Abstract
Thymic stromal lymphopoietin (TSLP) has been recently implicated as a key molecule for initiating allergic inflammation at the epithelial cell-dendritic cell (DC) interface. In humans, aberrant TSLP expression is observed in allergic tissues, such as lesional skins of atopic dermatitis, lungs of asthmatics, nasal mucosa of atopic rhinitis and nasal polyps, and ocular surface of allergic keratoconjunctivitis. TSLP is produced predominantly by damaged epithelial cells and stimulates myeloid DCs (mDCs). TSLP-activated mDCs can promote the differentiation of naïve CD4+ T cells into a Th2 phenotype and the expansion of CD4+ Th2 memory cells in a unique manner dependent on OX40L, one of the tumor necrosis factor superfamily members with Th2-promoting function, and lack of production of IL-12. From a genetic point of view, multiple genome-wide association studies have repeatedly identified the TSLP gene as one of the loci associated with susceptibility to allergic diseases. Thus, TSLP is a rational therapeutic target for the treatment of allergic disorders. Elucidating the mechanisms that regulate TSLP expression and the effects of TSLP on orchestrating the immune response toward a Th2 phenotype is essential for developing anti-TSLP therapy.
Keywords: allergic inflammation, dendritic cells (DCs), OX40L, Th2 cells, thymic stromal lymphopoietin (TSLP)
INTRODUCTION
The prevalence of allergic diseases, such as atopic dermatitis, bronchial asthma, and allergic rhinitis, has increased.1 These allergic diseases are the result of a complex immunological effector system that includes T cells, B cells, eosinophils, basophils, and mast cells. Recent progress in understanding the mechanism of these allergic diseases has highlighted the integral role of dendritic cells (DCs) in the cellular cascade responsible for allergic inflammation through the induction of inflammatory T helper type 2 (Th2) cells.2 DCs are considered the most effective cell population that induces distinct immune responses to different pathogens, which is often called “functional plasticity”.3,4 For instance, DCs induce interferon (IFN)-γ-secreting Thl-cells with high cytotoxicity in response to viral and intracellular bacterial infection, whereas the same cells induce Th2 cells that secret interleukin (IL)-4, IL-5, and IL-13, which are required for induction of IgE, hypersecretion of mucus, and recruitment of eosinophils in response to parasite infection. Allergy is a state in which the host overreacts to otherwise innocuous antigens (allergens) by inducing Th2-type inflammation.
Recently, TSLP, IL-25, and IL-33, all epithelial cell-derived cytokines, have been shown to play important roles in the initiation and maintenance of allergic responses in the epithelial surfaces of skin, airway, or gastrointestinal tract.5 Of these cytokines, TSLP represents a key link between epithelial cells and DCs at the interface of allergic inflammation by participating in the programming of DC-mediated Th2 polarization.6 TSLP is produced mainly by damaged epithelial cells and conditions the immune system to induce Th2-type immune responses.6,7 This TSLP-mediated epithelia-immune system axis was shown to be protective against helminth infection.8 However, if other epithelial cells such as keratinocytes and bronchial epithelial cells are part of this axis, it becomes harmful by enhancing Th2-type immunity. Multiple lines of evidence, mainly from mouse experiments, suggest that TSLP acts on several hematopoietic cell types such as DCs, mast cells, NKT cells, eosinophils and basophils to induce allergic inflammation.6,9 However in humans, DCs are the major cell type on which TSLP acts. In this review article, we first summarize the documented association between TSLP and human allergic disorders, and then we describe the cellular and molecular mechanisms by which TSLP activates DCs and induces allergic inflammation.
TSLP AND TSLP RECEPTOR
TSLP is an IL-7-like, four-helix bundle cytokine that was first isolated from a mouse thymic stromal cell line and shown to support lymphocyte development in the absence of IL-7.10,11 The human TSLP (hTSLP) was isolated using a database search method,12,13 and the hTSLP gene was mapped to chromosome 5q22.1, which is near the Th2 cytokine gene cluster loci 5q23–32. Although epithelial cells appear to be the major source of TSLP,7,13 other cell types such as fibroblasts, smooth muscle cells, mast cells and basophils have been shown to have the potential to produce TSLP as well.7,14
The TSLP receptor is a heterodimeric receptor complex consisting of the TSLPR and the IL-7Rα chains13,15–17 (Fig. 1). The TSLPR chain is most closely related to the IL-2Rγ chain (γc). The extracellular portion of the TSLPR chain likely comprises a cytokine recognition homology domain. The intracellular portion of the TSLPR chain harbors a Box 1 motif and a single tyrosine residue, both are involved in signal transduction upon TSLP binding.18,19 TSLP binds to the TSLPR chain with a low affinity, but does not show any affinity to the IL-7Rα chain alone.16,17 However, the combination of TSLPR and IL-7Rα chains results in high-affinity binding to TSLP and transduces, at least, activation of signal transducer and activator of transcription (STAT) 5 upon TSLP binding.13,15
Fig. 1.
