Abstract
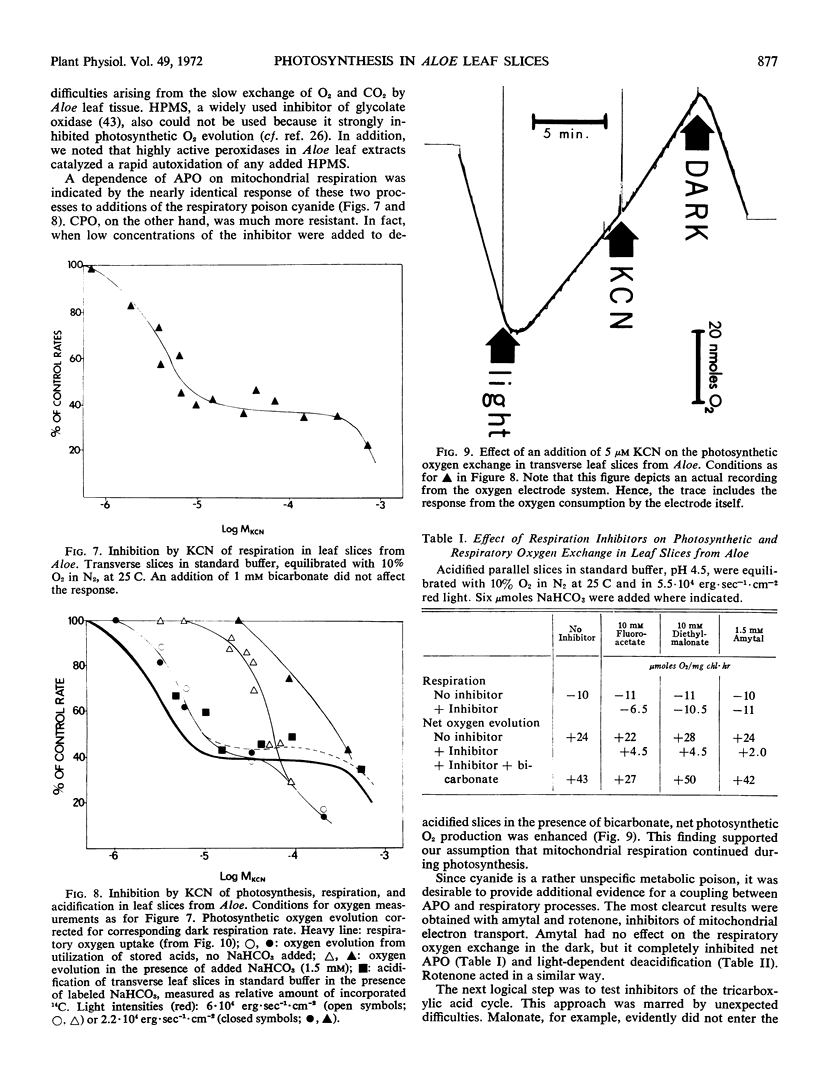
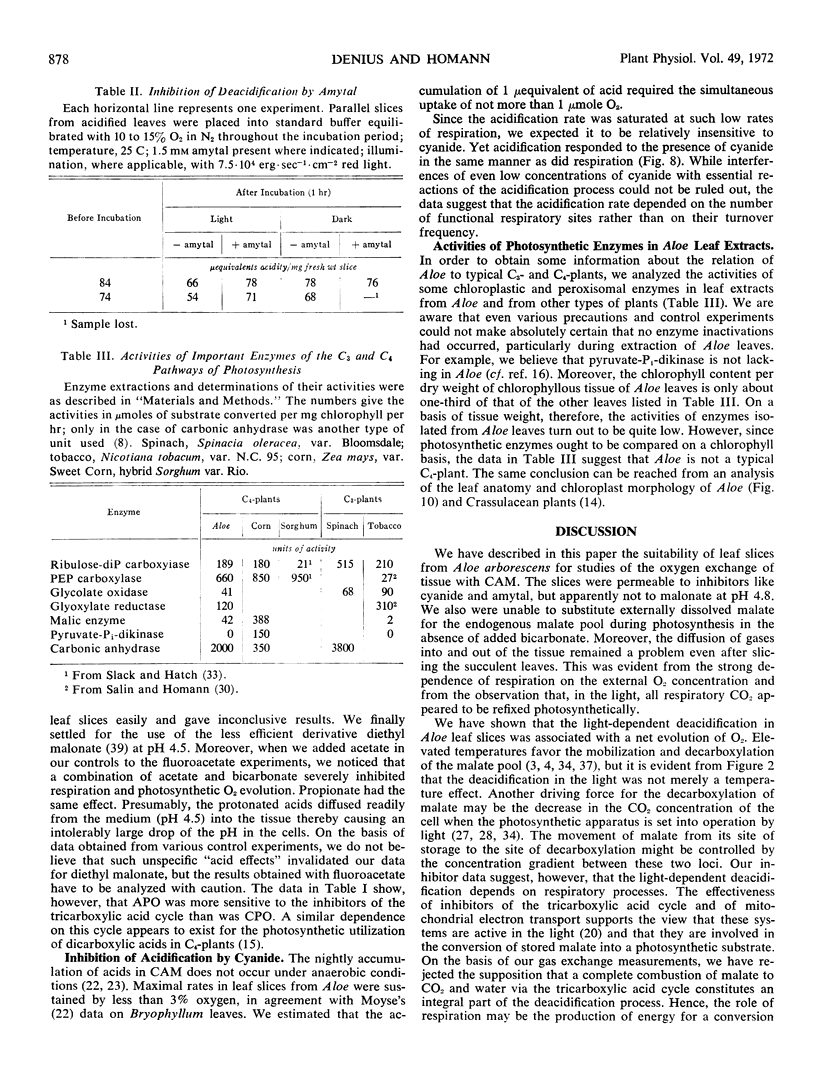
Leaves and leaf slices from Aloe arborescens Mill. were used to study the interrelations between Crassulacean acid metabolism, photosynthesis, and respiration. Oxygen exchange of leaf slices was measured polarographically. It was found that the photosynthetic utilization of stored malic acid resulted in a net evolution of oxygen. This oxygen production, and the decrease in acid content of the leaf tissue, were completely inhibited by amytal, although the rate of respiratory oxygen uptake was hardly affected by the presence of this inhibitor of mitochondrial electron transport. Other poisons of respiration (cyanide) and of the tricarboxylic acid cycle (trifluoroacetate, 2-diethyl malonate) also were effective in preventing acid-dependent oxygen evolution. It is concluded that the mobilization of stored acids during light-dependent deacidification of the leaves depends on the operation of the tricarboxylic acid cycle and of the electron transport of the mitochondria.
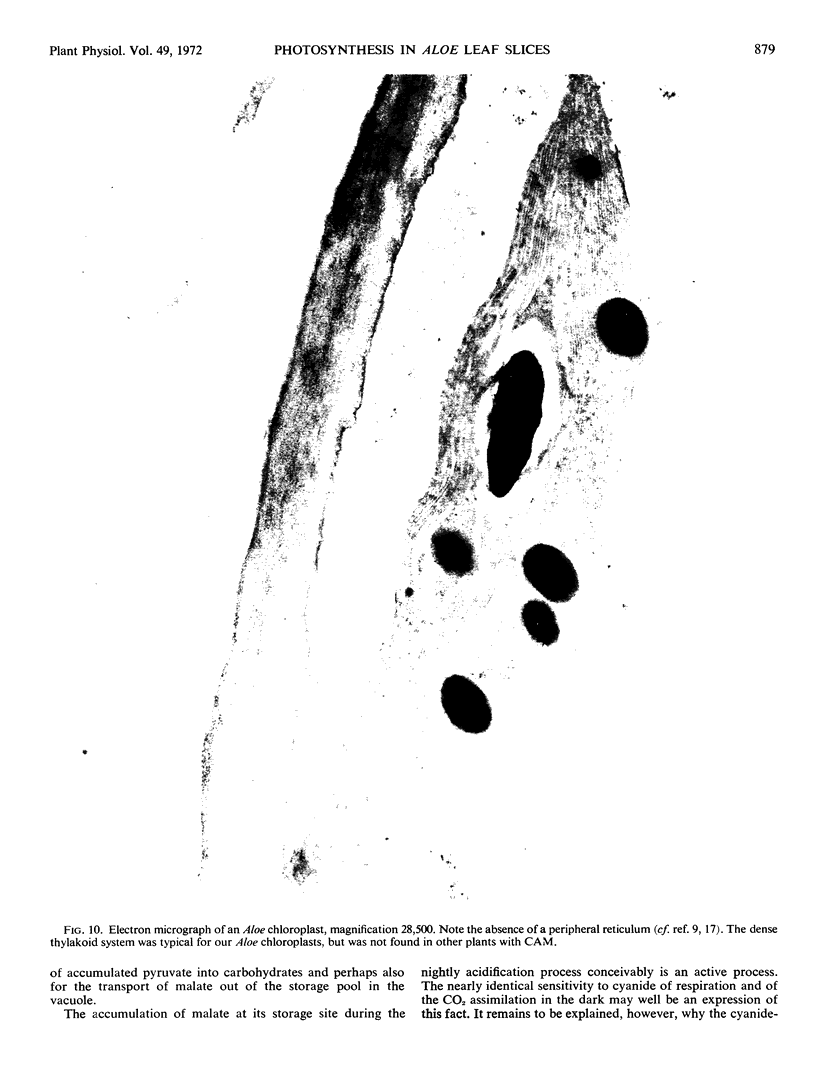
