Abstract
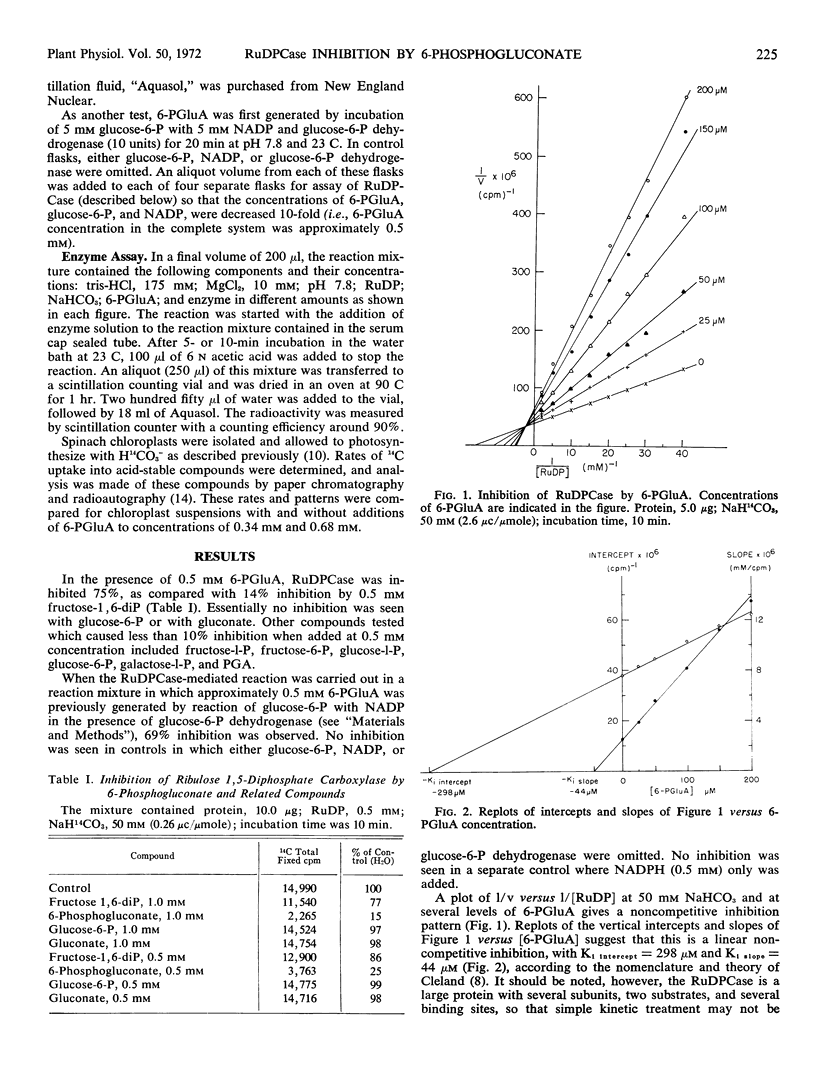
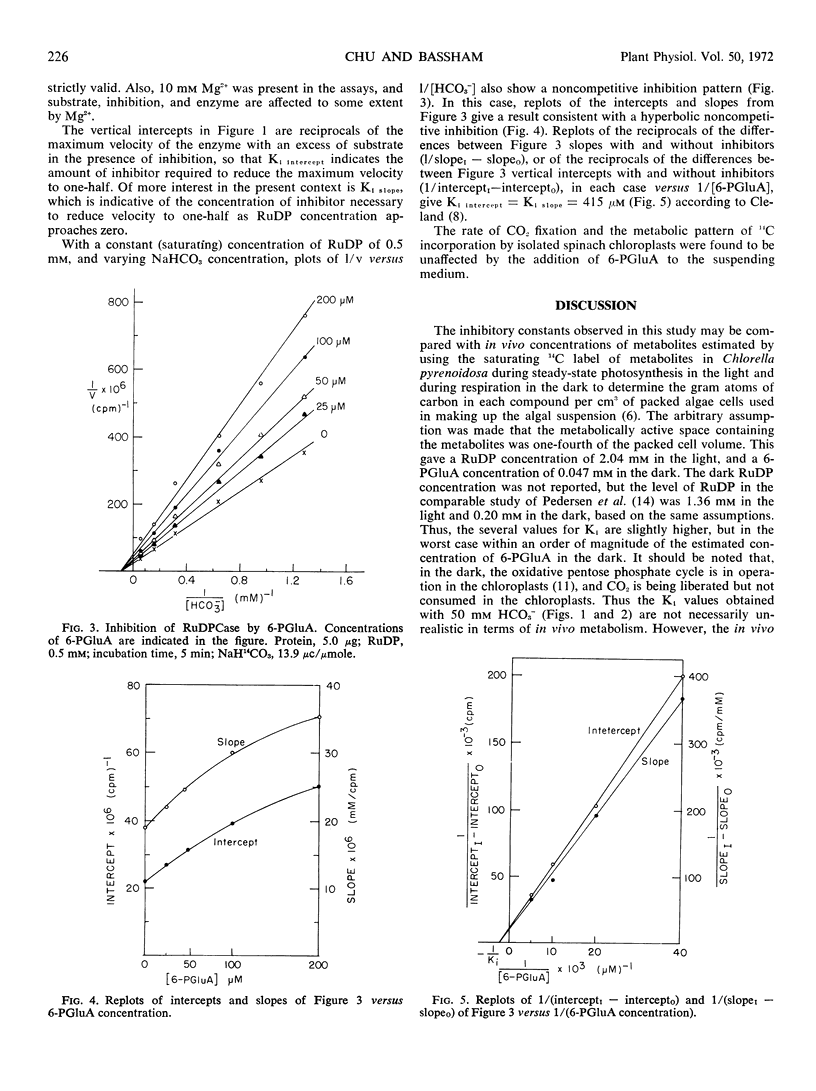
6-Phosphogluconate is a much more effective inhibitor of the photosynthetic carboxylation enzyme, ribulose-1, 5-diphosphate carboxylase, than other sugar phosphates and sugar acids of the reductive and oxidative pentose phosphate cycles. The inhibition appears to be noncompetitive with ribulose 1,5-diphosphate. Since 6-phosphogluconate is unique to the oxidative cycle and inhibits at concentrations comparable to those found in vivo, it is proposed that its inhibition of the carboxylase may be a regulatory factor. If so, it would operate during darkness as a different control factor from those factors postulated to activate the carboxylase during photosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassham J. A., Krause G. H. Free energy changes and metabolic regulation in steady-state photosynthetic carbon reduction. Biochim Biophys Acta. 1969 Oct 21;189(2):207–221. doi: 10.1016/0005-2728(69)90048-6. [DOI] [PubMed] [Google Scholar]
- Bassham J. A. Photosynthetic carbon metabolism. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2877–2882. doi: 10.1073/pnas.68.11.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham J. A. The control of photosynthetic carbon metabolism. Science. 1971 May 7;172(3983):526–534. doi: 10.1126/science.172.3983.526. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Heber U., Hallier U. W., Hudson M. A. Untersuchungen zur intrazellulären Verteilungen von Enzymen und Substraten in der Blattzelle. II. Lokalisation von Enzymen des reduktiven und dem oxydativen Pentosephosphat-Zyklus in den Chloroplasten und Permeabilität der Chloroplasten-Membran gegenüber Metaboliten. Z Naturforsch B. 1967 Nov;22(11):1200–1215. [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. 