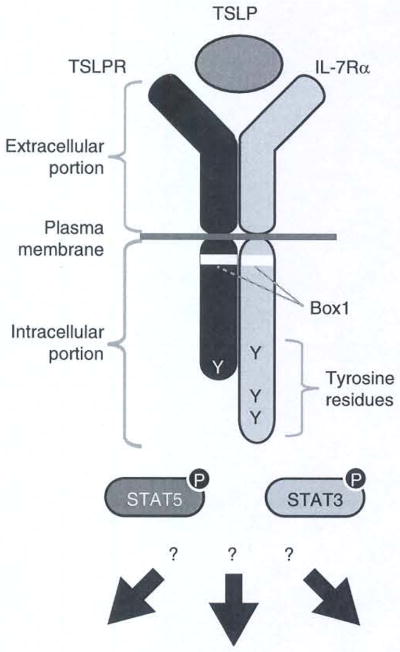
TSLP and TSLP receptor structure. The functional TSLP receptor complex consists of TSLPR and IL-7Rα chains. The extracellular portions of these chains comprise a TSLP-binding unit. The intracellular portions of both receptor chains contain membrane proximal Box1 domains and tyrosine residues that are involved in signal transduction. TSLP stimulation induces activation of several signal transduction pathways including STAT3 and STAT5. However, this activation alone cannot explain the unique function of TSLP in human dendritic cell (DC) activation.
ASSOCIATION OF TSLP WITH HUMAN ALLERGIC DISORDERS
Since 2003, several lines of evidence from mouse in vivo genetic data have solidified the concept that TSLP is a proallergic cytokine that plays a critical role in allergic inflammatory responses.6 At the same time, exaggerated TSLP production has been observed in multiple human allergic disorders such as atopic dermatitis, bronchial asthma, allergic rhinitis, and eosinophilic esophagitis. Moreover, recent large-scale human genetic studies have identified the TSLP gene locus as one of the allergy-susceptibility loci.
ATOPIC DERMATITIS
The link between TSLP and allergic disorders was first suggested just after the molecular cloning of human TSLP. Soumelis et al. demonstrated that the epidermis of lesional skin in patients with atopic dermatitis had higher TSLP expression than that of uninvolved skin or skin from patients with non-allergic dermatitis.7 Interestingly, TSLP expression in patients with atopic dermatitis was associated with the migration of Langerhans cells, which expressed abundant levels of the activation marker DC-LAMP, to the dermis. This suggests that TSLP may contribute to the activation of these cells and promote their migration to the draining lymph nodes, where they prime T-cell responses. A more recent study showed that TSLP protein expression was also upregulated in skin lesions from patients with Netherton syndrome, a severe, genetic skin disease with a constant atopic manifestation caused by mutations in the SPINK5 gene, illuminating a common role of TSLP in the pathogenesis of dermatitis with atopic characteristics.20 Multiple studies have investigated a possible correlation between serum TSLP levels and atopic dermatitis. In adults, serum TSLP levels were not increased in patients with atopic dermatitis,21,22 while one report showed that serum TSLP levels in children with atopic dermatitis were significantly higher than those in normal controls.23 However, serum TSLP levels did not significantly correlate with disease severity, blood eosinophil counts and serum total IgE levels, suggesting that TSLP does not mainly enter the blood circulation. This lack of quantitative correlation was also observed in an earlier study in which TSLP expression levels in lesional epidermis did not correlate with the degree of disease severity or serum IgE levels.7 TSLP produced within the epidermis might contribute in situ to the initiation of disease but not its progression. With regard to this point, Wang et al. suggested that another epithelial cell-derived cytokine, IL-25, plays a supportive functional role in the maintenance of adaptive Th2 memory cells induced by TSLP-activated DCs (TSLP-DCs), leading to the progression of chronic allergic disease24 (Fig. 2).
Fig. 2.
