Abstract
Cognitive reserve is hypothesized to help people withstand greater brain pathology without manifesting clinical symptoms, and may be regarded as a preventive factor of dementia. It is unclear whether the effect of cognitive reserve is evident only among the older adults or after conversion to dementia, or if it can also be seen earlier in life before the prominent effects of cognitive aging become apparent. While finding a main effect of cognitive reserve on cognitive outcome may be consistent with the reserve hypothesis, in our view, it is unnecessary to invoke the idea of reserve if only a main effect is present. Rather, it is the interaction between a measure of reserve and a brain measure on cognitive outcome that is key for confirming that the effects of brain pathology affect people differently according to their cognitive reserve. We studied whether general cognitive ability at an average age of 20 years, as a direct measure of cognitive reserve, moderates the association between hippocampal volume and episodic memory performance in 494 middle-aged men ages 51 to 60. Whereas there was no statistically significant direct relationship between hippocampal volume and episodic memory performance in middle age, we found a statistically significant interaction such that there was a positive association between hippocampal volume and episodic memory only among people with lower general cognitive ability at age 20, i.e., lower levels of cognitive reserve. Our results provide support for the hypothesis that cognitive reserve moderates the relationship between brain structure and cognition in middle age, well before the onset of dementia.
Keywords: cognitive reserve, general cognitive ability, episodic memory, hippocampus, verbal learning
1. Introduction
Why can some individuals with noticeable brain pathology maintain a relatively high level of cognitive performance while others with the same amount of brain damage have remarkable deficits in cognitive performance? (see e.g., Stern, 2009). This question, along with the observation that brain pathology and cognitive functioning do not always correlate (see e.g. Katzman et al., 1988), has led to the development of the concepts of cognitive and brain reserve. Cognitive and brain reserve may be viewed as buffers against the effects of brain pathology and protective factors against Alzheimer’s disease (AD) or other forms of dementia. Reserve may also explain individual differences in cognitive functioning in the context of AD. While there is evidence for the effect of reserve in healthy older adults (Brickman et al., 2011) and in context of AD (Stern, Albert, Tang, & Tsai, 1999) and other dementia-causing conditions like vascular pathology (Zieren et al., 2013), it is not clear whether the effect of reserve is present throughout adulthood or if it becomes apparent only later in life.
According to Stern (2002; 2009), the concept of reserve can be divided into passive (structural) and active (functional) models as represented by brain reserve and cognitive reserve, respectively. The passive model refers to a threshold at which brain pathology starts to affect cognition. An example of brain reserve would be the neuroanatomical measure of overall brain size (e.g., brain or intracranial volume), the idea being that larger brains can tolerate more pathology before the threshold where cognitive deficits start to occur is reached. The active model refers to compensatory processes that are invoked in order to tolerate or circumvent brain pathology. An example of cognitive reserve would be pre-existing cognitive or compensatory abilities (e.g., higher general intelligence or ability to use different cognitive strategies); in many studies these are approximated by the proxy measure of educational level. In contrast to the passive model, there is no certain threshold of brain pathology when cognition begins to be affected. Instead, higher cognitive reserve helps the individual to compensate and thus maintain a certain level of cognitive functioning despite brain pathology.
Two individuals can have the same amount of brain reserve (e.g., overall brain size), but can still differ in how much they can tolerate brain pathology because they differ in their cognitive reserve (e.g., premorbid general cognitive ability). Because cognitive functions do not operate in isolation from brain anatomy and brain structures are plastic throughout development, the distinction between active and passive models of reserve is not clear-cut. In sum, the reserve hypothesis states that individuals with higher levels of reserve, compared to individuals with lower levels of reserve, can better maintain cognitive functioning in the presence of brain pathology.
As suggested by Christensen et al. (2007), a full model to test the reserve hypothesis should include: 1) a direct or proxy measure of reserve, 2) a measure of brain pathology, and 3) a cognitive outcome. Their review showed that most studies to date have failed to include all of these measures when testing a reserve hypothesis. Instead, many studies have tested the main effects of measures of reserve on cognitive functioning. Such studies have demonstrated, for example, that higher education is related to better cognitive performance at middle age and at old age. These kinds of studies only report the main effect of a measure of reserve on later cognitive performance. Simply showing that more highly educated people (e.g., Le Carret et al., 2003; Singh-Manoux et al., 2011) or those with higher general cognitive ability (e.g., Corral et al. 2006) perform better on cognitive tests later in life does not address the question of whether people with higher levels of cognitive reserve tolerate brain pathology better than those with lower levels of reserve. Although this main effect is consistent with the reserve hypothesis, we think it is unnecessary—and therefore less parsimonious—to invoke the reserve hypothesis to account for this finding because those with more education or higher general cognitive ability will perform better on cognitive tests at any point in development, including childhood and young adulthood.
Some studies have suggested that the effect of reserve is supported by the findings that at any level of cognitive performance, people with higher reserve exhibit more brain pathology and will develop dementia later than people with lower levels of cognitive reserve. For example, by studying the Alzheimer’s Disease Neuroimage Initiative sample, Vemuri et al. (2011) found that the level of AD-related biomarkers (beta-amyloid, tau, and brain atrophy) was more pronounced at any level of cognitive functioning among people with higher cognitive reserve (based on the proxy measure of National Adult Reading Test [NART] performance) compared to those with lower cognitive reserve. This finding is important as it clearly shows that people with higher prior general cognitive ability develop dementia later and thus require more brain pathology before clinical manifestation of dementia than people with lower general cognitive ability. However, the effect of general cognitive ability was only on the intercepts, not on the slopes (i.e. individuals with higher cognitive reserve performed better at any level of pathology whereas the effect of pathology on cognition did not differ as a function of cognitive reserve).
