comosa extracts protect mice from ovariectomy-induced osteopenia with minimal effects on reproductive organs, and identified the diarylheptanoid (3R)-1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol (DPHD) as the major active component of C. comosa rhizomes. At 1–10 μM, DPHD increased differentiation in transformed mouse osteoblasts, but the effect of DPHD on normal bone cells was unknown. We examined the concentration dependency and mechanism of action of DPHD relative to 17β-estradiol in nontransformed human osteoblasts (h-OB). The h-OB were 10–100 fold more sensitive to DPHD than transformed osteoblasts: DPHD increased h-OB proliferation at 10 nM and, at 100 nM, activated MAP kinase signaling within 30 minutes. In long-term differentiation assays, responses of h-OB to DPHD were significant at 10 nM, and optimal response in most cases was at 100 nM. At 7–21 days, DPHD accelerated osteoblast differentiation, indicated by alkaline phosphatase activity and osteoblast-specific mRNA production. Effects of DPHD were eliminated by the estrogen receptor antagonist ICI182780. During differentiation, DPHD promoted early expression of osteoblast transcription factors, RUNX2 and osterix. Subsequently, DPHD accelerated production of bone structural genes, including COL1A1 and osteocalcin comparably to 17β-estradiol. In h-OB, DPHD increased the osteoprotegerin to RANKL ratio and supported mineralization more efficiently than 10 nM 17β-estradiol. We conclude that DPHD promotes human osteoblast function in vitro effectively at nanomolar concentrations, making it a promising compound to protect bone in menopausal women.
Keywords: Curcuma comosa, Diarylheptanoid, ERK1/2, Osteoporosis, Phytoestrogen, Selective estrogen receptor modulator
Introduction
Postmenopausal osteoporosis is a major public health problem that reduces the quality of life of many older women (Gallagher and Levine, 2011). An ideal therapy for prevention of bone loss would be a substance that reduces bone resorption, and enhances bone formation, with low toxicity (Stevenson et al., 2005). Development of osteoporosis, in major part, reflects the rapid decline in estrogen production (Riggs et al., 2002). Estrogen replacement therapy is effective in protecting bone loss in postmenopausal women (Bernstein, 2006). However, long term used it increases risk of breast and uterine cancers (Becker, 2008). Phytoestrogens, naturally occurring non-steroidal compounds from plants that have biological activities similar to those of estrogen in some, but not all, target tissues, are potential alternatives to estrogen replacement. Many phytoestrogens are selective estrogen receptor modulators (SERMS) that inhibit bone loss with limited effects on reproductive tissues (Hadji, 2012). But problems with phytoestrogens have included low potency, with micromolar concentrations of phytoestrogens required to achieve significant effects in most cases.
Curcuma comosa Roxb. (C. comosa) is ginger-family plant extensively used as a supplement to relieve unpleasant menopausal symptoms. Its hexane extract prevented bone loss in ovariectomized mice (Weerachayaphorn et al., 2011), with much less effect on uterine weight than 17β-estradiol (E2), highlighting its potential utility as a selective estrogen receptor modulator that might have fewer side effects than E2 itself. Recently, a diarylheptanoid was isolated from the hexane extract of C. comosa, (3R)-1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol (hereafter DPHD), and identified as the major active compound (Suksamrarn et al., 2008). DPHD exerts the estrogenic activity at the transcription level similar to that of E2 both in vitro and in vivo (Winuthayanon et al., 2009a,b). DPHD increased differentiation of transformed mouse osteoblasts, but only at high concentrations, 1–10 micromolar. The mechanism, in transfected transformed mouse cells, included activation of estrogen receptor/Akt/glycogen syntheses kinase 3β activation of Wnt/β-catenin signaling (Bhukhai et al., 2012). On the other hand, the response of normal human bone cells to DPHD was untested.
This work tested DPHD using nontransformed bone-forming cells in steroid-free media. 17β-estradiol (E2) was used as a positive control, and the antiestrogen ICI182780 was used to control for the possibility that some effects might occur by non-estrogen receptor-dependent mechanisms. To assure relevancy of the results to human metabolism, we used osteoprogenitor-enriched human (hOB) cells (Zaidi et al., 2012). This cell system allows evaluation of bone formation in the presence or absence of cytokines and metabolic activators or inhibitors targeting specific pathways (Robinson et al., 2012); the precursor cells can be expanded in vitro so that experiments compare nontransformed, but identical, cell preparations, to avoid pitfalls of differences in cell isolate activity or response. We report that DPHD at 10–100 nM produced significant increases in h-OB proliferation and promoted the production of bone-specific proteins and of mineralized bone in vitro, in some cases to a greater extent than E2 itself, indicating unique potency and efficacy relative to other phytoestrogens.