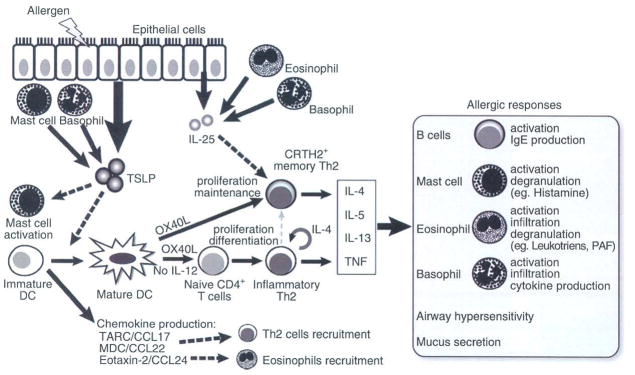
Schematic illustration of the TSLP-mediated allergic inflammation. Insults from allergens or certain pathogens trigger the aberrant expression of TSLP by epithelial cells exposed to the environment (such as skin keratinocytes and airway epithelium), mast cells and basophils. TSLP in turn activates DCs residing in the epithelium to migrate to draining lymph nodes, where they prime naïve T cells. During this process, TSLP-activated DCs (TSLP-DCs) preferentially express OX40L without IL-12 production and concomitantly produce chemokines including TARC/CCL17, MDC/CCL22, and eotaxin-2/CCL24, which function as chemoattractants for Th2 cells and eosinophils. DC-expressing OX40L strongly primes naïve CD4+ T cells to differentiate into inflammatory Th2 cells with the capacity to produce IL-4, IL-5 and IL-13 and TNF, but little or no IL-10. OX40L is the DC-derived trigger to induce the generation of such TNF++ IL-10− inflammatory Th2 cells, while IL-4 derived from the developing Th2 cells is an autocrine enhancer of the inflammatory Th2 response. Furthermore, OX40L on TSLP-DCs and IL-25 from epithelia or infiltrated eosinophils and basophils work synergistically in maintaining the memory Th2 cell response by expansion and further enhancement of Th2 cytokine production. Inflammatory Th2 cytokines eventually initiate allergic responses by activation of B cells, mast cells, eosinophils, basophils, and airway epithelium.
BRONCHIAL ASTHMA
Ying et al. showed by in situ hybridization that TSLP mRNA expression was increased in asthmatic airways and correlated with both the expression of Th2-attracting chemokines and disease severity, which provided the first link between TSLP and human asthma.25 More recently, it was demonstrated that TSLP protein expression in bronchoalveolar lavage fluids of patients with allergic asthma was indeed elevated than in fluids from healthy controls or non-allergic asthmatics.26 However, there is no report regarding serum TSLP levels in asthmatics. Epithelial cells are considered to be the main source of TSLP.6,9 In the lungs, the production of TSLP by bronchial epithelial cells can be enhanced in response to a wide variety of allergens and viral infections. This may particularly explain why respiratory viral infections exacerbate the symptoms of bronchial asthma.27 Augmented TSLP expression may contribute to the conditioning of the response by the local environment to incoming allergens.
ALLERGIC RHINITIS AND NASAL POLYPS
Allergic rhinitis and nasal polyps are chronic inflammatory diseases of the upper airways often associated with asthma and are characterized by increased serum IgE and numbers of eosinophils, Th2 cells, goblet cells, and mast cells. TSLP expression was augmented in the nasal mucosa of allergic rhinitis patients, and TSLP production correlated well with severity of the disease and numbers of eosinophils.28,29 The number of TSLP-expressing cells was significantly greater in the nasal mucosa of allergic rhinitis patients than that of non-allergic rhinitis patients. Furthermore, it was reported that expression of TSLP and Th2-recruiting chemokines, such as thymus- and activation-regulated chemokine (TARC/CCL17) and macrophage-derived chemokines (MDC/CCL22), was markedly higher in nasal polyps than allergic nasal mucosa.30 Importantly, a strong correlation existed between the number of TSLP-expressing cells in nasal polyps and eosinophil counts and IgE levels. Interestingly, an infiltration of IL-4+TNF+ CD4+ T cells was found in the nasal polyposis mucosa of patients with allergic rhinitis, whereas an infiltration of IFN-γ+TNF+ CD4+ T cells was found in the same types of sample from patients with non-allergic rhinitis.31 These observations are consistent with a proposed scenario by which TSLP induces TNF+ inflammatory Th2-type immune response described below.