A comparison of enzyme activities in extracts from Aloe leaves and from other plants and studies of leaf anatomy and chloroplast morphology revealed typical characteristics of C3−, as well as C4−, plants in Aloe.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Hatch M. D. Properties and mechanism of action of pyruvate, phosphate dikinase from leaves. Biochem J. 1969 Aug;114(1):117–125. doi: 10.1042/bj1140117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon P. C. Temperature features of enzymes affecting crassulacean Acid metabolism. Plant Physiol. 1967 Jul;42(7):977–984. doi: 10.1104/pp.42.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYSE A., JOLCHINE G. Les variations quantitatives des acides organiques des feuilles de Bryophyllum, à l'obscurité et a la lumière, en fonction de la tension partielle de l'oxygène. Bull Soc Chim Biol (Paris) 1956 Jul 10;38(4):761–784. [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C. B., Avadhani P. N. Inhibition of the beta-Carboxylation Pathway of CO(2) Fixation by Bisulfite Compounds. Plant Physiol. 1970 Feb;45(2):228–230. doi: 10.1104/pp.45.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., Homann P. H. Changes of photorespiratory activity with leaf age. Plant Physiol. 1971 Aug;48(2):193–196. doi: 10.1104/pp.48.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid G. H., Gaffron H. Light metabolism and chloroplast structure in chlorophyll-deficient tobacco mutants. J Gen Physiol. 1967 Jan;50(3):563–582. doi: 10.1085/jgp.50.