Abstract
Natural killer (NK) cells play a prominent role at the intersection between innate and cognate immunity, thus influencing the development of multiple pathological conditions including HIV-1-induced AIDS. Not only NK cells directly kill HIV-1-infected cells, but also control the maturation and/or elimination of dendritic cells (DCs). These functions are regulated by the delicate balance between activating and inhibiting receptors expressed at the NK-cell surface. Among the former, NKp30 has raised significant interest since the alternative splicing of its intracellular domain leads to differential effector functions, dictating the prognosis of patients bearing gastrointestinal sarcoma, and B7-H6 has recently been identified as its main ligand. Since NKp30 is downregulated in CD56-/CD16+ NK cells expanded in viremic, chronically infected HIV-1+ patients, we decided to investigate the predictive value of NKp30 splice variants for spontaneous disease progression in 89 therapy-naïve HIV-1-infected individuals enrolled in an historical cohort of patients followed since diagnosis (ANRS SEROCO cohort). We found no difference in the representation of NK-cell subsets (CD56bright, CD56dim, CD56neg) in HIV-1-infected patients as compared with healthy subjects. NKp30 downregulation was detected in CD56dim and CD56neg NK-cell subsets, yet this did not convey any prognostic value. None of the NKp30 isoforms did affect disease progression, as measured in terms of time-to-loss of circulating CD4+ T cells, time-to-AIDS-defining events and overall survival. NKp30 isoforms do not seem to play a major role in the outcome of HIV-1 infection, but the heterogeneity of the immuno-virological status of patients at enrollment could have to be taken into account.
Keywords: HIV-1, immunosurveillance, natural course of infection, NK, NKp30 isoforms
Introduction
Accumulating evidence suggests an important role for innate immunity in the control of acute HIV-1 infection prior to the establishment of adaptive immune responses, as well as in the subsequent rate of viral replication and disease progression.1
A vast array of receptors with either inhibitory or activating functions regulates the interaction between natural killer (NK) cells and other cells. Uninfected and untransformed “self” cells are recognized by inhibitory NK-cell receptors that sense normal HLA class I molecules expression levels and prevent NK-cell activation.2 Killer-cell immunoglobulin-like receptors (KIRs) are the main receptors for HLA class I molecules (i.e., HLA-A, HLA-B, HLA-C and HLA-E).2 The major NK-cell activating molecules include natural cytotoxicity receptors (NCRs) (i.e., NKp46, NKp30 and NKp44) and NKG2D, which are readily triggered by ligands expressed at the surface of infected and transformed cells.3 The activating NK-cell receptor NKp30 is involved in both dendritic cells (DC) killing and DC maturation,4 and appears not only to be critical for tumor-cell recognition5 but also to influence the prognosis of different infectious diseases.6-12 The human NKp30-encoding gene (NCR3) is transcribed in six different splice variants,13 among which the most highly expressed are NKp30a, b and c.14