KERATOCONJUNCTIVITIS
Similar to other types of epithelial cells, human corneal epithelial cells were shown to be capable of producing TSLP in response to an array of Toll-like receptor (TLR) ligands or proinflammatory cytokines.32 Atopic keratoconjunctivitis and vernal keratoconjunctivitis are types of severe, chronic allergic inflammation at the ocular surface in which giant papillae formation is frequently observed and is characterized by local infiltration of eosinophils, Th2 cells, and mast cells. The giant papillae tissues obtained from patients with keratoconjunctivitis had in vivo expression of TSLP mRNA and protein in the epithelium, whereas control conjunctivae specimens did not.33
GENETIC ASSOCIATION OF TSLP GENE AND ALLERGIC DISORDERS
Besides the in vivo TSLP expression data described above, there is another line of evidence suggesting that TSLP plays some roles in human allergic disorders. First, multiple genome-wide studies have found that polymorphisms near or within the TSLP gene were associated with various aspects of allergic inflammation such as IgE levels,34 eosinophil numbers,35 pediatric eosinophilic esophagitis,36 and bronchial asthma.37,38 Importantly, two large meta-analyses of asthma genome-wide association studies involving European and diverse US populations similarly highlighted a robust association of four loci, 17q21 locus, IL1RL1/IL18R, IL33, and TSLP, with asthma.37 These results suggest that epithelial cell-derived factors (IL-33 and TSLP; IL1RL1 encodes an IL-33 receptor) play important roles in the heritable traits of asthma pathogenesis. Furthermore, several “candidate gene approaches” have been taken to identify the genes associated with susceptibility to allergic diseases and have found genetic links between polymorphisms near or within the TSLP gene and atopic dermatitis,39 bronchial asthma,40,41 and allergic rhinitis.42 Functionally, one of the polymorphisms in the regulatory element of the TSLP gene creates a binding site for activating protein-1 (AP-1) and affects the transcriptional efficiency of TSLP induced by stimulation with double-stranded RNA, which mimics viral infection, in bronchial epithelial cells, indicating that a genetic factor could affect environmental-driven TSLP production.43 A genetic association between the TSLPR gene and eosinophilic esophagitis was also reported, substantiating that the TSLP/ TSLPR system is an important disease-susceptible factor for eosinophilic esophagitis, a chronic Th2-associated inflammatory disease.44
TSLP-ACTIVATED DCs MEDIATE Th2 INFLAMMATION THROUGH OX40L
TSLP is a key cytokine that initiates DC-mediated Th2 immune response in the allergic cascade (Fig. 2). TSLP-DCs can induce naïve T cells to differentiate into a new type of effector Th2 cells producing IL-4, IL-5, IL-13, and TNF, but not IL-10.7 Historically, Th2 cells are defined as effector T cells with the capacity to produce IL-4, IL-5, IL-13, and IL-10.45,46 However, IL-10 is recognized as an anti-inflammatory cytokine that suppresses allergic inflammation.47,49 Many studies have demonstrated that such conventional Th2 cells are not involved in allergic inflammation.50–52 In contrast to IL-10, TNF has been implicated in Th2 cell-mediated allergic diseases.53–56 In this context, the TSLP-DC-induced TNF-highly positive and IL-10-negative “inflammatory Th2” cells most likely represent the pathogenic Th2 cells that cause allergic inflammation.57
OX40 ligand (OX40L) was identified as the critical downstream mediator of TSLP-DCs that induces inflammatory Th2 cell responses.57 It belongs to the TNF superfamily that has been implicated in the initiation of Th2 cell responses.58–60 In contrast to TLR ligands such as poly (I:C) and LPS, TSLP stimulates human myeloid DCs to upregulate OX40L preferentially.57 Blockade of OX40L using neutralizing antibodies to OX40L dramatically inhibited production of IL-4, IL-5, IL-13, and TNF and promoted IL-10 production by CD4+ T cells primed by TSLP-DCs.57 In addition, recombinant OX40L-transfected cells, like TSLP-DCs, strongly primed naïve CD4+ T cells to produce IL-4, IL-5, IL-13 and TNF, but little IL-10. These data confirm that OX40L is the original trigger of TSLP-DCs to induce the generation of TNF++IL-10− inflammatory Th2 cells. On a transcriptional basis, it was determined that OX40L expressed by TSLP-DCs induced the expression of GATA-3 in T cells, further supporting their critical roles in Th2 polarization.57
In addition to preferential induction of OX40L expression, another specific feature of TSLP-DCs is a lack of proinflammatory cytokine production including IL-12 as well as type I IFNs, both of which are factors required for Thl differentiation.4,7 The OX40L-mediated inflammatory Th2 cell differentiation depends critically on the absence of IL-12, as IL-12 shows dominant effect over OX40L in Th cell differentiation.57 Thus, the development of inflammatory Th2 cells leading to allergic inflammation requires two immunological conditions, an expression of OX40L and an absence of IL-12.
Although IL-4 is the key Th2-polarizing signal,4,46,61 human DCs activated by TSLP or other stimuli have no capacity to produce IL-4,7,57 Thus, IL-4 is not the DC-derived original trigger of Th2 responses. However, blockade of IL-4 using a neutralizing antibody to IL-4 in a coculture system of TSLP-DCs and naïve T cells demonstrated that IL-4 contributed to the generation of Th2 cells induced by TSLP-DCs but had no effect on TNF or IL-10 production in the Th2 cells.57 These findings suggest that IL-4 derived from the developing Th2 cells is an autocrine enhancer, and DC-derived OX40L and Th2 cell-derived IL-4 could work synergistically in driving Th2 cell responses.