The simplest explanation is that people who started out with higher prior cognitive ability will continue to have higher cognitive performance at any point before developing dementia. Also, they develop dementia later simply because they have farther to fall before they reach that threshold of cognitive impairment, just as an object dropped from the sixth floor will take longer to reach the ground than one dropped from the third floor. We argue that it is not necessary or particularly useful to invoke the reserve hypothesis to account for these findings. Like the objects being dropped from different heights, the outcome is virtually guaranteed. It is difficult, if not impossible, to conceive of a situation in which individuals with higher pre-existing general cognitive capacity (cognitive reserve) would not have better cognitive performance than those with lower reserve when they both have the same level of current pathology. If one shifts the scale so that two individuals with different cognitive reserve levels are equated on current cognitive performance, it is self-evident that the one with higher reserve will have greater pathology.
What is more meaningful is to test the hypothesis of a moderating effect of cognitive reserve, which means testing for the presence of a significant interaction between measures of cognitive reserve and brain pathology in prediction of cognitive performance (as suggested by Christensen et al., 2007; see also Stern, 2012). A significant interaction would suggest that the association between a brain (or biomarker of dementia or age) and the cognitive measure differs as a function of cognitive reserve. In terms of the cognitive reserve hypothesis, one would expect that greater brain atrophy would be associated more strongly with poorer cognitive performance among people with low levels of cognitive reserve compared to people with high levels of cognitive reserve. If the effects of brain atrophy on cognitive functioning were less pronounced among people with high cognitive reserve, it would suggest that individuals with high cognitive reserve can compensate more in the presence of brain atrophy, thus resulting in a weaker or zero correlation between brain atrophy and the cognitive measure.
An interaction between education and white matter pathology on cognitive functions has been reported among older adults. A significant relationship between white matter hyperintensities and cognition has been reported to be stronger in individuals with low levels of education compared to those with higher levels of education (Dufouil, Alperovitch, & Tzourio, 2003: Nebes et al., 2006). Similarly, senile plaques have been reported to be more strongly associated with poorer cognitive functioning among those with lower education levels (Bennet et al., 2003). These findings support the cognitive reserve hypothesis because the brain pathology and cognition relationships were more pronounced among people with lower cognitive reserve. However, a longitudinal study found no significant interaction between brain atrophy/white matter hyperintensities and education on cognitive functioning, either at baseline (60 – 64 years) or at follow up after four years (Christensen et al., 2007; Christensen et al., 2009). One important reason for the conflicting reports on the effects of cognitive reserve may be the use of education, which may be too crude an estimate, particularly in relatively younger adults who have less brain atrophy and greater ability to compensate than their older counterparts.
As noted, relatively few studies have tested the interaction model. To our knowledge, no studies have tested whether cognitive reserve moderates the more specific relationship between hippocampal volume and episodic memory performance, despite the well known importance of these measures in the context of aging and AD. Impaired episodic memory performance is characteristic of AD and common in persons with mild cognitive impairment (MCI) (Petersen et al. 1999). Age has been shown to affect episodic memory performance among healthy individuals (Kramer et al., 2003), but there are large individual differences in episodic memory performance change in older adults (Christensen et al., 1999). Hippocampal structure and function are both crucial to episodic memory. People with AD show hippocampal atrophy and there is also evidence of hippocampal atrophy in normal aging (Jack et al., 2000; Jernigan et al., 2001). Individuals with MCI have also been reported to have smaller hippocampal volume compared to cognitively healthy older adults (Wolf et al., 2004). A study by Petersen et al. (2000) suggested that smaller hippocampal volume is related to poorer episodic memory performance in persons with AD, but not among non-AD individuals. On the other hand, Kramer et al. (2007) found that decrease in hippocampal volume was related to decrease in episodic memory among healthy older adults.
Although among all reviewed brain-behavior relationships in healthy older adults, the association of hippocampal volume and memory was one of the more consistent findings (Kaup, Mirzakhanian, Jeste, & Eyler, 2011), a meta-analysis of these studies suggested that the relationship between hippocampal volume and episodic memory performance among healthy older adults is weak and there is variability in findings (Van Petten, 2004). Cognitive reserve could account for the increased individual differences in episodic memory at older ages and explain the inconsistent results regarding relationship between hippocampal volume and episodic memory among non-demented individuals. In the context of cognitive aging, it is important to detect subgroups that might be more vulnerable to brain pathology such as hippocampal atrophy. Indeed, cognitive reserve-based subgrouping might allow detection of individuals whose cognition will be affected by brain pathology before clinical signs of dementia.