Materials and Methods
Isolation of the active compound from C. comosa
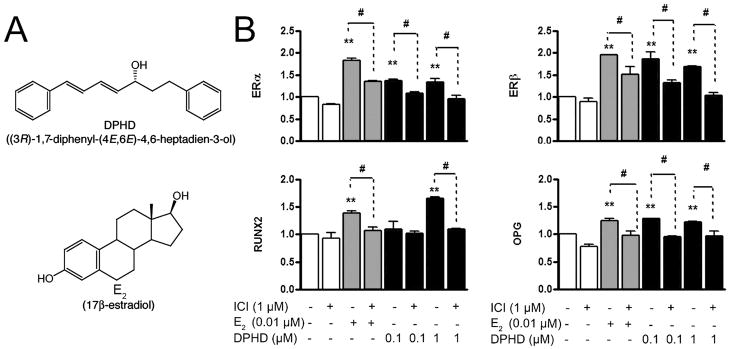
Diarylheptanoid (3R)-1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol, DPHD (Fig. 1A), was prepared as described (Suksamrarn et al., 2008). Briefly, rhizomes of C. comosa were purchased from Kampaengsaen district, Nakhon Pathom province, Thailand, and subjected to taxonomic identification with voucher herbarium specimen (SCMU No. 300) deposited at the Department of Plant Science, Faculty of Science, Mahidol University, Bangkok. The rhizomes of C. comosa were cut into small pieces, dried and ground to powder. The powder was extracted with n-hexane in a Soxhlet extractor and, after removal of the solvent in vacuo, pale brown viscous oil was obtained. The DPHD was isolated from the hexane extract as a major component (23.9%) by repeated silica gel column chromatography, eluting with hexane-dichloromethane, each step with an increasing quantity of the more polar solvent. The structure of DPHD was confirmed and the absolute stereochemistry at the 3-position was determined to be R, the same as that of DPHD previously isolated, by nuclear magnetic resonance and mass spectroscopy (Suksamrarn et al., 2008).
Fig. 1. Effect of DPHD on osteoblast mRNAs at seven days.
A. Structures of (3R)-1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol (DPHD) and 17β-estradiol (E2).
B. Expression of estrogen receptors (ER) α and β, RUNX2 and OPG mRNA in total RNA from h-OB not treated (open bars), treated with E2 (0.010 μM, gray bars) or treated with DPHD (0.10 or 1.0 μM, filled bars), with and without the ER antagonist ICI182780 (1 μM) for 7 days. The qPCR data were normalized to GAPDH. Each value is Mean±SEM from 3 independent experiments; **p < 0.01 relative to controls (open bars); #p < 0.05 relative to addition of ICI187280.
Materials
Thymidine, [methyl-3H] was from New England Nuclear (Waltham, MA). Media were from Lonza (Walkersville, MD). Test compounds were added in dimethylsulfoxide (DMSO) at final DMSO concentrations <0.05%; controls contained equal DMSO. Rabbit monoclonal anti-phospho-ERK1/2 (p-Thr202/Tyr204, clone D13.14.4E, #4370) was from Cell Signaling (Danvers, MA). Rabbit polyclonal anti ERK1/2 (C-terminal 16-mer antigen, #SC93) and anti-c-Src (clone H-12, #SC5266) were from Santa Cruz (Santa Cruz, CA). Rabbit polyclonal anti-phospho-Src family (#2101, to p-Tyr416), rabbit monoclonal anti-phospho-Akt (to p-Thr308, clone C31E5E, #2965), and polyclonal rabbit anti-Akt (#9272, C-terminal peptide-specific), were from Cell Signaling. Mouse monoclonal anti-β-actin (clone AC74) was from Sigma (St Louis, MO).
Cell cultures
Human osteoprogenitor cells from Lonza, #CC-2538, were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. These cells are isolated from the bone of human donors and are a sub-population that remains division-competent for ten or more generations, but in which the majority of cells produce bone alkaline phosphatase at low levels, and thus are a nontransformed, pre-osteoblastic proliferation-competent bone cell enriched fraction (Zaidi et al., 2012). Serum was charcoal stripped to eliminate endogenous steroids. Cells were expanded twice and differentiated at confluence in the third passage. Differentiation medium added 200 nM hydrocortisone, 10 mM 2-glycerol phosphate, 50 μg/ml ascorbic acid, 1 nM 1,25 dihydroxyvitamin D3, and contained 2 mM total calcium. Media were replaced at three day intervals.
Growth assays
The MTT assay for viable cells was performed in 2 cm2 wells with 18,000 cells; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 0.5 mg/ml, was added and cultures were incubated at 37 °C for 4 hours. DMSO was added to solubilize the formazan crystals and the blue reaction product was quantified as absorbance at 570 nm. Thymidine incorporation assay was conducted similar to that of the MTT assay but [methyl-3H]-thymidine was included in the media, 1.0 μCi/ml, for 18 hours; cells were washed, trypsinized, transfered to microfuge tubes, and incubated in cold 5% trichloroacetic acid for 30 minutes. Precipitates were collected, washed, dissolved in 1 M NaOH, and label was determined by scintillation counting.
PCR
RNA was extracted using phenol and guanidine isothiocyanate (Trizol, Invitrogen, Grand Island, NY) and quantified by absorbance at 260 nm. Reverse transcription used 500 ng of RNA, random hexamer primers, and MMLV reverse transcriptase (Superscript III, Invitrogen) in a final volume of 20 μl. Quantitative PCR (qPCR) was performed using the indicator SYBR III green (Stratagene, La Jolla, CA) (Robinson et al., 2012). RNAs were quantified using cycle threshold relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Blair et al., 1996). For PCR primers, see Table 1.