Recent studies have suggested that memory Th2 cells are the principal cell population responsible for the maintenance of chronic allergic inflammation and the relapse of allergic inflammation upon re-exposure to allergens.62–65 Elevated TSLP expression in inflamed keratinocytes of skin samples from patients with atopic dermatitis,7 together with the findings that CRTH2+ memory Th2 cells had infiltrated the dermis of legional skin and were present at increased frequency in the blood of atopic dermatitis patients66 indicate the pathophysiological importance of the link between TSLP and memory Th2 cells in allergic diseases (Fig. 2). Also in regard to this point, TSLP-DCs play a role in the homeostatic expansion of allergen-specific Th2 memory cells and in the further polarization of the Th2 phenotypes, contributing to the maintenance of chronic allergic inflammation.67 OX40L on TSLP-DCs also aids this process and enhances DC survival by allowing formation of prolonged DC-T cell conjugates.67 Moreover, TSLP equips DCs with the capacity to produce a variety of chemokines such as TARC/CCL17, MDC/CCL22, I-309/CCL1, eotaxin-2/CCL24 as well as IL-8/CXCL8,7,57 which function as chemoattractants for Th2 cells, eosinophils, and neutrophils, contributing to aggravation or maintenance of chronic allergic inflammation. In this process, IL- 25, another epithelial cell-derived cytokine aberrantly expressed in atopic dermatitis skin lesion and asthmatic lung tissues, enhances memory Th2 cell expansion and function induced by TSLP-DCs24 (Fig. 2). While TSLP is further produced by mast cells and basophils,7,14,68 IL-25 is further produced by eosinophils and basophils.24 Thus, epithelial cell-derived cytokines collaboratively conduct the Th2 cell-mediated inflammation that tailors the activation of mast cells and eosinophils in the development and maintenance of allergic disorders.
TSLP SIGNAL TRANSDUCTION IN HUMAN DCs
Upon binding to TSLP, the TSLP receptor complex generates intracellular signaling. Earlier studies demonstrated that TSLP induces STAT3 and STAT5 phosphorylation, resulting in transcription of the STAT-responsive genes, such as CIS15,13,69 (Fig. 1). However, the precise kinase responsible for TSLP-mediated STAT phosphorylation has long remained controversial.15,18,69 Moreover, the pleiotropic function of TSLP in human myeloid DCs apparently cannot be explained by activation of STAT3 and STAT5 alone, which is ubiquitous in many cytokine signaling pathways. Thus, we performed a large-scale isolation of human primary DCs to study intracellular signaling triggered by TSLP70 (Fig. 3). In myeloid DCs, hTSLP induced robust and sustained (−1 h) phosphorylation of JAK1 and JAK2, while hIL-7 induced only transient (−5 min) phosphorylation of JAK1. Consequently, hTSLP induced broad and sustained (>2 h) phosphorylation of STAT1, STAT3, STAT4, STAT5, and STAT6. Direct STAT6 activation by TSLP seemed to be the responsible mechanism for inducing TARC/CCL17 production.70 In addition, hTSLP induceed phosphorylation of AKT and the MAPKs ERK and JNK, all are sensitive to JAK inhibitors. hTSLP also induced a slow but robust NF-κB activation, as revealed by sustained nuclear localization of p50, p52, and RelB.
Fig. 3.
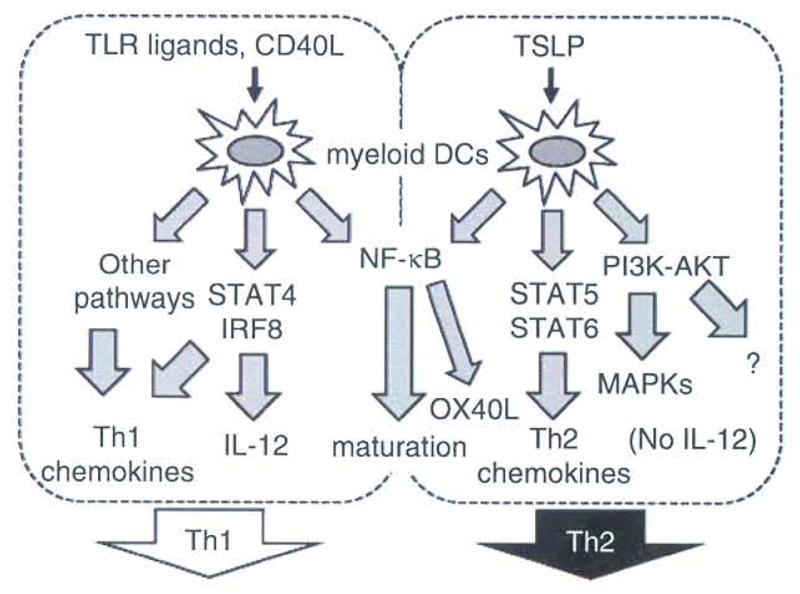
Distinct signal codes generate the functional plasticity of human myeloid DCs (mDCs). DC activators such as TLR ligands and CD40L induce mDC maturation and IL-12 production, thus promoting Th1 responses. This IL-12 production is granted by co-expression and activation of STAT4 and IRF8. By contrast, TSLP induces mDC maturation that is uncoupled from IL-12 production due to lack of induction of protein expression of STAT4 and IRF8. TSLP robustly activates other STATs including STAT6, which leads to production of TARC/CCL17, a Th2-attracting chemokine. Furthermore, TSLP induces OX40L expression by mDCs owing to unique activation of the NF-κB pathway. Consequently, TSLP-activated mDCs induce Th2 responses. TSLP also activates PI3K-AKT and some MAPK pathways, whose functions remain elusive.