Here we investigate the association between hippocampal volume and episodic memory performance among middle-aged men in the context of cognitive reserve. We examined the possible moderating effect of cognitive reserve on the hippocampal volume-episodic memory relationship. In line with the cognitive reserve hypothesis, we hypothesized that smaller hippocampal volume would be more strongly associated with poorer episodic memory performance among individuals with low cognitive reserve relative to those with higher cognitive reserve, or that the hippocampal volume-episodic memory relationship would be evident only among those with lower levels of cognitive reserve. In the context of a full model of reserve (Christensen et al., 2007) we assessed general cognitive ability, hippocampal volume, and episodic memory as measures of cognitive reserve, brain anatomy, and cognitive outcome, respectively. In order to relate our results specifically to cognitive reserve independent of brain reserve, we used total intracranial volume—a measure of brain reserve—as a covariate. Importantly, our study used a direct measure of cognitive reserve in the form of a measure of general cognitive ability administered at an average age of 20 years rather than indirect proxies such as education or the NART. In contrast to previous cognitive reserve studies using relatively general measures, we used specific brain and cognitive measures (hippocampal volume and episodic memory) that are particularly relevant to age-related cognitive decline and progression to dementia, and we tested for moderation effects of cognitive reserve in a middle-aged sample in which early detection and intervention is most relevant.
2. Methods
2.1. Participants
Participants were 534 middle-aged males (at the time of recruitment from 51 to 59 years) from the ongoing Vietnam Era Twin Study of Aging (VETSA; Kremen et al., 2006; Kremen et al., 2010). The VETSA participants served in the military but are representative of men of similar age in US (Lyons et al., 2006). The mean level of education in years was 13.8 (SD 2.1). Participants traveled to University of California, San Diego or Boston University for an extensive laboratory study protocol that included cognitive testing and magnetic resonance imaging (MRI) of the brain. MRIs for the Boston participants were administered at Massachusetts General Hospital. The study protocol was approved by the institutional review boards at each site. All participants gave written informed consent before participation. The MRI study sample size has been increased since the report of Kremen et al. (2010). Of the 534 participants for whom data were available, we excluded 32 scans due to technical errors in scan acquisition or image processing, yielding 502 participants with adequate hippocampal volume data. Of these, 494 participants had available cognitive reserve data for the present study (age M = 55.67, SD = 2.62). The MRI study was started in the third year of data collection of the VETSA study. Approximately 95% of those invited agreed to undergo the MRI and about 80% met the inclusion criteria for MRI safety.
2.2. Cognitive reserve measure
General cognitive ability in young adulthood (average age of 20 years) was measured with the Armed Forces Qualification Test (AFQT20), a 100-item multiple-choice paper-and-pencil test administered just before military induction (Bayroff & Anderson, 1963). The AFQT has a high correlation of 0.84 with Wechsler Adult Intelligence Scale (McGrevy, Knouse, & Thompson, 1974; Lyons et al., 2009). The test consists of items that cover domains of vocabulary, arithmetical ability, tool/mechanical knowledge and reasoning, and visual-spatial ability. Military policy excluded everyone who scored in the bottom 10th percentile. In addition, the highest end of the cognitive ability scale has traditionally been slightly under-represented in the military. As a result, the AFQT20 scores approximate a truncated normal distribution (Lyons et al., 2009).
The total score on the AFQT20 was used as a continuous measure of cognitive reserve. In addition to the continuous measure we used an extreme groups approach that categorized individuals into low (n=131) and high (n=114) cognitive reserve groups. As such, we contrasted individuals in lowest and highest quartiles according to the AFQT20 score (see characteristics of low and high reserve groups in Table 1). We used both the continuous and categorical measures of AFQT20 because effects might be subtle in this relatively young sample, which might mean that the effects would be observable only in a comparison of extreme groups.
Table 1.
Characteristics of low (lowest 25%) and high (highest 25%) cognitive reserve (CR) groups according to their age 20 general cognitive ability as measured with Armed Forces Qualification Test (AFQT20).
| Low CR (n=131) | High CR (n=114) | |||
|---|---|---|---|---|
|
| ||||
| M (SD) | M (SD) | F | p | |
| Age | 55.34 (2.60) | 55.82 (2.59) | 0.08 | 0.78 |
| Years of education | 12.91 (1.92) | 14.89 (2.17) | 44.81 | <.001 |
| APOE ε4+/ε4− (% of ε4+)a | 33/95 (26%) | 36/73 (33%) | 1.13 | 0.29 |
| Hippocampus volume (mm3)aa | 8096 (852) | 8282 (759) | 0.01 | 0.99 |
Note. Comparisons based on mixed models, except APOE ε4+ prevalence comparison based on Rao-Scott chi-square test. Twin relatedness controlled in all comparisons.
number of individuals with and without APOE ε4 allele and the prevalence of APOE ε4 carriers in parentheses among low (n=128) and high (n=109) CR individuals.
controlled also for estimated total intracranial volume.
2.3. Episodic memory measures
Episodic memory at middle age was measured with the second edition of the California Verbal Learning Test (CVLT-2) (Delis, Kramer, Kaplan, & Ober, 2000). In this test, there are five trials with immediate recall after every trial. Each trial consists of a 16-item list that is read to participants, and each time they are asked to recall as many items as they can. In the next phase participants are asked to recall all items from a single presentation of a different 16-item list (distraction stimuli). After that participants are asked to recall all the items from the first list that was presented five times, but this time the list is not presented (short delay free recall). Further, participants are asked to recall all the items from this list again after 20 minutes (long delay free recall). We used three CVLT-2 measures. Total number of recalled words in trials 1 – 5 was used as a verbal learning measure. This measure captures both acquisition and retrieval components of episodic memory performance. We also used number of recalled words in the short and long delay free recall. These measures have only the retrieval component because the word list was not presented before the recall in these conditions.
