Abstract
The pool sizes of the common amino acids in purified intact chloroplasts from Vicia faba L. were measured (nanomoles per milligram chlorophyll). The three amino acids present in the highest concentrations were glutamate, aspartate, and threonine. Alanine, serine, and glycine were each present at levels between 15 and 20 nanomoles per milligram chlorophyll and 13 other amino acids were detectable at levels below 10.
Only aspartate, alanine, glycine, serine, threonine, and lysine became labeled during photosynthetic 14CO2 fixation by isolated chloroplasts: the label in aspartate represented over 60% of the total 14C found in the amino acids. Glutamate dehydrogenase, glutamate-oxaloacetate, and glutamate-pyruvate aminotransferases were present in the chloroplasts, but no other transferase activities from glutamate could be detected. The chloroplasts were able to synthesize a total of 17 other protein amino acids from either alanine or aspartate, but no synthesis of leucine by aminotransferase reactions could be detected. The synthesis of aspartate was studied in more detail. The enzyme systems required for the generation of oxaloacetate from triose phosphate were virtually absent from the chloroplasts but present in the leaf cytoplasmic fraction. Addition of either a leaf “cytoplasmic” fraction or an oxaloacetate generating system resulted in an increased proportion of the total 14C fixed being found in the amino acid fraction during photosynthetic 14CO2 fixation.
It is suggested that the supply of oxaloacetate from the cytoplasm is one of the important factors controlling the synthesis of amino acids by the chloroplast.
Full text
PDF



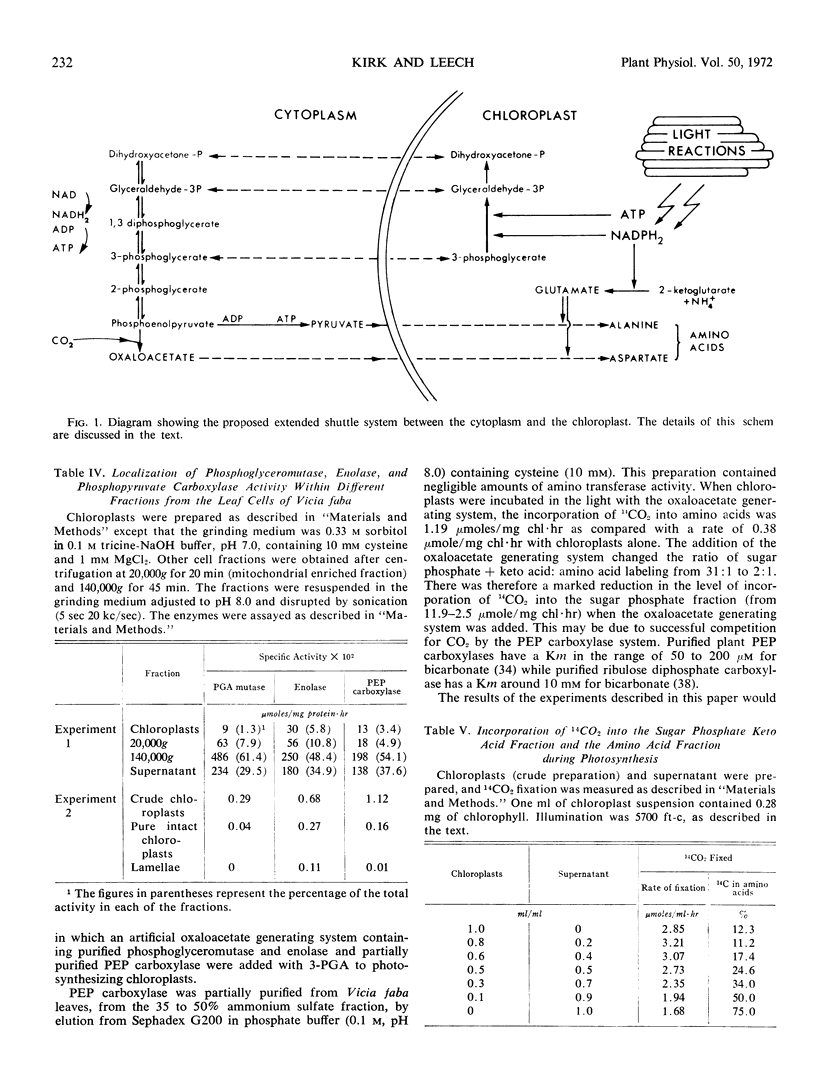


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff S., Benson A., Hassid W. Z., Calvin M. Distribution of C14 in Photosynthesizing Barley Seedlings. Science. 1947 Jun 27;105(2739):664–665. doi: 10.1126/science.105.2739.664. [DOI] [PubMed] [Google Scholar]
- BASSHAM J. A., KIRK M. PHOTOSYNTHESIS OF AMINO ACIDS. Biochim Biophys Acta. 1964 Sep 4;90:553–562. doi: 10.1016/0304-4165(64)90234-x. [DOI] [PubMed] [Google Scholar]
- Bucke C., Walker D. A., Baldry C. W. Some effects of sugars and sugar phosphates on carbon dioxide fixation by isolated chloroplasts. Biochem J. 1966 Dec;101(3):636–641. doi: 10.1042/bj1010636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. H., Tolbert N. E. Distribution of C in Serine and Glycine after CO(2) Photosynthesis by Isolated Chloroplasts. Modification of Serine-C Degradation. Plant Physiol. 1965 Nov;40(6):1048–1052. doi: 10.1104/pp.40.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson R. G., Gibbs M. Photosynthetic assimilation of carbon dioxide and acetate by isolated chloroplasts. Plant Physiol. 1967 Aug;42(8):1153–1154. doi: 10.1104/pp.42.8.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan C. V., Givan A. L., Leech R. M. Photoreduction of alpha-Ketoglutarate to Glutamate by Vicia faba Chloroplasts. Plant Physiol. 1970 May;45(5):624–630. doi: 10.1104/pp.45.