Table 1.
Primers for PCR assays
| Gene | Product size | Primers |
|---|---|---|
| GAPDH | 238 bp | f-GAGTCAACGGATTTGGTCGT r-TTGATTTTGGAGGGATCTCG |
| RUNX2 | 247 bp | f-CCTCGGAGAGGTACCAGATG r-TTCCCGAGGTCCATCTACTG |
| OSX | 161 bp | f-GCCAGAAGCTGTGAAACCTC r-GCTGCAAGCTCTCCATAACC |
| ALP | 182 bp | f-CCTTGCTCACTCACTCACTCC r-TTTTTTTTGCCGTTCCAAAC |
| COL1A1 | 108 bp | f-AGGGCCAAGACGAAGACATCCC r-TGTCGCAGACGCAGATCCG |
| Osteocalcin | 152 bp | f-GTGCAGAGTCCAGCAAAGGT r-TCAGCCAACTCGTCACAGTC |
| OPG | 160 bp | f-AACGCCAACACAGCTCACAAGAAC r-TGCTCGAAGGTGAGGTTAGCATGT |
| RANKL | 152 bp | f-ATCGTTGGATCACAGCACATC r-AGACTCACTTTATGGGAACCAGA |
| ERα | 125 bp | f- TGATGAAAGGTGGGATACGA r- AAGGTTGGCAGCTCTCATGT |
| ERβ | 165 bp | f-TCTTGGAGAGCTGTTGGATG r- AAGTAGTTGCCAGGAGCATG |
Western blots
Cells were lysed in 150 mM NaCl, 10 mM EDTA, 50 mM Tris, 1 mM EDTA, 1 mM orthovanadate, 15 mM NaF, 1% polyoxyethyleneoctylphenyl ether, 10% glycerol, and proteinase inhibitors, pH 7.4, for 10 minutes, on ice. Lysates were centrifuged to remove cell debris. Protein concentrations were determined using by bicinchoninic acid dye binding (BCA assay, Pierce, Rockford, IL). For blots, each sample contained 20 μg of protein; lysates were diluted with 6X sample buffer, denatured at 100 °C, 5 minutes, separated by SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. Nonspecific binding was blocked, 1 hour in 5% BSA in Tris-buffered saline-Tween 20. Membranes were incubated in primary antibody at 1:1000 18 hours at 4 °C. After washing the membranes, horseradish peroxidase-conjugated-FAB secondary antibodies were added at 1:5000 (anti-rabbit or mouse IgG), 1 hour at 20 °C, and visualized by enhanced chemiluminescence (Pierce ECL, Thermo- Fischer, Rockford, IL). For re-probing, blots were stripped with Restore Plus (Thermo-Fischer).
Enzyme assays and Stains
Lysate alkaline phosphatase (ALP) activity was assayed using p-nitrophenolphosphate as the substrate, at pH 10.2, measuring the yellow product as absorbance at 405 nm. ALP in cell cultures was determined using naphthol phosphate substrate and fast violet B to visualize the product, at pH 9.5 (alkaline phosphatase kit, Sigma, St. Louis, MO). Silver (von Kossa) to stain phosphate deposits black was done, after rinsing cultures with PBS, using 2% AgNO3 under UV light for 15 minutes and fixing with 2.5% Na2S2O3.
Statistical analysis
Data are expressed as mean±SEM. Individual comparisons used the unpaired Student’s t-test; multiple data sets were analyzed by one-way analysis of variance (Newman-Keuls) using Prism 5.0 (GraphPad, San Diego, CA).
Results
DPHD promotes ER-dependent transcription of genes in nontransformed human osteoblasts
The phytoestrogen DPHD lacks the steroid skeleton and A ring hydroxylation of 17β-estradiol (Fig. 1A), but despite this, DPHD showed ER promoter activity in plasmid reporter assays (Winuthayanon et al., 2009). In keeping, in h-OB treated with E2 (0.01 μM) or DPHD (0.1 or 1 μM) for seven days, mRNAs for ERs α and β, the osteoblast transcription factor RUNX2, and the RANKL-binding decoy receptor (Fig. 1B) were upregulated; these differences were all blocked by ICI182780.