2.4. Image acquisition and processing of hippocampus volume measure
Detailed description of magnetic resonance imaging (MRI) data collection, acquisition and processing in VETSA has been previously described in Kremen et al. (2010). In short, brain images were acquired with Siemens 1.5 Tesla scanners (sagittal T1-weighted MPRAGE sequences with a TI=1000 ms, TE=3.31 ms, TR=2730 ms, flip angle=7 degrees, slice thickness=1.33mm, and voxel size=1.3×1.0×1.3mm). Image analysis based on the publicly available FreeSurfer software was used for volumetric segmentation (Fischl et al., 2002). We used left and right hippocampus volumes and a total hippocampus volume (left + right). Estimated total intracranial volume (eTIV) was used as a covariate to control for individual differences in head size.
2.5. Statistical analysis
Although we have a twin sample, the analyses for this article were not twin-based. That is, the unit of analysis was the individual rather than the twin pair. Linear mixed-effects models (Proc Mixed, SAS version 9.3) were used to test the main and interaction effects of cognitive reserve and brain measures on episodic memory performance at middle age. The moderator effect is supported if the interaction term is significant (see Baron and Kenny, 1986). In other words, the relationship between predictor (hippocampal volume) and outcome variable (episodic memory) differs as a function of the moderator variable (AFQT20). The moderator effect can be present in the absence of significant main effects. In this study, we hypothesized that the association between hippocampal volume and episodic memory would be stronger or would be evident only among those with lower levels of cognitive reserve. These models controlled for the nested family data (twins within families), age, and eTIV. Both study sites used only one MRI scanner; because there was no scanner effect on hippocampal volume measures, we did not use scanner variable as a covariate in these analyses (see Panizzon et al., 2012). Because the mixed models controlled for the twin relatedness, the degrees of freedom in these models refer to number of families (twin pairs).
We first separately tested the main effects of bilateral hippocampal volume and continuous AFQT20 on CVLT measures. The effect of age on CVLT measures was reported based on these models. Next we ran the same models by adding the AFQT20 x hippocampal volume interaction term in order to see whether the hippocampal volume-episodic memory relationship was more prominent among people with lower cognitive reserve. For all interaction analyses both continuous and categorical measures of AFQT20 were used. Finally, we tested the interaction terms separately for left and right hippocampal volumes and included APOE genotype (ε4 carrier vs. ε4 non-carrier) as an additional covariate in the models with the interaction terms. Total hippocampal volume and left and right volumes separately as well as eTIV were centered to mean values. Likewise, AFQT20 and age were centered at mean.
3. Results
As expected, years of education correlated positively with the continuous AFQT20 measure (r = .35, p <.0001). Continuous AFQT20 measure was not significantly related to hippocampal volume when adjusted for age and eTIV (F(1,209) = 0.01, p = .92). The APOE ε4+ genotype did not have an effect on continuous AFQT20 measure (F(1,199) = 0.05, p = .82). The categorical AFQT20 measure had a significant association with education, showing that those with higher cognitive reserve had higher education compared to those with lower cognitive reserve. Individuals with low and high cognitive reserve, as categorized by the categorical AFQT20 measure, did not differ in the prevalence of APOE ε4 carriers or in hippocampal volume. The means for demographic and hippocampal volume variables and the prevalence of APOE ε4+/ε4− genotypes among individuals with low and high cognitive reserve by the categorical AFQT20 measure can be seen from Table 1. The prevalence of ε4+ genotypes was consistent with the general population prevalence (Blair et al., 2005; Ghebranious, Ivacic, Mallum, & Dokken, 2005). Participants with one or two ε4+ alleles were grouped together because there were too few homozygous ε4+ participants to analyze them separately.
3.1. Verbal learning
There was no significant main effect of hippocampal volume on CVLT total recalled words in trials 1-5 (F(1,205) = 0.27, p = .6037). The main effect of the continuous AFQT20 measure on CVLT total recalled words in trials 1-5 was significant (F(1,205) = 41.82, p < .0001), showing that better AFQT20 performance was related to better CVLT total recalled words in trials 1-5 performance (see also Table 2 for similar categorical AFQT20 result). Age was a significant predictor of CVLT total recalled words in trials 1 – 5 (F(1,205) = 14.80, p < .001), indicating that the number of total recalled words was reduced by 0.62 per year.
Table 2.
California Verbal Learning Test (CVLT) performance among low (lowest 25%) and high (highest 25%) cognitive reserve (CR) groups according to their age 20 general cognitive ability as measured with Armed Forces Qualification Test (AFQT20).
| Low CR (n=131) | High CR (n=114) | |||
|---|---|---|---|---|
|
| ||||
| M (SD) | M (SD) | F | p | |
| CVLT Total words trials 1-5 | 39.68 (8.48) | 44.81 (9.24) | 16.37 | <.001 |
| CVLT Short delay free recall | 7.73 (2.75) | 9.21 (2.99) | 12.65 | <.001 |
| CVLT Long delay free recall | 8.05 (2.93) | 9.89 (3.10) | 15.75 | <.001 |
Note. Comparisons based on mixed models. Twin relatedness controlled in all comparisons.
As presented in Table 3, there was a significant AFQT20 x hippocampal volume interaction on CVLT total recalled words in trials 1-5 with the continuous AFQT20 measure (F(1,204) = 7.47, p < .01). Similarly, there was a statistically significant AFQT20 x hippocampal volume interaction (F(1,59) = 4.63, p < .05) when we used categorical AFQT20 measure (Model 1 in Table 3).