The significance of NK-cell antiviral activity in vivo is indicated by the fact that HIV-1 evolved specific strategies to evade NK-cell responses. Indeed, the viral protein Nef acts on infected cells by selectively downregulating the expression of HLA-A and HLA-B (preventing cells to be recognized and eliminated by T cells), but not of HLA-C and HLA-E (protecting cells from NK-cell cytotoxicity).15 Nef also induces the downregulation of ligands for the activating NK receptors NKG2D (i.e., MICA, ULBP1 and ULBP2)16 and NKp44.17 During chronic infection, HIV-1 mutants are detected that enhance the binding of the inhibitory receptor KIR2DL2 to its ligands, thus avoiding the recognition of infected cells by NK cells.18 The relative amounts of activating and inhibitory KIRs play a role in the containment of viral replication in HIV-1-infected individuals.19 Furthermore, NK cells seem to be relevant determinants for the outcome of HIV-1 infection, as the deletion of the gene encoding the NK-cell activating receptor NKG2C is a risk factor for HIV-1 infection,20 and increased NK-cell activity has been correlated with protection from infection in several cohorts of highly-exposed seronegative subjects.21
HIV-1 infection is associated with a functional impairment of NK cells that is evident early after infection and persists during disease progression,22 leading to alterations of the DC/NK crosstalk.6,23,24 In viremic HIV-1-infected patients, reduced NK-cell function is associated with low expression of NCRs25 as well as with the expansion of the “anergic” CD56-/CD16+ (CD56neg) NK-cell subset,26 which is characterized by reduced NKp30 expression, decreased cytolytic functions and low cytokine production capacity.26,27 The exhaustion of NK cells in chronic HIV-1-infected patients leads to altered DC editing, manifesting with an impaired killing of autologous immature DCs (iDCs). In particular, the markedly impaired expression and function of NKp30 among CD56neg NK cells subset largely accounts for the highly defective NK cell-mediated lysis of autologous iDCs.6 In turn, mature DCs generated from HIV-1 viremic patients are substantially impaired in their ability to induce the proliferation of autologous NK cells, which consequently fail to secrete adequate amounts of interferon γ (IFNγ).6 On the contrary, HIV-1-infected chimpanzees, which control infection when exposed to human-adapted HIV-1 variants, maintain functionally competent NK cells with high NCR expression during the course of infection,7 confirming the importance of NCRs in this setting.
We have recently characterized NKp30 isoforms, demonstrating functional differences among the three major NKp30 splice variants: whereas NKp30a-transfected NKL cells (a human NK cell line)28 block the proliferation of tumor cells harboring the NKp30 ligand B7-H6,5 exhibit granule exocytosis into the microenvironment and kill tumor cells as well as iDCs, NKL cells that express NKp30b or NKp30c fail to do so (though the former preserve the capacity to respond to B7-H6-harboring cells by secreting TH1 cytokines).29 Most interestingly, in a retrospective analysis of 80 patients affected by gastrointestinal stromal tumor (GIST), a neoplasm that expresses NKp30 ligands and is sensitive to NK cell-mediated lysis, a predominant expression of the NKp30c isoform was associated with reduced patient survival, decreased NKp30-dependent tumor necrosis factor α (TNFα) and CD107a release, as well as defective IFNγ and interleukin (IL)-12 secretion in the NK-DC crosstalk, which could be restored by blocking IL-10. In line with this notion, the NKp30 status has been shown to predict the clinical outcome of patients with GIST.29
Considering (1) the critical role of NKp30 during NK-dependent DC maturation or killing and the subsequent polarization of immune responses, (2) the alteration of NKp30 and NKp46 expression on NK cells following HIV-1 infection and (3) the different functions of the three major isoforms of NKp30, we sought to determine the potential prognostic impact of the genetically determined NKp30 status on the control of HIV-1 infection in a historical cohort of HIV-1-infected untreated patients, the ANRS SEROCO.