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Hallier U. W., Hudson M. A. Untersuchungen zur intrazellulären Verteilungen von Enzymen und Substraten in der Blattzelle. II. Lokalisation von Enzymen des reduktiven und dem oxydativen Pentosephosphat-Zyklus in den Chloroplasten und Permeabilität der Chloroplasten-Membran gegenüber Metaboliten. Z Naturforsch B. 1967 Nov;22(11):1200–1215. [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A., Hudson M. A., Hallier U. W. Untersuchungen zur intrazellulären Verteilung von Enzymen und Substraten in der Blattzelle. I Intrazellulärer Transport von Zwischenprodukten der Photosynthese im Photosynthese-Gleichgewicht und im Dunkel-Licht-Dunkel-Wechsel. Z Naturforsch B. 1967 Nov;22(11):1189–1199. [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- LEECH R. M., ELLIS R. J. Coprecipitation of mitochondria and chloroplasts. Nature. 1961 May 27;190:790–792. doi: 10.1038/190790a0. [DOI] [PubMed] [Google Scholar]
- LEECH R. M. THE ISOLATION OF STRUCTURALLY INTACT CHLOROPLASTS. Biochim Biophys Acta. 1964 May 25;79:637–639. doi: 10.1016/0926-6577(64)90235-9. [DOI] [PubMed] [Google Scholar]
- Leech R. M., Kirk P. R. An NADP-dependent L-glutamate dehydrogenase from chloroplasts of Vicia faba L. Biochem Biophys Res Commun. 1968 Aug 21;32(4):685–690. doi: 10.1016/0006-291x(68)90293-3. [DOI] [PubMed] [Google Scholar]
- Marino G., Greco A. M., Scardi V., Zito R. Purification and general properties of aspartate aminotransferase of ox heart. Biochem J. 1966 Jun;99(3):589–594. doi: 10.1042/bj0990589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P. S., Wang C. T. Amino acid permeability of pea chloroplasts as measured by osmotically determined reflection coefficients. Biochim Biophys Acta. 1970 Jul 7;211(1):79–87. doi: 10.1016/0005-2736(70)90125-2. [DOI] [PubMed] [Google Scholar]
- Ongun A., Stocking C. R. Effect of Light on the Incorporation of Serine into the Carbohydrates of Chloroplasts and Nonchloroplast Fractions of Tobacco Leaves. Plant Physiol. 1965 Sep;40(5):819–824. doi: 10.1104/pp.40.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Stocking C. R. Oxygen evolution and the permeability of the outer envelope of isolated whole chloroplasts. Plant Physiol. 1968 Oct;43(10):1597–1604. doi: 10.1104/pp.43.10.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Stocking C. R. Intracellular localization of enzymes in leaves and chloroplast membrane permeability to compounds involved in amino acid syntheses. Z Naturforsch B. 1969 Sep;24(9):1170–1179. doi: 10.1515/znb-1969-0915. [DOI] [PubMed] [Google Scholar]
- Stepka W., Benson A. A., Calvin M. The Path of Carbon in Photosynthesis: II. Amino Acids. Science. 1948 Sep 17;108(2803):304–304. doi: 10.1126/science.108.2803.304. [DOI] [PubMed] [Google Scholar]
- Stocking C. R., Larson S. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochem Biophys Res Commun. 1969 Oct 8;37(2):278–282. doi: 10.1016/0006-291x(69)90731-1. [DOI] [PubMed] [Google Scholar]
- Urbach W., Hudson M. A., Ullrich W., Santarius K. A., Heber U. Verteilung und Wanderung von Phosphoglycerat zwischen den Chloroplasten und dem Cytoplasma während der Photosynthese. Z Naturforsch B. 1965 Sep;20(9):890–898. [PubMed] [Google Scholar]
- WEISSBACH A., HORECKER B. L., HURWITZ J. The enzymatic formation of phosphoglyceric acid from ribulose diphosphate and carbon dioxide. J Biol Chem. 1956 Feb;218(2):795–810. [PubMed] [Google Scholar]
- Walker D. A. Improved rates of carbon dioxide fixation by illuminated chloroplasts. Biochem J. 1964 Sep;92(3):22C–23C. doi: 10.1042/bj0920022c. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]