How is OX40L selectively upregulated by TSLP-DCs? The promoter region of OX40L has two potential NF-κB binding elements. We found that TSLP induced a predominant and persistent nuclear translocation of p50, an NF-κB molecule, which is not prolonged when induced by CD40L or TLR ligands. Indeed, the OX40L promoter preferentially bound p50.70 In a promoter-reporter assay, we also demonstrated that p50 plus RelB activated the OX40L promoter. These data suggest that predominant p50 activation triggered by TSLP is the determinant for selective upregulation of OX40L as described previously.
Why TSLP-mediated DC maturation is uncoupled from IL-12 production, also as described above, was another critical issue to be addressed because most DC activators, such as CD40L and TLR ligands, induce both DC maturation and IL-12 production to induce Th1 responses.3 Unlike the DC activators capable of inducing expression of both STAT4 and interferon regulatory factor (IRF) 8 that are required for IL-12 production, TSLP simply fails to induce protein expression of these transcription factors. It is of great interest that TSLP has the capacity to activate STAT6, a transcription factor involved in a Th2 response, but not STAT4 in DCs.70 In T helper cell differentiation, STAT4 and STAT6 show the opposite capacity to promote Th1- versus Th2-type immune responses, respectively, functioning within T cells. In addition, STAT4 critically functions also in DCs for a Th1 response.71 Our study suggests that STAT6 functions not only in T cells, but also in DCs to shape Th2-type immune responses. Taken together, TSLP programs DCs to induce a Th2 response through selective activation and inactivation of multiple signaling pathways, forming a unique signal code.
FUTURE PERSPECTIVES
TSLP expression in epithelia closely links the initiation and maintenance of inflammatory Th2 cell responses to its unique DC activation capacity. Furthermore, OX40L expression by TSLP-activated DCs appears to be a key pathway leading to allergic inflammation. Thus, the TSLP-OX40L axis represents a potential molecular target against allergic disorders. Studies using neutralizing antibody to OX40L or OX40L-deficient mice have demonstrated the importance of OX40-OX40L interaction for triggering Th2 cell immune responses leading to allergic inflammation in vivo.72–75 Particularly, Seshasayee et al. demonstrated that treatment with OX40L-neutralizing antibodies inhibited antigen-driven Th2 immune responses, eosinophil infiltration, and IgE production induced by TSLP in mice and rhesus monkeys.75 This strongly suggests that, also in vivo, TSLP-induced OX40L appears to be a critical mediator of allergic inflammation.
Two adjuvants have been reported to alter the ability of TSLP to induce the pathogenic inflammatory Th2 cell responses in vitro. One is Bacillus Calmette-Guérin (BCG), an attenuated derivative of a virulent strain of Mycobacterium bovis, and the other is imidazoquinoline, a specific ligand for TLR7 and TLR8,76 that is used to topically treat viral-mediated skin diseases by cream formulation. BCG and imidazoquinoline serve as strong immune adjuvants leading to a Th1 response via maturation of DCs.77–79 Both agents could function as potent inhibitors of OX40L expression on human DCs, while they induce IL-12 production by DCs, even in the presence of TSLP.80,81 As a result, they could cancel the DC-mediated Th2-permissive condition and in turn induce the generation of IL-10+IFN-γ+ regulatory Th1 cells.80,81 This type of Th1-like T cells has been reported to possess the ability to inhibit airway hyperresponsiveness.82 Moreover, imidazoquinoline can block the functional characteristics of CRTH2+ CD4+ Th2 memory cells that are expanded and maintained by TSLP-DCs.81 In terms of signal transduction of TSLP in DCs, a JAK inhibitor clearly shut down the TSLP-dependent signals.70 Thus, using a JAK inhibitor for treatment of allergic diseases proposed recently83 might also dampen the TSLP signal transduction, leading to alleviation of disease symptoms.
In summary, TSLP/OX40L blockade is an attractive therapeutic approach for the treatment of severe allergic diseases. The use of drugs to treat allergic disorders has historically focused on the effector phase of the allergic immune cascade including activation of T cells, mast cells, and eosinophils. The newly emergent concept to target TSLP, DCs, and/or OX40L as key players at the initiation phase of the allergic immune cascade is novel and thus might pave the way for the development of new treatment for allergic disorders.
Acknowledgments
We thank Ms. Melissa J. Wentz for critical reading of the manuscript.