Results
Expression of NKp30 and NKp46 receptors on peripheral blood NK cells
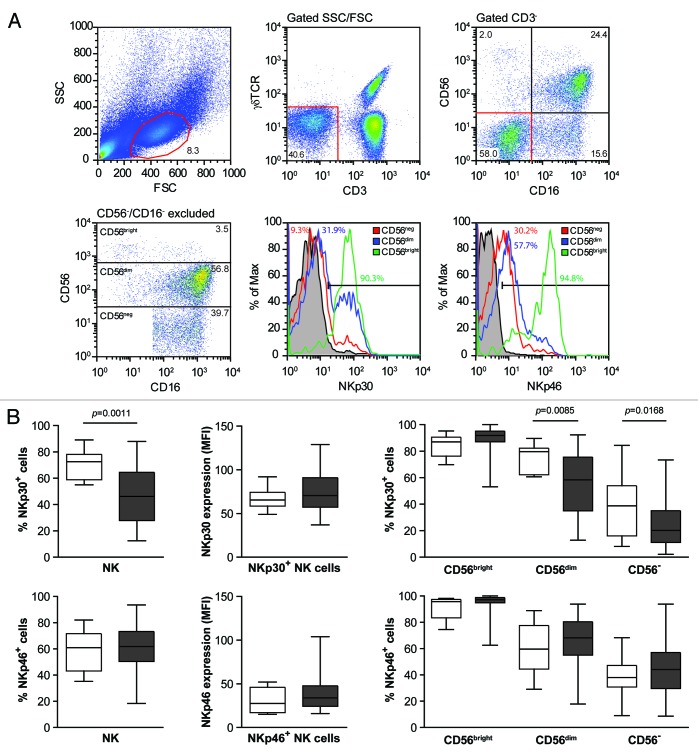
Peripheral blood mononuclear cells (PBMCs) from HIV-1+ patients (n = 89) and healthy donors (HDs) (n = 10) were analyzed by flow cytometry to determine the relative abundance of NK-cell subsets as well as their expression levels of NKp30 and NKp46 (Fig. 1A). NK cells were identified as CD3-, TCRγδ-, CD56+ and/or CD16+ cells (Fig. 1). Among total NK cells, three subpopulations were defined based on the levels of expression of CD56 and CD16: CD56bright/CD16- (CD56bright), CD56dim/CD16+/− (CD56dim) and CD56-/CD16+ (CD56neg). For each of these NK-cell subtypes, the percentage of NKp30+ or NKp46+ cells was evaluated together with the mean fluorescence intensity (MFI) of NKp30 or NKp46 expression on positive cells (Fig. 1).
Figure 1. HIV-1+ seroconverters exhibit lower percentage of NK cells expressing NKp30 on their membrane, as compared with healthy donors. (A and B) Frozen peripheral blood mononuclear cells (PBMCs) isolated from HIV-1+ patients and healthy donors (HDs) were stained with CD3, CD16, CD56, γδTCR, NKp30 and NKp46-specific antibodies and analyzed by flow cytometry. (A) Gating procedure and NKp30 and NKp46 expression among natural killer (NK) cells of the indicated subsets (one representative experiment out of 89 is shown). (B) White and gray box plots represent data for HDs (n = 10) and HIV-1+ subjects (n = 74), respectively (middle bars = median values, box plots = 25% and 75% percentiles, whiskers = minimum and maximum values). Statistically significant p values are reported (unpaired, two-tailed Student’s t-test).
We found no significant difference in the percentage of NK cells nor in the distribution of NK-cell subsets (CD56bright, CD56dim, CD56neg) between HIV-1+ patients and HDs (Fig. S1). In particular, the average percentage of CD56neg cells in HDs was 15.6% (6.0–26.7%) and in HIV+ patients 26.9% (4.0–77%). Nevertheless, we observed a decrease in the percentage of NK cells expressing NKp30 in HIV-1+ patients as compared with HDs (p = 0.0011) (Fig. 1B), while the expression level of NKp30 on a per cell basis (evaluated by MFI on positive cells) remained stable (Fig. 1B). A reduction in the percentage of NKp30+ cells was also observed among CD56dim and CD56neg cells of HIV-1-infected subjects compared with HD-derived cells (p = 0.085 and p = 0.0168, respectively) (Fig. 1B). Meanwhile, no significant differences were detected in the expression levels of the activating receptor NKp46, regardless of the NK-cell subset considered (Fig. 1B). Hence, HIV+ individuals exhibit a downregulation of NKp30 expression on peripheral NK cells.
NKp30 expression levels and clinical predictors
To analyze whether a reduced expression of NKp30 was related to transcriptional defects, we isolated total RNA from the PBMCs of 89 HIV-1+ patients and 87 HDs, and quantified the expression levels of the three major NKp30 isoforms (a, b and c) using quantitative reverse transcription-PCR (qRT-PCR) and the ΔΔCt analysis method. The mean ΔCT (Ct NKp30 - Ct β2M) values for NKp30a, NKp30b and NKp30c in the HD group were 9.69, 7.35 and 8.83, respectively.