Effect of DPHD on key kinase pathways, and on proliferation, in nontransformed h-OB cells
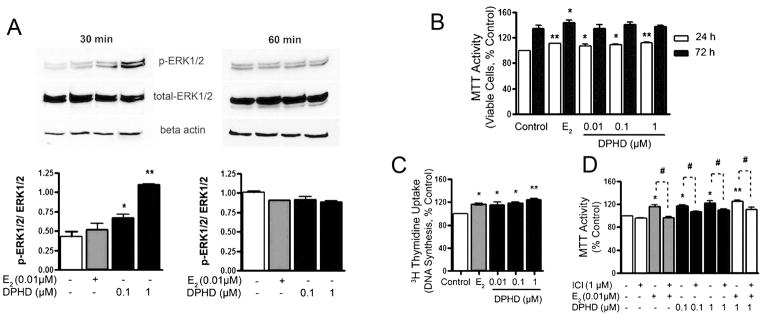
ERs also participate in non-nuclear pathways including regulation of mitogen activated protein (MAP) kinase (Erk1/2), Akt (protein kinase B, a serine/threonine-specific protein kinase), and the intracellular tyrosine kinase Src. These typically occur in ~30 minutes and reflect estrogen effects not dependent on transcription, also called nongenomic estrogen receptor-mediated responses. We studied the effect of DPHD on these kinases at 30 and 60 minutes by determining the phosphorylated target followed by whole enzyme. DPHD convincingly induced ERK phosphorylation at 30 minutes in E2 and in 0.1 and 1 μM DPHD with concentration dependence. DPHD effects at 1 μM were larger than those with E2 (Fig. 2A). The effects faded at 60 minutes.
Fig. 2. Effect of DPHD on cell proliferation and on Erk1/2 kinase.
A. Expression of phospho-Erk1/2 were shown at 30 minutes (left) and at 60 minutes (right). Reprobing for total Erk1/2 and β-actin show equivalent Erk and total protein. The bar graphs (bottom) show densitometry for p-Erk/Erk.
B. Human osteoblast precursor cells in growth medium containing compounds for 24 hours (open bars) and 72 hours (filled bars), evaluated using the MTT assay.
C. Thymidine incorporation at 24 hours. n=4.
D. To confirm estrogen-receptor dependence, MTT assays at 24 hours were done using ICI182780 without treatment (open bars) and with 10 nM E2 (gray bars) or 0.1–1 μM DPHD (filled bars). Each value is Mean±SEM from 3 independent experiments, otherwise indicated; *p < 0.05 or **p < 0.01 relative to controls.
The h-OB cells retain capacity for cell division until they differentiate, and since MAP kinases are important in growth (Noda-Seino et al., 2012), we queried whether DPHD would replicate effects of E2 on cell survival or proliferation. Addition of DPHD increased the number of viable h-OB at 0.01, 0.1, and 1 μM at 24 hours (p < 0.05, Fig. 2B), with 0.01 μM E2 and 1 μM DPHD havingindistinguishable effects. At 72 hours, differences between E2, DPHD, and controls were reduced. This reflects that, in differentiating cells, growth tails off. The MTT method quantifies viable cells. That this difference reflected increased cell proliferation and not cell death was confirmed by 3H-thymidine uptake (Fig. 2C). This sensitive assay showed that even the lowest dose of DPHD, 0.01 μM, had measurable effects on proliferation. Further to confirm that the cell proliferation effect was estrogen-receptor dependent, additional MTT assays were done using ICI182780 controls. In all cases, ICI182780 reduced proliferation (p < 0.05), and there was no additive effect of E2 and DPHD together (Fig. 2D). These results indicate that E2 or DPHD increase the rate of entry into cell division by non-cooperative pathways that are blocked by ICI182780.
Effect of DPHD on nontransformed osteoblast cell differentiation and maturation
A key indicator of osteoblast maturation is the membrane-bound ectoenzyme alkaline phosphatase (ALP). ALP activity was measured in cell lysates (Fig. 3A). Estrogen and all concentrations of DPHD promoted alkaline-phosphatase expression in osteoblasts compared to controls (p < 0.01). In situ activity, using naphthol phosphatase substrate, they gave consistent results (Fig. 3B). Thus, DPHD at concentrations as low as 0.01 μM (10 nM) may have significant anabolic effects on bone formation.
Fig. 3. Effect of DPHD on alkaline phosphatase (ALP) activity.
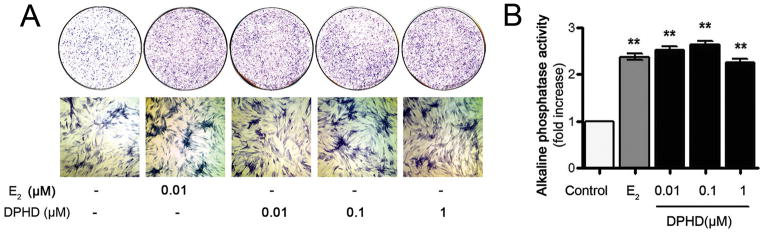
A. In situ ALP activity in cells in differentiation medium containing the compounds indicated for 21 days. The upper row shows alkaline phosphatase activity of 4 cm whole osteoblast cultures. The lower row shows photomicrographs of fields of the same cultures, 1.6 mm across, in transmitted light.
B. Quantitative ALP activity in cell lysates at 21 days. Data are fold increase of ALP activity of E2 (gray bar) and all concentrations of DPHD (closed bars) treatment relative to controls (open bar). Each value is Mean±SEM from 3 independent experiments; ** p < 0.01 relative to controls.
