Abstract
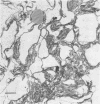
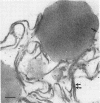
Spherosomes are bounded by unusual single-line “membranes” which measure 2 to 3.5 nanometers in width, contrasted to the well known tripartite unit-membranes which measure 6 to 8.5 nanometers in over-all thickness. Juxtaposed externally (from the side addressing the hyaloplasm), two spherosomal membranes adjoin to form a thicker single line, but apposed internally (the sides that contact stored lipid) two single-line membranes touch to form a tripartite structure resembling a unit-membrane. Morphologically, we interpret the single-line membranes of spherosomes as half unit-membranes whose polar surfaces face the hyaloplasm and whose lipoidal nonpolar surfaces contact internal storage lipid.
Corroboration of this interpretation was shown biochemically by demonstrating the presence of membrane structural protein in peanut spherosomes. In addition, an immunological identity between membrane protein isolated from spherosomes of quiescent seeds and membrane protein extracted from the mitochondrial fraction of 10-day germinated seedlings was observed. We conclude that the atypical, single-line membranes bounding spherosomes are in fact biological membranes that correspond to half unit-membranes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel A. Studies on the compartmentation of lipid in adipose cells. I. Subcellular distribution, composition, and transport of newly synthesized lipid: liposomes. J Lipid Res. 1970 Sep;11(5):420–432. [PubMed] [Google Scholar]
- Bowen C. C., Jensen T. E. Blue-Green Algae: Fine Structure of the Gas Vacuoles. Science. 1965 Mar 19;147(3664):1460–1462. doi: 10.1126/science.147.3664.1460. [DOI] [PubMed] [Google Scholar]
- Daussant J., Neucere N. J., Yatsu L. Y. Immunochemical Studies on Arachis hypogaea Proteins With Particular Reference to the Reserve Proteins. I. Characterization, Distribution, and Properties of alpha-Arachin and alpha-Conarachin. Plant Physiol. 1969 Apr;44(4):471–479. doi: 10.1104/pp.44.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., TISDALE H. D., CRIDDLE R. S., CHEN P. Y., BOCK R. M. Isolation and properties of the structural protein of mitochondria. Biochem Biophys Res Commun. 1961 Jun 2;5:109–114. doi: 10.1016/0006-291x(61)90021-3. [DOI] [PubMed] [Google Scholar]
- Jacks T. J., Yatsu L. Y., Altschul A. M. Isolation and characterization of peanut spherosomes. Plant Physiol. 1967 Apr;42(4):585–597. doi: 10.1104/pp.42.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mani R. S., Zalik S. Physiochemical studies of bean and wheat chloroplast structural protein. Biochim Biophys Acta. 1970 Jan 20;200(1):132–137. doi: 10.1016/0005-2795(70)90051-6. [DOI] [PubMed] [Google Scholar]
- Mollenhauer H. H., Totten C. Studies on seeds. II. Origin and degradation of lipid vesicles in pea and bean cotyledons. J Cell Biol. 1971 Feb;48(2):395–405. doi: 10.1083/jcb.48.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neucere N. J. Isolation of alpha-arachin, the major peanut globulin. Anal Biochem. 1969 Jan;27(1):15–24. doi: 10.1016/0003-2697(69)90215-2. [DOI] [PubMed] [Google Scholar]
- Palmer F. B., Verpoorte J. A. The phosphorus components of solubilized erythrocyte membrane protein. Can J Biochem. 1971 Mar;49(3):337–347. doi: 10.1139/o71-050. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Zilversmit D. B. The surface coat of chylomicrons: electron microscopy. J Lipid Res. 1968 Mar;9(2):187–192. [PubMed] [Google Scholar]
- Singh J., Wasserman A. R. Detection of aggregation and non-destructive disaggregation of membranous proteins using polyacrylamide gel electrophoresis with non-ionic detergents. Biochim Biophys Acta. 1970 Nov 17;221(2):379–382. doi: 10.1016/0005-2795(70)90281-3. [DOI] [PubMed] [Google Scholar]
- Woodward D. O., Munkres K. D. Alterations of a maternally inherited mitochondrial structural protein in respiratory-deficient strains of Neurospora. Proc Natl Acad Sci U S A. 1966 Apr;55(4):872–880. doi: 10.1073/pnas.55.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J., Hensarling T. P. Isolation of spherosomes (oleosomes) from onion, cabbage, and cottonseed tissues. Plant Physiol. 1971 Dec;48(6):675–682. doi: 10.1104/pp.48.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler W. L., Saito A., Fleischer S. Removal of structural protein from mitochondria. Biochem Biophys Res Commun. 1968 Aug 13;32(3):512–518. doi: 10.1016/0006-291x(68)90692-x. [DOI] [PubMed] [Google Scholar]