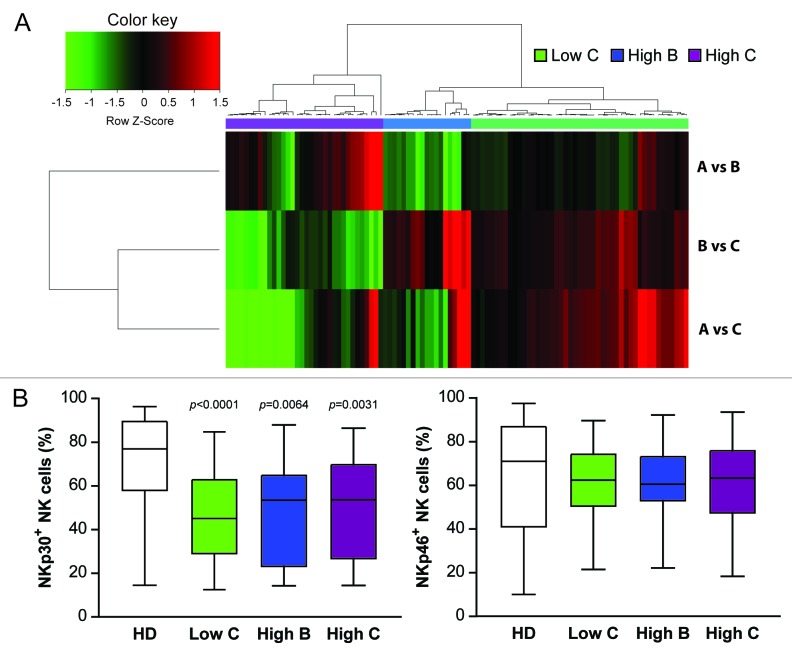
By means of an unsupervised hierarchical clustering based on log-transformed and median-centered data, HIV-1+ patients were then clustered into three groups reflecting the mRNA expression level of the three NKp30 isoforms, compared with HDs (ΔΔCt cluster): high NKp30 (n = 20, ΔCT NKp30a: 9.66; NKp30b: 7.49; NKp30c: 8.95, comparable to the values of HDs), intermediate NKp30 (n = 42, ΔCT NKp30a: 12.36; NKp30b: 10.02; NKp30c: 10.95) and low NKp30 (n = 23, ΔCT NKp30a: 16.20; NKp30b: 13.46; NKp30c: 15.83) (Fig. 2A and Table 1). NKp30 mRNA expression level could not be determined in 4 patients.
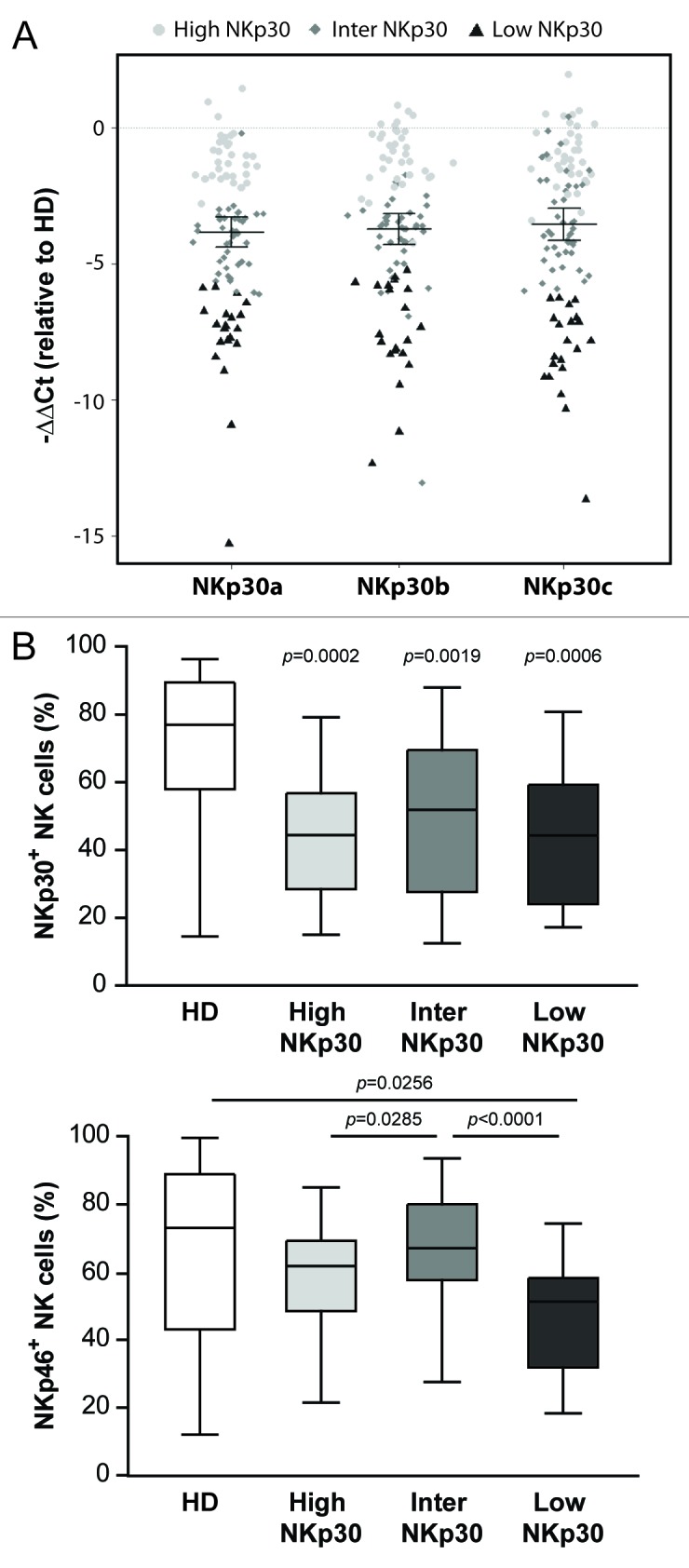
Figure 2. Seroconverters clustered based on NCR3 mRNA expression levels do not differ in terms of relative abundance of NKp30+ NK cells. (A and B) Total RNA was isolated from the peripheral blood mononuclear cells (PBMCs) of HIV-1+ patients and healthy donors (HDs) and quantified by qRT-PCR. (A) HIV-1+ patients were clustered into three groups based on NCR3 expression levels as compared with the HD group (ΔΔCt cluster). The difference between NKp30 levels in HIV+ patients and HDs is reported. (B) Percentage of NKp30+ or NKp46+ natural killer (NK) cells for each of the three patient groups as identified by the ΔΔCt cluster are shown. Middle bars = median values, box plots = 25% and 75% percentiles, whiskers = minimum and maximum values. Statistically significant p values are reported (unpaired, two-tailed Student’s t-test). In the upper panel, p values refer to the difference between each group of HIV-1+ subjects and HDs.
Table 1. Patients’ characteristics and ΔΔCt cluster.