Table 3.
Interaction effects of age 20 Armed Forces Qualification Test (AFQT20) and hippocampal volume on CVLT total words in trials 1 – 5. Statistics presented separately for total, left, and right hippocampal volume.
| Covariates | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| age, eTIV | age, eTIV, APOE | |||||
|
| ||||||
| df | F | p | df | F | p | |
| Total hippocampal volume | ||||||
| Interaction with continuous AFQT20 | 204 | 7.47 | 0.007 | 191 | 7.41 | 0.007 |
| Interaction with categorical AFQT20 | 59 | 4.63 | 0.035 | 54 | 4.72 | 0.034 |
| Left hippocampal volume | ||||||
| Interaction with continuous AFQT20 | 204 | 8.05 | 0.005 | 191 | 8.70 | 0.004 |
| Interaction with categorical AFQT20 | 59 | 4.52 | 0.038 | 52 | 5.51 | 0.023 |
| Right hippocampal volume | ||||||
| Interaction with continuous AFQT20 | 204 | 5.95 | 0.016 | 191 | 5.40 | 0.021 |
| Interaction with categorical AFQT20 | 59 | 4.28 | 0.043 | 52 | 3.64 | 0.062 |
Note. Model 1 covariates include age and estimated total intracranial volume (eTIV). Model 2 includes also APOE ε4 status (carrier vs. non-carrier) as an additional covariate. Twin relatedness controlled in all analyses. Type three effects controlled also for the main effects of AFQT and hippocampal volume.
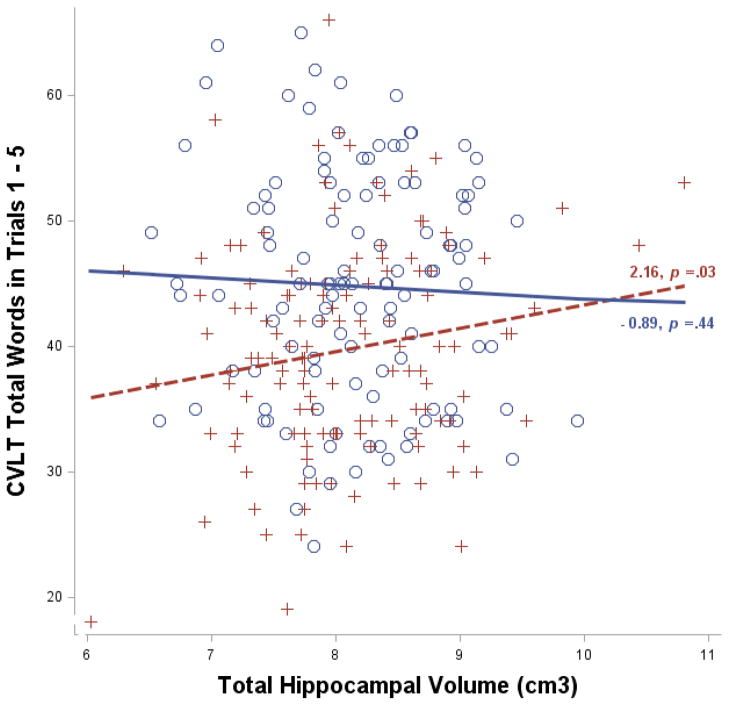
These analyses reflect the fact that there was a positive relationship between hippocampal volume and total words recalled in trials 1-5 only among individuals with low cognitive reserve, while no association was evident among those with high cognitive reserve (Fig 1). In other words, AFQT20 moderated this relationship. In the low reserve group, number of total words recalled increased 2.16 words per cubic centimeter of hippocampal volume.
Fig. 1.
Relationship between California Verbal Learning Test (CVLT) total number of words in trials 1 – 5 and bilateral hippocampal volume (in cubic centimeters, adjusted for age and estimated total intracranial volume) among individuals with high (blue circles and solid line) and low (red crosses and dotted line) cognitive reserve. High and low reserve individuals are those in the upper and lower quartiles according to their age 20 general cognitive ability, respectively. The slope estimates denote the change in CVLT score as a function of one cm3 increase in hippocampal volume.
The significant interaction effect between AFQT20 and hippocampal volume on CVLT total recalled words in trials 1-5 remained when we used APOE genotype as an additional covariate (both with continuous and categorical AFQT measures, model 2 in Table 3). When we tested left and right hippocampal volumes separately, the interactions were significant with the exception of categorical AFQT20 measure x right hippocampal volume in model where APOE genotype was used as an additional covariate (see Table 3).
3.2. Free recall
As in trials 1-5, there was a significant main effect of the continuous AFQT20 measure on CVLT short-delay free recall (F(1,205) = 32.34, p <.0001), showing that better AFQT20 performance was related to better CVLT short-delay free recall performance (see also Table 2 for similar categorical AFQT20 results). Hippocampal volume had no significant main effect on CVLT short-delay free recall (F(1,205) = 0.01, p = .9831). Age was a significant predictor of CVLT short-delay free recall (F(1,205) = 6.03, p = .0149), indicating that the number of total recalled words was reduced by 0.13 per year. There was no significant interaction between AFQT20 and hippocampal volume on CVLT short-delay free recall, either for the continuous (F(1,204) = 0.54, p = .4623) or categorical (F(1,59) = 0.09, p = .7634) AFQT20 measure.