Footnotes
Conflict of interest: No potential conflict of interest was disclosed.
References
- 1.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–74. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 2.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce TH2 and tolerogenic responses. Nat Immunol. 2010;11:647–55. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Moser M, Murphy KM. Dendritic cell regulation of TH1–TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 5.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–90. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu YJ, Soumelis V, Watanabe N, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 7.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 8.Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–67. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–93. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–8. [PubMed] [Google Scholar]
- 11.Sims JE, Williams DE, Morrissey PJ, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;192:671–80. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quentmeier H, Drexler HG, Fleckenstein D, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–92. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 13.Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 14.Sokol CL, Barton GM, Fair AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin SD, Koelling RM, Friend SL, et al. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677–83. [PubMed] [Google Scholar]
- 16.Park LS, Martin U, Garka K, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–70. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 18.Carpino N, Thierfelder WE, Chang MS, et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–92. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaksen DE, Baumann H, Zhou B, et al. Uncoupling of proliferation and Stat5 activation in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 2002;168:3288–94. doi: 10.4049/jimmunol.168.7.3288. [DOI] [PubMed] [Google Scholar]
- 20.Briot A, Deraison C, Lacroix M, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–47. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyagaki T, Sugaya M, Fujita H, Saeki H, Tamaki K. Increased serum thymic stromal lymphopoietin levels in patients with cutaneous T cell lymphoma. Clin Exp Dermatol. 2009;34:539–40. doi: 10.1111/j.1365-2230.2008.02990.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Miyata M, Ohba T, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to TH2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122:1208–14. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, Kim KE. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21:e457–60. doi: 10.1111/j.1399-3038.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin Immunol. 2010;6:4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welliver RC, Kaul TN, Ogra PL. The appearance of cell-bound IgE in respiratory-tract epithelium after respiratory-syncytial-virus infection. N Engl J Med. 1980;303:1198–202. doi: 10.1056/NEJM198011203032103. [DOI] [PubMed] [Google Scholar]
- 28.Mou Z, Xia J, Tan Y, et al. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. 2009;129:297–301. doi: 10.1080/00016480802225884. [DOI] [PubMed] [Google Scholar]
- 29.Zhu DD, Zhu XW, Jiang XD, Dong Z. Thymic stromal lymphopoietin expression is increased in nasal epithelial cells of patients with mugwort pollen sensitive-seasonal allergic rhinitis. Chin Med J (Engl) 2009;122:2303–7. [PubMed] [Google Scholar]
- 30.Kimura S, Pawankar R, Mori S, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011;3:186–93. doi: 10.4168/aair.2011.3.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T, Li TL, Zhao F, et al. Role of thymic stromal lymphopoietin in the pathogenesis of nasal polyposis. Am J Med Sci. 2011;341:40–7. doi: 10.1097/MAJ.0b013e3181f20489. [DOI] [PubMed] [Google Scholar]
- 32.Ma P, Bian F, Wang Z, et al. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol Vis Sci. 2009;50:2702–9. doi: 10.1167/iovs.08-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda A, Ebihara N, Yokoi N, et al. Functional role of thymic stromal lymphopoietin in chronic allergic kerato-conjunctivitis. Invest Ophthalmol Vis Sci. 2010;51:151–5. doi: 10.1167/iovs.09-4183. [DOI] [PubMed] [Google Scholar]
- 34.Hunninghake GM, Lasky-Su J, Soto-Quiros ME, et al. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177:830–6. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gudbjartsson DF, Bjornsdottir US, Halapi E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–7. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 36.Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–91. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao PS, Rafaels NM, Mu D, et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol. 2010;125:1403–7. doi: 10.1016/j.jaci.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunninghake GM, Soto-Quiros ME, Avila L, et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010;65:1566–75. doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harada M, Hirota T, Jodo AI, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44:787–93. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunyavanich S, Melen E, Wilk JB, et al. Thymic stromal lymphopoietin (TSLP) is associated with allergic rhinitis in children with asthma. Clin Mol Allergy. 2011;9:1. doi: 10.1186/1476-7961-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada M, Hirota T, Jodo AI, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:368–74. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 44.Sherrill JD, Gao PS, Stucke EM, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:160–5. doi: 10.1016/j.jaci.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 46.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 47.Zuany-Amorim C, Haile S, Leduc D, et al. Interleukin-10 inhibits antigen-induced cellular recruitment into the airways of sensitized mice. J Clin Invest. 1995;95:2644–51. doi: 10.1172/JCI117966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 49.Oh JW, Seroogy CM, Meyer EH, et al. CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J Allergy Clin Immunol. 2002;110:460–8. doi: 10.1067/mai.2002.127512. [DOI] [PubMed] [Google Scholar]