| Characteristics | Total (n = 89) |
#NKp30High (n = 20) |
#NKp30Int (n = 42) |
#NKp30Low (n = 23) |
Statistics | |||
|---|---|---|---|---|---|---|---|---|
|
Sex* |
p1 |
|||||||
| Men |
67 (75%) |
15 (75%) |
31 (74%) |
17 (74%) |
1 |
|||
| Women |
22 (25%) |
5 (25%) |
11 (26%) |
6 (26%) |
||||
|
Mode of transmission* |
||||||||
| Men from Men |
31 (69%) |
13 (65%) |
27 (64%) |
17 (74%) |
0. 69 |
|||
| Men from Women |
6 (7%) |
2 (10%) |
4 (10%) |
0 (0%) |
||||
| Women from Men |
22 (25%) |
5 (25%) |
11 (26%) |
6 (26%) |
||||
|
Time (in months) from infection to ** |
p2 |
|||||||
| Inclusion |
6.2 [1.3–43.5] |
5.4 [2.7–41.2] |
8.9 [1.3–43.5] |
6.1 [2.1–10.1] |
0.08 |
|||
| NKp30 study |
38.3 [3.6–44.4] |
40.0 [8.5–44.0] |
28.0 [3.6–44.4] |
41.8 [17.2–44.4] |
0.008 |
|||
|
Biological markers** |
p2 |
p3 |
||||||
|
At inclusion |
||||||||
| CD4+ cells |
556 [72–1330] |
458.5 [268–1260] |
542 [72–1193] |
616 [224–1330] |
0.05 |
0.09a |
||
| Log(HIV-1 RNA) |
4.1 [2.4–5.6] |
4.3 [3.5–5.6] |
4.1 [3.1–5.3] |
3.9 [2.4–5.5] |
0.18 |
0.18a |
||
| Log(HIV-1 DNA) |
2.8 [1.7–4.0] |
2.8 [1.8–3.6] |
2.7 [1.7–3.7] |
2.9 [1.7–4.0] |
0.57 |
0.60a |
||
|
At NKp30 status assessment |
||||||||
| CD4+ cells |
478 [19–1193] |
479 [244–797] |
490 [72–1193] |
456 [19–917] |
0.60 |
0.62b |
||
| Log(HIV-1 RNA) |
4.0 [2.4–5.6] |
3.7 [3.0–5.5] |
4.0 [3.0–5.1] |
4.0 [2.7–5.6] |
0.99 |
0.99b |
||
| Log(HIV-1 DNA) | 2.9 [1.6–5.2] | 3.0 [2.0–5.2] | 2.9 [1.9–3.7] | 3.0 [1.6–4.0] | 0.75 | 0.45b | ||
# ΔΔCt cluster groups; *number (%); **median [min-max]; 1Fisher’s exact test; 2Kruskal-Wallis rank sum test; 3Linear regression adjusted for: amean time from contamination to inclusion in the study, bmean time from contamination to NKp30 status assessment.
For each group of patients defined by the ΔΔCt cluster, the percentage of NK cells expressing membrane NKp30 or NKp46 was determined by flow cytometry (Fig. 2B). Among the three ΔΔCt cluster groups, the percentages of NKp30+ NK cells were comparable (Fig. 2B, upper panel), although significant differences were found in the percentage of NKp46+ NK cells (Fig. 2B, lower panel), suggesting that the reduced expression of NKp30 on the surface of NK cells from HIV+ individuals does not result from transcriptional alterations of the three main NKp30 isoforms.
We then addressed whether the mRNA expression levels of NCR3 may influence the progression of HIV-1 infection. For each of the three ΔΔCt cluster groups, we evaluated the time- to-CD4+ T-cell loss (based on the number of patients whose CD4+ cell count fell below 200 cells/mm3 at two consecutive visits) (Fig. 3A), the time-to-first AIDS-defining illness (Fig. 3B) and survival (Fig. 3C). We observed no association between NCR3 mRNA levels in the three ΔΔCt cluster groups and these parameters (p = 0.89, p = 0.93, p = 0.54, for CD4+ T-cell count fall, AIDS and survival, respectively).
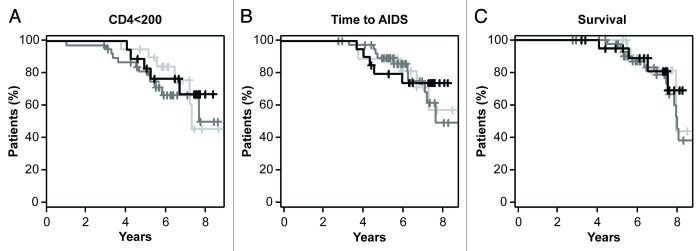
Figure 3.NCR3 mRNA expression levels neither correlate with the loss of CD4+ T cells nor constitute a prognostic factor for time-to-AIDS or overall survival. For each of the three groups of patients as identified by the ΔΔCt cluster (light gray = High NKp30, dark gray = Inter NKp30, black = Low NKp30), Kaplan-Meier curves for time-to-loss of CD4+ T cells (< 200/mm3) time-to-first AIDS-defining event and survival are shown.