Effect of DPHD on expression of characteristic osteoblast mRNAs
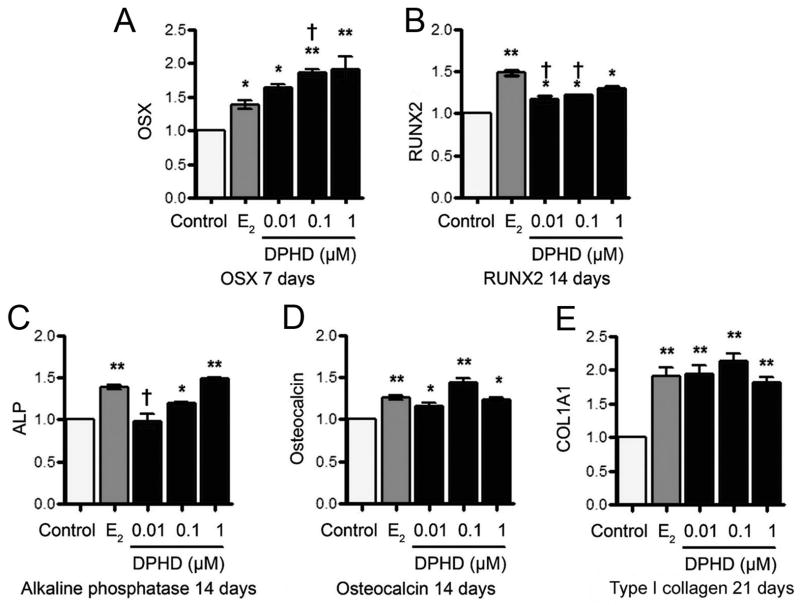
Despite the similarity of response of h-OB to E2 and DPHD in Erk1/2 response, cell proliferation, and ALP expression, it was likely that quantitative differences in specific transcriptional targets exist. We compared response of E2 to DPHD for characteristic osteoblast mRNAs at 7, 14, and 21 days in differentiation medium. Key findings are summarized in Fig. 4A–D. In differentiation medium, 10 nM E2 or 1 μM DPHD increased mRNA for the major osteoblast transcription factors osterix and RUNX2 at 1, 2, and 3 weeks. The most consistent and largest overall effects were seen at 7 days for OSX and at 14 days for RUNX2 (Fig. 4A–B). The increases in osterix and RUNX2 were highly significant and averaged ~50% over controls. This was consistent with an increased rate of osteoblast differentiation while not affecting osteoblast features. In this regard, osterix was more strongly induced by DPHD than by estrogen, while RUNX2 responded better to E2.
Fig. 4. Effect of DPHD on the mRNAs in differentiating osteoblasts.
The mRNA expression of osteoblast transcription factors, osterix (A), RUNX2 (B), ALP (C), Osteocalcin (D) and type I collagen (COL1A1) (E) are shown at 7 and 14 days, respectively. In each case 17β-estradiol (E2) was the positive control; results by qPCR are normalized to GAPDH. Each value is Mean±SEM from 3 independent experiments; *p < 0.05, *p < 0.01 versus control; †p < 0.05 versus E2.
Functional bone-forming and structural genes; ALP, osteocalcin, and type I collagen (COL1A1) (Fig. 4C–E) were also increased by DPHD and E2 consistently beginning at 14 days, at or after the peak of increased transcription factor expression, in keeping with structural gene expression being in major part dependent on osterix and RUNX2. At 7 day type I collagen was not increased relative to the controls and only the highest concentration of DPHD showed significant increases in ALP or osteocalcin (not shown). By 14 days ALP and OCN were increased in DPHD similarly to E2 (Fig. 4C–D). At 21 days, COL1A1 reached the highest and most consistent transcription in all concentrations of DPHD and E2 (Fig. 4E). These findings suggest that DPHD at 0.1–1 μM has effects comparable to E2 on osteoblast differentiation.
Unique effects of DPHD on bone matrix production and on osteoclast regulatory proteins
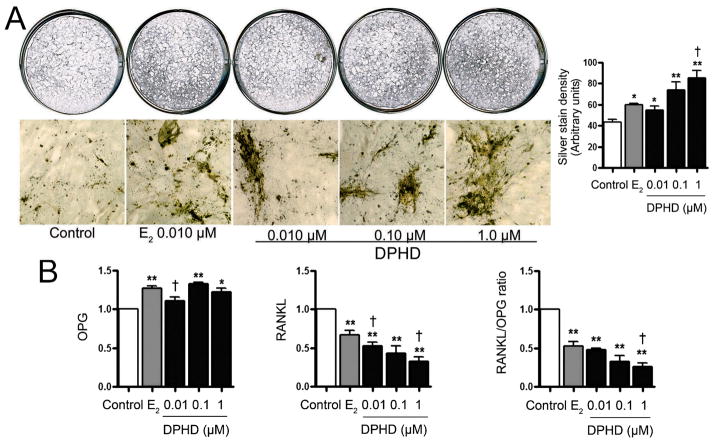
To evaluate the effect of DPHD on terminal differentiation of nontransformed osteoblasts, we evaluated mineralization of osteoblast-produced bone matrix using von Kossa silver staining at 21 days. In Fig. 5A, whole culture and low power photomicrographs and quantification of staining show that E2 induces extracellular mineral deposition, which show as dark material in transmitted light. All of the 10 nM–1 μM DPHD treated h-OB cultures had increased matrix production, and at 1 μM matrix in DPHD exceeded that in 10 μM E2 (p < 0.05). We also examined the effect of estrogen and DPHD on osteoprotegerin (OPG) and RANKL mRNAs. An increase in the ratio of OPG to RANKL in osteoblast cells is reported to mediate a bone sparing effect (Wang et al., 2012). We found that in hOB cells at 21 days in differentiation medium, both E2 and DPHD increased OPG and suppressed RANKL production significantly (p < 0.05–0.01, left and middle panels, Fig. 5B). DPHD showed a dose responsive effect on RANKL mRNA that significantly exceeded effect of E2. Thus, DPHD selective effects supporting bone preservation differed from, and in some conditions exceeded, E2 response.