- 50.Sloan-Lancaster J, Evavold BD, Allen PM. Th2 cell clonal anergy as a consequence of partial activation. J Exp Med. 1994;180:1195–205. doi: 10.1084/jem.180.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Constant SL, Bottomly K. Induction of Thl and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 52.O’Garra A, Steinman L, Gijbels K. CD4+ T-cell subsets in autoimmunity. Curr Opin Immunol. 1997;9:872–83. doi: 10.1016/s0952-7915(97)80192-6. [DOI] [PubMed] [Google Scholar]
- 53.Artis D, Humphreys NE, Bancroft AJ, Rothwell NJ, Potten CS, Grencis RK. Tumor necrosis factor alpha is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J Exp Med. 1999;190:953–62. doi: 10.1084/jem.190.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen L, Martinez O, Overbergh L, Mathieu C, Prabhakar BS, Chan LS. Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin Exp Immunol. 2004;138:375–87. doi: 10.1111/j.1365-2249.2004.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwasaki M, Saito K, Takemura M, et al. TNF-α contributes to the development of allergic rhinitis in mice. J Allergy Clin Immunol. 2003;112:134–40. doi: 10.1067/mai.2003.1554. [DOI] [PubMed] [Google Scholar]
- 56.Kips JC. Cytokines in asthma. Eur Respir J Suppl. 2001;34:24s–33s. doi: 10.1183/09031936.01.00229601. [DOI] [PubMed] [Google Scholar]
- 57.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohshima Y, Yang LP, Uchiyama T, et al. OX40 costimulation enhances interleukin-4 (IL4) expression at priming and promotes the differentiation of naive human CD4+ T cells into high IL4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 59.Akiba H, Miyahira Y, Atsuta M, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191:375–80. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193:387–92. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol. 2003;15:620–6. doi: 10.1016/j.coi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Bohle B, Schwihla H, Hu HZ, et al. Long-lived Th2 clones specific for seasonal and perennial allergens can be detected in blood and skin by their TCR-hypervariable regions. J Immunol. 1998;160:2022–7. [PubMed] [Google Scholar]
- 63.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 64.Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-lived Th2 memory in experimental allergic asthma. J Immunol. 2002;169:4788–96. doi: 10.4049/jimmunol.169.9.4788. [DOI] [PubMed] [Google Scholar]
- 65.Morimoto M, Whitmire J, Xiao S, et al. Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J Immunol. 2004;172:2424–30. doi: 10.4049/jimmunol.172.4.2424. [DOI] [PubMed] [Google Scholar]
- 66.Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972–9. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 67.Wang YH, Ito T, Homey B, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–38. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 68.Moon PD, Kim HM. Thymic stromal lymphopoietin is expressed and produced by caspase-1/NF-κB pathway in mast cells. Cytokine. 2011;54:239–43. doi: 10.1016/j.cyto.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–7. [PubMed] [Google Scholar]
- 70.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukao T, Frucht DM, Yap G, Gadina M, O’Shea JJ, Koyasu S. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J Immunol. 2001;166:4446–55. doi: 10.4049/jimmunol.166.7.4446. [DOI] [PubMed] [Google Scholar]
- 72.Arestides RS, He H, Westlake RM, et al. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–80. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 73.Hoshino A, Tanaka Y, Akiba H, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33:861–9. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 74.Salek-Ardakani S, Song J, Halteman BS, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seshasayee D, Lee WP, Zhou M, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–78. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jurk M, Heil F, Vollmer J, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 77.Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–12. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 79.Uehira K, Amakawa R, Ito T, et al. Dendritic cells are decreased in blood and accumulated in granuloma in tuberculosis. Clin Immunol. 2002;105:296–303. doi: 10.1006/clim.2002.5287. [DOI] [PubMed] [Google Scholar]
- 80.Yokoi T, Amakawa R, Tanijiri T, et al. Mycobacterium bovis Bacillus Calmette-Guerin suppresses inflammatory Th2 responses by inducing functional alteration of TSLP-activated dendritic cells. Int Immunol. 2008;20:1321–9. doi: 10.1093/intimm/dxn094. [DOI] [PubMed] [Google Scholar]
- 81.Torii Y, Ito T, Amakawa R, et al. Imidazoquinoline acts as immune adjuvant for functional alteration of thymic stromal lymphopoietin-mediated allergic T cell response. J Immunol. 2008;181:5340–9. doi: 10.4049/jimmunol.181.8.5340. [DOI] [PubMed] [Google Scholar]
- 82.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyperreactivity. Nat Immunol. 2004;5:1149–56. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 83.Matsunaga Y, Inoue H, Fukuyama S, et al. Effects of a Janus kinase inhibitor, pyridone 6, on airway responses in a murine model of asthma. Biochem Biophys Res Commun. 2011;404:261–7. doi: 10.1016/j.bbrc.2010.11.104. [DOI] [PubMed] [Google Scholar]