The results for CVLT long-delay free recall were similar to those for CVLT short-delay free recall. There was a significant main effect of the continuous AFQT20 measure on CVLT long-delay free recall (F(1,205) = 41.51, p < .0001), showing that better AFQT20 performance was related to better CVLT long-delay free recall performance (see also Table 2 for categorical AFQT20 results). There was no significant main effect of hippocampal volume on CVLT long-delay free recall (F(1,205) = 0.13, p = .7181). Age was a significant predictor of CVLT long-delay free recall (F(1,205) = 5.07, p = .0254), indicating that the number of total recalled words was reduced by 0.13 per year. Finally, there was no significant AFQT20 x hippocampal volume interaction on CVLT long-delay free recall, with the continuous (F(1,204) = 0.66, p = .4182) or categorical (F(1,59) = 0.52, p = .4721) AFQT20 measures.
4. Discussion
Individuals with more cognitive reserve, based on higher general cognitive ability in young adulthood, had better memory performance at middle age. This result demonstrates the expected main effect of a measure of cognitive reserve on cognitive outcome. Also, age was a significant predictor of all episodic memory measures even in our narrow age range with older individuals performing more poorly than younger individuals. More importantly, we tested whether cognitive reserve moderates the association between hippocampal volume and memory. The main finding of this study was a significant interaction between general cognitive ability at age 20 and midlife hippocampal volume on the learning component of episodic memory showing that smaller hippocampal volume was related to poorer midlife episodic memory performance only among individuals with lower levels of cognitive reserve. We controlled for estimated total intracranial volume, a commonly used proxy measure of brain reserve, which suggests that the effect of cognitive reserve is evident when the level of brain reserve is held constant across individuals. This is important because individuals who have the same level of brain reserve might still differ in their level of cognitive reserve. More efficient neural networks and use of different brain structures and networks have been suggested as underlying brain mechanisms for high cognitive reserve (Stern, 2009). It is possible that individuals who have a small hippocampus but high cognitive reserve can compensate by utilizing other brain areas (e.g. more frontal control) to improve or maintain performance on episodic memory tests. Those with small hippocampus and lower levels of cognitive reserve may be more dependent on their hippocampal size alone because they may have less ability to invoke other networks.
These results are important in several ways. First, they stress the importance of detecting subgroups that might be more vulnerable to the cognitive and functional effects of brain pathology. If we had only looked at the brain-cognition association in the whole sample, we would have simply concluded that there is no relationship between hippocampal volume and episodic memory performance among non-demented middle-aged adults. A meta-analysis of studies on healthy individuals (Van Petten, 2004) has indicated variability in findings on the association between hippocampal volume and episodic memory among healthy older adults. Our results indicate that cognitive reserve can be a useful way of elucidating heterogeneity in the context of cognitive aging, and that the lack of overall association between a brain measure and cognitive performance can mask important subgroup differences. Early predictors of cognitive decline and AD may not show any association with cognitive functioning in the population as a whole, but may do so in some subgroups.
Second, our results show that the effect of cognitive reserve may already be evident in non-demented individuals and before they reach old age. Although there is evidence of the effect of cognitive reserve among younger adults with conditions that affect cognition, like human immunodeficiency virus (Stern et al., 1996) and traumatic brain injury (Kesler et al., 2003), our results demonstrate that the effect of cognitive reserve can be seen among healthy individuals as well. Given the age range of 51 – 60, our results are unlikely to be complicated by brain diseases that become increasingly common in older samples (e.g., AD or cerebrovascular disease). While an earlier study did not detect a significant interaction between measures of brain pathology and education on early old-age cognition (Christensen et al., 2007 & Christensen et al., 2009), we note that that study differed from our study in several ways. They used a nonstandard administration of CVLT as their immediate and delayed CVLT recall measures were based on presenting only the first trial of the CVLT. They also examined white matter pathology and estimated total brain volume atrophy rather than targeting specific gray matter structures. No earlier studies have used hippocampal volume as a brain measure when testing the interaction approach in the context of the reserve hypothesis, although hippocampal volume is especially relevant when the cognitive outcome is memory.
Further, Christensen et al. used education as a proxy measure of cognitive reserve. It is possible that proxy measures such as educational level are not sensitive enough for detecting significant interaction effects with brain measures among middle-aged non-demented individuals. For example, there were 195 VETSA participants (39% of the sample of this study) who all had 12 years of education. Despite the absence of educational variability, there was considerable variability (covering virtually the full range [11th–99th percentiles]) of AFQT scores.
It is noteworthy that we detected the interaction between general cognitive ability and hippocampal volume only for one of the three episodic memory measures in our study. The interaction was significant only for CVLT-2 total recalled words in trials 1 – 5. Like the Christensen et al. studies, we did not find the effect for short- or long-delay free recall. These measures differ in which memory processes are required to perform them. They all require retrieval of material from memory, whereas total words recalled in trials 1 – 5 also has an acquisition component as this measure requires learning of new words. Similar to our results, Reitz et al. (2009) reported that hippocampal volume predicted total, but not delayed, recall in the Selective Reminding Test, although this finding was nonsignificant when individuals with dementia were excluded. However, by using the CVLT, Genon et al. (2013) concluded that acquisition deficits are evident already at the predementia stage of AD.