Fig. 5. Effect of DPHD on matrix mineralization and on RANKL and osteoprotegerin mRNAs.
A. Cell cultures at 21 days (top, 4 cm diameter) and photomicrographs of cultures (bottom, 1.6 mm fields) show mineralizing matrix by AgNO3 stain (black), quantified by densitometry in the bar graph (right). E2 (gray bar) and DPHD (closed bars) increased mineralization relative to controls. Mean±SEM from four independent measurements; *p < 0.05, *p < 0.01 versus control; †p < 0.05 versus 10 nM E2.
B. Effect of DPHD (closed bars) on Osteoprotegerin, RANKL mRNAs and the ratio of RANKL/OPG. Values are Mean±SEM from three experiments; *p < 0.05, *p < 0.01 versus control; †p < 0.05 versus E2.
Discussion
Although phytoestrogens in the diet have been promoted widely for many years, clinical studies generally show modest or marginal effects on bone mass in older adults including postmenopausal women (Kuhnle et al., 2011; Shedd-Wise et al., 2011). This reflects, in large part, that the effective concentrations of the nutritional supplements are higher than those achievable, and indicate the importance of seeking out compounds that have the desired estrogen like effects on bone but minimal effects on reproductive organs at sub-micromolar concentrations. Here we demonstrate promising osteogenic effects, at nanomolar concentrations, of a recently discovered phytoestrogen diarylheptanoid, (3R)-1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol (DPHD) isolated from C. comosa, (Suksamrarn et al., 2008) using nontransformed human osteoblasts. In these cells, DPHD promoted proliferation, differentiation, and reduced production of RANKL relative to OPG at concentrations as low as 10 nM. This is 100 to 300 fold lower than concentrations that produce similar effects using isoflavone phytoestrogens (Robinson et al., 2009). Human nontransformed cells were 10–100 fold more sensitive to DPHD than transformed murine osteoblasts (Bhukhai et al., 2012).
The effects of DPHD on human osteoblasts were similar to those of E2, and included both nongenomic and transcriptional effects. The transcriptional effects, evaluated by qPCR, were effectively reversed by the ER antagonist ICI182780, confirming that these effects of DPHD are by an ER-dependent pathway despite the structural dissimilarity of DPHD and E2 (Fig. 1). The magnitude of the response varied, depending on the target, between E2 and DPHD, with RUNX2 responding only the higher concentration of DPHD, and with stronger response to E2 in most assays. The effect on ERs α and β shows that h-OBs did not respond to E2 or DPHD by down-regulation of ERs.
Studies in nontransformed human osteoblasts showed that DPHD, at low concentrations, had selective effects of bone preserving pathways that exceed the effects of E2, specifically the effect on RANKL and OPG mRNAs. This is important because ERβ mediates a bone sparing effect via an increase in the ratio of OPG to RANKL, in a manner differentially affected by steroids other than E2, including dihydroepiandrosterone (Wang et al., 2012). Transformed osteoblast lines have poor response of OPG and RANKL even to E2 (Cao et al., 2003). But in h-OB cells in differentiation medium, both E2 and DPHD increased OPG and suppressed RANKL, but DPHD gave a dose response for RANKL mRNA that extended well beyond the effect of E2 (p < 0.05 relative to E2, Fig 4B). Thus, low concentrations of DPHD, such as found in the traditional medicine, might have effects in preserving bone mass, via inhibiting RANKL relative to OPG, that exceed those of estrogen itself.
Estrogen and phytoestrogens affect both bone formation and resorption by a wide variety of nongenomic and genomic effects (Dang and Lowik, 2005) that vary widely between agents and between specific tissue targets. Here, we show that DPHD at concentrations as low as 10 nM positively affects the differentiation in nontransformed human osteoblasts. Bone loss in postmenopausal women reflects, in a major part, impairment of bone formation (Raisz, 2005). Matched cell cultures comparing E2 and DPHD treatment to controls showed that E2 and DPHD similarly promoted osteoblast proliferation as well as differentiation. However, DPHD had stronger effects on MAP kinase activation and on some gene transcription, particularly reducing RANKL mRNA (Fig. 5B), which may make DPHD an excellent selective agent to reduce bone loss in the menopause. Importantly, these effective doses of DPHD in promoting bone formation did not stimulate the specificity of the estrogenic response in a highly-estrogen sensitive human breast epithelial cell line, MCF-7 (Winuthayanon et al., 2009b). Further, the hexane extract of C. comosa in ovariectomized mice (Weerachayaphorn et al., 2011) prevented bone loss with much less effect on the increased uterine weight than E2, highlighting its potential utility as a selective estrogen receptor modulator.