3. Light activation of the carboxylation reaction. Biochim Biophys Acta. 1968 Jan 15;153(1):227–234. doi: 10.1016/0005-2728(68)90164-3. [DOI] [PubMed] [Google Scholar]
- Krause G. H., Bassham J. A. Induction of respiratory metabolism in illuminated Chlorella pyrenoidosa and isolated spinach chloroplasts by the addition of vitamin K5. Biochim Biophys Acta. 1969 Apr 8;172(3):553–565. doi: 10.1016/0005-2728(69)90151-0. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Pedersen T. A., Kirk M., Bassham J. A. Inhibition of photophosphorylation and photosynthetic carbon cycle reactions by fatty acids and esters. Biochim Biophys Acta. 1966 Feb 7;112(2):189–203. doi: 10.1016/0926-6585(66)90320-7. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Racker E. The reductive pentose phosphate cycle. III. Enzyme activities in cell-free extracts of photosynthetic organisms. Plant Physiol. 1961 Jul;36(4):409–414. doi: 10.1104/pp.36.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWN P. W. AN IMPROVED METHOD FOR THE ISOLATION OF CARBOXYDISMUTASE. PROBABLE IDENTITY WITH FRACTION I PROTEIN AND THE PROTEIN MOIETY OF PROTOCHLOROPHYLL HOLOCHROME. Biochemistry. 1965 May;4:908–918. doi: 10.1021/bi00881a018. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HORECKER B. L., HURWITZ J. The enzymatic formation of phosphoglyceric acid from ribulose diphosphate and carbon dioxide. J Biol Chem. 1956 Feb;218(2):795–810. [PubMed] [Google Scholar]