Earlier results from the VETSA sample indicate that CVLT-2 performance in trials 1 – 5 total words recalled is influenced by some unique genetic effects that are not shared with the genetic effects acting on short- and long-delay free recall performance (Panizzon et al., 2011). Together with our current results, these findings emphasize the specificity of these different memory measures and stress the importance of studying different components of episodic memory in the context of aging. The learning measures can also be valuable predictors of AD. Indeed, some recent findings suggest that learning measures of episodic memory may be better than recall measures at predicting conversion to AD from MCI (Chang et al., 2010) or from questionable AD to AD (Lekeu et al., 2010). Further, it is possible that encoding and acquisition are affected earlier than ability to retrieve material in persons with low cognitive reserve.
Our study had some limitations. First, because the sample included only men, we do not know if these results can be generalized to women (CVLT is a cognitive measure with reported sex difference showing that women perform on average slightly better than men; Kramer, Yaffe, Lengenfelder, & Delis, 2003). Second, as noted, this sample is slightly under-represented at both the lowest and highest extremes of AFQT scores. However, it seems likely that, if anything, the lack of the most extreme groups would attenuate the interaction effects. Therefore, our results demonstrating the reserve effect may well be somewhat conservative. The cross-sectional nature of these analyses is also a limitation, but this work is part of a longitudinal study with follow-up assessments being conducted at the time of this writing.
The strengths of our study were the use of a direct measure of cognitive reserve and a large sample of individuals within a narrow age range. As already indicated by the range of scores among those with 12 years of education, by using education as a measure of reserve, there will be great heterogeneity in general cognitive ability within low and high reserve groups. Another reason for some studies’ failure to detect effects of reserve might be their wide age range. For example level of education among 49 – 82 year olds or baseline cognitive ability among 18 – 97 year olds did not affect the rate of cognitive change in studies of Van Gerven, Van Boxtel, Ausems, Bekers, & Jolles (2012) and Salthouse (2012), respectively. The narrow age range in our study has the advantage that the sample is more homogeneous. If, for example, MCI is due to a different set of factors at 50 than at 70, detecting either set of factors could prove difficult in a sample with a wide age range. Finally, our design avoids the difficulty of equating educational attainment in different age cohorts. For example, 80 year olds with 12 years of education will have more education relative to their age peers than would 40 year olds with the same level of education. Christensen et al. (2007; 2009) also had a narrow age range (60–64 years at baseline), but, as noted, they did not have a direct index of cognitive reserve and they did not examine specific gray matter structures.
The current results show that even if there is no association in the whole sample, there may well be subgroups where significant brain-cognition relationships can be detected. As demonstrated here, cognitive reserve can be one very useful approach to subgrouping. Studies on the effects of cognitive reserve should test for interaction effects in addition to testing for main effects. Whenever possible, direct measures of cognitive reserve should be used in favor of proxies. Finally, we cannot know precisely when in the aging process an interaction effect as function of cognitive reserve may manifest itself. Therefore, continued longitudinal assessment will be essential for elucidating the reserve phenomenon.
Hippocampal volume is related to memory in those with low cognitive reserve
Hippocampal volume is not related to memory in those with high cognitive reserve
Reserve effects can be seen in midlife if a direct measure of reserve is used
Acknowledgments
Funding
This work was supported by grants from the National Institute on Aging [R01 AG022982, R01 AG018386, and R01 AG022381, R01 AG018384]; National Institute of Drug Abuse [DA029475]; National Institute of Neurological Disorders and Stroke [NS056883], National Center for Research Resources [P41-RR14075, BIRN002, U24 RR021382]; National Institute for Biomedical Imaging and Bioengineering [EB006758]; National Center for Alternative Medicine [RC1 AT005728-01]; National Institute for Neurological Disorders and Stroke [NS052585-01, 1R21NS072652-01, 1R01NS070963]; the Ellison Medical Foundation; Academy of Finland; and a Sigrid Juselius Foundation Fellowship (E.V.). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA or the NIH.