The best characterized of many non-genomic estrogen-activated cytoplasmic pathways is rapid activation of the MAP kinases Erk1/2 (Endoh et al., 1997). This response is, according to most studies, mediated in major part by a short transcriptional variant of ERα, ERα-36 (Kang et al., 2010). The direct estrogen action stimulating Erk1/2 was clearly seen in the h-OB cells and this effect was particularly strongly stimulated by DPHD (Fig. 2), more strongly than by 17β-estradiol itself, suggesting strongly that the response of DPHD and E2 are diverge significantly and that the nongenomic activity may be particularly a DPHD target. This might reflect greater potential for nongenomic effects of compounds, such as DPHD, with structures less similar to E2 (Fig. 1A).
In keeping with the MAP kinase activation, E2 and DPHD increased cell number and DNA synthesis at one day (Fig. 2). Subsequently, this effect faded as the cells differentiated, reflecting that differentiating cells cease proliferation. However, an effect on osteoblast differentiation was retained, as reflected in ALP activity, and this effect was note dependent on an increase in cell number, but reflected increased activity per cell protein (Fig. 2B–C). In longer-term h-OB cultures, DPHD accelerated osteoblast differentiation overall, indicated by ALP activity, mineral production and osteoblast-specific mRNAs including the osteoblast transcription factors osterix and RUNX2, as well as the functional bone matrix-related proteins ALP, osteocalcin, and type I collagen (Aubin, 1998) (Fig. 4C–E). In only one respect DPHD was more effective than E2 in regulating osteoblast gene expression, in the ratio of osteoprotegerin to RANKL mRNA (Fig. 5B). SERMs often have idiosyncratic effects on specific transcription products (Doran et al., 2001) that can only be determined experimentally. This finding holds promise that DPHD might reduce bone turnover at concentrations that avoid significant effects on reproductive organs (Winuthayanon et al., 2009b).
In conclusion, our study using nontransformed human osteoblasts provides the first clear evidence that DPHD has osteogenic activity on human cells via ER-dependent pathways. At nanomolar concentrations, DPHD promoted h-OB proliferation and differentiation. The effects of DPHD on h-OB included ER-dependent nongenomic short term effects on Erk1/2 phosphorylation and activation of osteoblastic genes. The genomic effects were selective; effects on osteoblast development at DPHD concentrations as low as 10 nM. The high sensitivity of h-OB and the much smaller effects of DPHD on breast or uterine targets highlight the potential of DPHD as a bone-specific agent.
Acknowledgments
This study was supported by Thailand Research Fund (TRF), Royal Golden Jubilee PhD Program grant PHD/0103/2549 (DS and PP); Office of the Higher Education Commission and Mahidol University, National Research Universities Initiative, Strategic Basic Research Grant, TRF (AS); National Institutes of Health (USA) awards AR053566 (LJR), AR055208 (HCB); Department of Veteran’s Affairs (USA) (HCB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aubin JE. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76:899–910. [PubMed] [Google Scholar]
- Becker C. Pathophysiology and clinical manifestations of osteoporosis. Clin Cornerstone. 2008;9:42–47. doi: 10.1016/s1098-3597(09)62038-x. discussion 48–50. [DOI] [PubMed] [Google Scholar]
- Bernstein L. The Risk of Breast, Endometrial and Ovarian Cancer in Users of Hormonal Preparations. Basic & Clinical Pharmacology & Toxicology. 2006;98:288–296. doi: 10.1111/j.1742-7843.2006.pto_277.x. [DOI] [PubMed] [Google Scholar]
- Bhukhai K, Suksen K, Bhummaphan N, Janjorn K, Thongon N, Tantikanlayaporn D, Piyachaturawat P, Suksamrarn A, Chairoungdua A. A phytoestrogen diarylheptanoid mediates ER/Akt/GSK-3beta-dependent activation of Wnt/beta-catenin signaling pathway. J Biol Chem. 2012 doi: 10.1074/jbc.M112.344747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HC, Jordan SE, Peterson TG, Barnes S. Variable effects of tyrosine kinase inhibitors on avian osteoclastic activity and reduction of bone loss in ovariectomized rats. J Cell Biochem. 1996;61:629–637. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C629::AID-JCB17%3E3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Cao L, Bu R, Oakley JI, Kalla SE, Blair HC. Estrogen receptor-β modulates synthesis of bone matrix proteins in human osteoblast-like MG63 cells. J Cell Biochem. 2003;89:152–164. doi: 10.1002/jcb.10486. [DOI] [PubMed] [Google Scholar]