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Reich R., Witt H. T. Electrochromism of chlorophylls and carotenoids in multilayers and in chloroplasts. Naturwissenschaften. 1971 Aug;58(8):414–414. doi: 10.1007/BF00591523. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Vickery H. B. The Effect of Temperature on the Behavior of Malic Acid and Starch in Leaves of Bryophyllum calycinum Cultured in Darkness. Plant Physiol. 1954 Jul;29(4):385–392. doi: 10.1104/pp.29.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. A. Physiological studies on acid metabolism. 4. Phosphoenolpyruvic carboxylase activity in extracts of Crassulacean plants. Biochem J. 1957 Sep;67(1):73–79. doi: 10.1042/bj0670073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B. Energy conservation associated with cyanide-insensitive respiration in plant mitochondria. Biochim Biophys Acta. 1970 Dec 8;223(2):383–387. doi: 10.1016/0005-2728(70)90195-7. [DOI] [PubMed] [Google Scholar]
- ZELITCH I. The role of glycolic acid oxidase in the respiration of leaves. J Biol Chem. 1958 Dec;233(6):1299–1303. [PubMed] [Google Scholar]
- ZELITCH I. alpha-Hydroxysulfonates as inhibitors of the enzymatic oxidation of glycolic and lactic acids. J Biol Chem. 1957 Jan;224(1):251–260. [PubMed] [Google Scholar]