The Cooperative Studies Program of the U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. This material was, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. We also appreciate the time and energy of many staff and students on the VETSA projects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayroff AG, Anderson AA. Development of Armed Forces Qualification Tests 7 and 8. Arlington, VA: U.S. Army Research Institute; 1963. Technical Research Report 1122. [Google Scholar]
- Bennet DA, Wilson RS, Scheider JA, Evans DA, Mendes de Leon CF, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Blair CK, Folson AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E. APOE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005;64:268–276. doi: 10.1212/01.WNL.0000149643.91367.8A. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, et al. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiology of Aging. 2011;32:1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YL, Bondi MW, Fennema-Notestine C, McEvoy LK, Hagler DJ, Jr, Jacobson MW, et al. Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to alzheimer’s disease. Neuropsychologia. 2010;48:1237–1247. doi: 10.1016/j.neuropsychologia.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Anstey KJ, Parslow RA, Maller J, Mackinnon A, Sachdev P. The brain reserve hypothesis, brain atrophy and aging. Gerontology. 2007;53:82–95. doi: 10.1159/000096482. [DOI] [PubMed] [Google Scholar]
- Christensen H, Batterham PJ, Mackinnon AJ, Anstey KJ, Wen W, Sachdev PS. Education, atrophy, and cognitive change in an epidemiological sample in early old age. The American Journal of Geriatric Psychiatry. 2009;17:218–226. doi: 10.1097/JGP.0b013e3181961a84. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, et al. An analysis of diversity in the cognitive performance of elderly community dwellers: Individual differences in change scores as a function of age. Psychology and Aging. 1999;14:365–379. doi: 10.1037//0882-7974.14.3.365. [DOI] [PubMed] [Google Scholar]
- Corral M, Rodríguez M, Amenedo E, Sánchez JL, Díaz F. Cognitive reserve, age, and neuropsychological performance in healthy participants. Developmental Neuropsychology. 2006;29:479–491. doi: 10.1207/s15326942dn2903_6. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, Texas: The Psychological Corporation; 2000. [Google Scholar]
- Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60:831–836. doi: 10.1212/01.wnl.0000049456.33231.96. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Genon S, Collette F, Moulin CJ, Lekeu F, Bahri MA, Salmon E, et al. Verbal learning in Alzheimer’s disease and mild cognitive impairment: Fine-grained acquisition and short-delay consolidation performance and neural correlates. Neurobiology of Aging. 2013;34:361–372. doi: 10.1016/j.neurobiolaging.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Ivacic L, Mallum J, Dokken C. Detection of ApoE E2, E3 and E4 alleles using MALDI-TOF mass spectrometry and the homogeneous mass-extend technology. Nucleic Acids Research. 2005;33:e149. doi: 10.1093/nar/gni155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful aging. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Applied Neuropsychology. 2003;10:153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Yaffe K, Lengenfelder J, Delis DC. Age and gender interactions on verbal memory performance. Journal of the International Neuropsychological Society. 2003;9:97–102. doi: 10.1017/s1355617703910113. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, et al. Genetic and environmental influences on the size of specific brain regions in midlife: The VETSA MRI study. Neuroimage. 2010;49:1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YM, Grant MD, Franz CE, Eisen SA, et al. Genes, environment, and time: The vietnam era twin study of aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Le Carret N, Lafont S, Letenneur L, Dartigues JF, Mayo W, Fabrigoule C. The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Developmental Neuropsychology. 2003;23:317–337. doi: 10.1207/S15326942DN2303_1. [DOI] [PubMed] [Google Scholar]
- Lekeu F, Magis D, Marique P, Delbeuck X, Bechet S, Guillaume B, et al. The california verbal learning test and other standard clinical neuropsychological tests to predict conversion from mild memory impairment to dementia. Journal of Clinical and Experimental Neuropsychology. 2010;32:164–173. doi: 10.1080/13803390902889606. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, et al. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological Science. 2009;20:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrevy DF, Knouse SB, Thompson RA. Relationships among an individual intelligence test and two air force screening and selection tests. San Antonio, TX: Personnel Research Division, Air Force Human Resources Laboratory, Brooks Air Force Base; 1974. Technical Report AFHRL-TR-74–25. [Google Scholar]
- Nebes RD, Meltzer CC, Whyte EM, Scanlon JM, Halligan EM, Saxton JA, et al. The relation of white matter hyperintensities to cognitive performance in the normal old: Education matters. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology, and Cognition. 2006;13:326–340. doi: 10.1080/138255890969294. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Lyons MJ, Jacobson KC, Franz CE, Grant MD, Eisen SA, et al. Genetic architecture of learning and delayed recall: A twin study of episodic memory. Neuropsychology. 2011;25:488–498. doi: 10.1037/a0022569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger RL, Eaves LJ, Chen CH, Dale AM, Eyler LT, et al. Genetic influences on hippocampal volume differ as a function of testosterone level in middle-aged men. Neuroimage. 2012;59:1123–1131. doi: 10.1016/j.neuroimage.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O’Brien PC, Smith GE, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Reitz C, Brickman AM, Brown TR, Manly J, DeCarli C, Small SA, et al. Linking hippocampal structure and function to memory performance in an aging population. Archives of Neurology. 2009;66:1385–1392. doi: 10.1001/archneurol.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Does the direction and magnitude of cognitive change depend on initial level of ability? Intelligence. 2012;40:352–361. doi: 10.1016/j.intell.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimäki M, Dugravot A. Does cognitive reserve shape cognitive change? Annals of Neurology. 2011;70:296–304. doi: 10.1002/ana.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Silva SG, Chaisson N, Evans DL. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Archives of Neurology. 1996;53:148–153. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Van Gerven PW, Van Boxtel MP, Ausems EE, Bekers O, Jolles J. Do apolipoprotein E genotype and educational attainment predict the rate of cognitive decline in normal aging? A 12-year follow-up of the Maastricht Aging Study. Neuropsychology. 2012;26:459–472. doi: 10.1037/a0028685. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Weigand SD, Przybelski SA, Knopman DS, Smith GE, Trojanowski JQ, et al. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain. 2011;134:1479–1492. doi: 10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, Hensel A, Kruggel F, Riedel-Heller SG, Arendt T, Wahlund LO, et al. Structural correlates of mild cognitive impairment. Neurobiology of Aging. 2004;25:913–924. doi: 10.1016/j.neurobiolaging.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Zieren N, Duering M, Peters N, Reyes S, Jouvent E, Herve D, et al. Education modifies the relation of vascular pathology to cognitive function: Cognitive reserve in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiology of Aging. 2013;34:400–407. doi: 10.1016/j.neurobiolaging.2012.04.019. [DOI] [PubMed] [Google Scholar]