- Dang ZC, Lowik C. Dose-dependent effects of phytoestrogens on bone. Trends Endocrinol Metab. 2005;16:207–213. doi: 10.1016/j.tem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Doran PM, Riggs BL, Atkinson EJ, Khosla S. Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. J Bone Miner Res. 2001;16:2118–2125. doi: 10.1359/jbmr.2001.16.11.2118. [DOI] [PubMed] [Google Scholar]
- Endoh H, Sasaki H, Maruyama K, Takeyama K, Waga I, Shimizu T, Kato S, Kawashima H. Rapid activation of MAP kinase by estrogen in the bone cell line. Biochem Biophys Res Commun. 1997;235:99–102. doi: 10.1006/bbrc.1997.6746. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Levine JP. Preventing osteoporosis in symptomatic postmenopausal women. Menopause. 2011;18:109–118. doi: 10.1097/gme.0b013e3181e324a6. [DOI] [PubMed] [Google Scholar]
- Hadji P. The evolution of selective estrogen receptor modulators in osteoporosis therapy. Climacteric. 2012 doi: 10.3109/13697137.2012.688079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnle GG, Ward HA, Vogiatzoglou A, Luben RN, Mulligan A, Wareham NJ, Forouhi NG, Khaw KT. Association between dietary phyto-oestrogens and bone density in men and postmenopausal women. Br J Nutr. 2011;106:1063–1069. doi: 10.1017/S0007114511001309. [DOI] [PubMed] [Google Scholar]
- Noda-Seino H, Sawada K, Hayakawa J, Ohyagi-Hara C, Mabuchi S, Takahashi K, Nishio Y, Sakata M, Kurachi H, Kimura T. Estradiol and Raloxifene induce the proliferation of osteoblasts through G-protein-coupled receptor GPR30. J Endocrinol Invest. 2012 doi: 10.3275/8301. [DOI] [PubMed] [Google Scholar]
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocrine Reviews. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Yaroslavskiy BB, Griswold RD, Zadorozny EV, Guo L, Tourkova IL, Blair HC. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-alpha with BCAR1 and Traf6. Exp Cell Res. 2009;315:1287–1301. doi: 10.1016/j.yexcr.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Mancarella S, Songsawad D, Tourkova IL, Barnett JB, Gill DL, Soboloff J, Blair HC. Gene disruption of the calcium channel Orai1 results in inhibition of osteoclast and osteoblast differentiation and impairs skeletal development. Lab Invest. 2012 doi: 10.1038/labinvest.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedd-Wise KM, Alekel DL, Hofmann H, Hanson KB, Schiferl DJ, Hanson LN, Van Loan MD. The soy isoflavones for reducing bone loss study: 3-yr effects on pQCT bone mineral density and strength measures in postmenopausal women. J Clin Densitom. 2011;14:47–57. doi: 10.1016/j.jocd.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M, Jones ML, De Nigris E, Brewer N, Davis S, Oakley J. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9:1–160. doi: 10.3310/hta9220. [DOI] [PubMed] [Google Scholar]
- Suksamrarn A, Ponglikitmongkol M, Wongkrajang K, Chindaduang A, Kittidanairak S, Jankam A, Yingyongnarongkul BE, Kittipanumat N, Chokchaisiri R, Khetkam P, Piyachaturawat P. Diarylheptanoids, new phytoestrogens from the rhizomes of Curcuma comosa: Isolation, chemical modification and estrogenic activity evaluation. Bioorg Med Chem. 2008;16:6891–6902. doi: 10.1016/j.bmc.2008.05.051. [DOI] [PubMed] [Google Scholar]
- Wang YD, Tao MF, Wang L, Cheng WW, Wan XP. Selective Regulation of Osteoblastic OPG and RANKL by Dehydroepiandrosterone Through Activation of the Estrogen Receptor beta-mediated MAPK Signaling Pathway. Horm Metab Res. 2012;44:494–500. doi: 10.1055/s-0032-1311567. [DOI] [PubMed] [Google Scholar]
- Weerachayaphorn J, Chuncharunee A, Mahagita C, Lewchalermwongse B, Suksamrarn A, Piyachaturawat P. A protective effect of Curcuma comosa Roxb. on bone loss in estrogen deficient mice. J Ethnopharmacol. 2011;137:956–962. doi: 10.1016/j.jep.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Piyachaturawat P, Suksamrarn A, Ponglikitmongkol M, Arao Y, Hewitt SC, Korach KS. Diarylheptanoid phytoestrogens isolated from the medicinal plant Curcuma comosa: biologic actions in vitro and in vivo indicate estrogen receptor-dependent mechanisms. Environ Health Perspect. 2009a;117:1155–1161. doi: 10.1289/ehp.0900613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winuthayanon W, Suksen K, Boonchird C, Chuncharunee A, Ponglikitmongkol M, Suksamrarn A, Piyachaturawat P. Estrogenic activity of diarylheptanoids from Curcuma comosa Roxb. Requires metabolic activation. J Agric Food Chem. 2009b;57:840–845. doi: 10.1021/jf802702c. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Sun L, Blair Harry C. Special Stem Cells for Bone. Cell stem cell. 2012;10:233–234. doi: 10.1016/j.stem.2012.02.012. [DOI] [PubMed] [Google Scholar]