Relative NKp30 isoform expression levels and clinical predictors
The levels of expression of the three major NKp30 isoforms were measured by qRT-PCR using RNA extracted from PBMCs, purified total NK cells, CD56bright, CD56dim and CD56neg NK-cell subsets from 10 HIV-1+ patients. The relative expression of the different isoforms compared with each other was calculated using the “ratio” formula: NKp30x / NKp30y = 2^(ΔΔCt NKp30y − ΔΔCt NKp30x). The relative expression of NKp30 isoforms was similar in all cell subsets analyzed (Fig. S2). Furthermore, the NKp30 isoform profile was stable over time, as shown by a longitudinal analysis performed in the 10 HIV-1+ patients at two time points with a mean temporal distance of 5.5 y (Fig. S3).
Unsupervised hierarchical clustering was subsequently performed on the relative NKp30 isoform expression data from 56 HDs and 89 HIV-1+ patients. The A vs. B, B vs. C and A vs. C distribution on HDs is shown in Figure S4. The clustering of HIV+ subjects resulted in the definition of three groups of patients with distinct NKp30 profile (ratio cluster): patients presenting as the most remarkable feature a low expression level of the c isoform (Low C, n = 40), a high expression level of the b isoform (High B, n = 15) and a high expression level of the c isoform (High C n = 28) (Fig. 4A and Table 2). The NKp30 isoform profile could not be determined in 6 patients.
Figure 4. Patients clustered based on their NKp30 isoform profile do not differ in terms of percentage of NKp30+ NK cells. (A and B) Total RNA was isolated from the peripheral blood mononuclear cells (PBMCs) of HIV-1+ patients and healthy donors (HDs) and quantified by qRT-PCR. (A) HIV-1+ patients were clustered in three groups based on the relative expression levels of the three major NKp30 isoforms (ratio cluster). (B) Percentage of NKp30+ or NKp46+ natural killer (NK) cells for each of the three ratio cluster groups. Boxes = 25% and 75% percentiles, middle bars = median values; whiskers = minimum and maximum values. Statistically significant p values are reported (unpaired, two-tailed Student’s t-test). In the left panel, p values refer to the difference between each of the three groups of HIV-1+ subjects and HD.
Table 2. Patients’ characteristics and ratio cluster.
| Characteristics |
#Low C (n = 40) |
#Low B (n = 15) |
#High C (n = 28) |
Statistics | |||
|---|---|---|---|---|---|---|---|
|
Sex* |
|
|
|
|
p1 |
|
|
| Men |
30 (75%) |
11 (73%) |
22 (79%) |
|
0.89 |
|
|
| Women |
10 (25%) |
4 (27%) |
6 (21%) |
|
|
|
|
|
Mode of transmission* |
|||||||
| Men from Men |
27 (68%) |
11 (73%) |
19 (68%) |
|
0.87 |
|
|
| Men from Women |
3 (8%) |
0 (0%) |
3 (11%) |
|
|
|
|
| Women from Men |
10 (25%) |
4 (27%) |
6 (21%) |
|
|
|
|
|
Time (in months) from infection to** |
|
p2 |
|
||||
| Inclusion |
6.0 [1.3–43.5] |
6.8 [1.8–35.5] |
6.6 [1.3–36.5] |
|
0.72 |
|
|
| NKp30 study |
38.7 [4.7–44.3] |
35.5 [10–44] |
39.2 [3.6–44.4] |
|
0.75 |
|
|
|
Biological markers** |
|
|
|
p2 |
|
p3 |
|
|
At inclusion |
|
|
|
|
|
|
|
| CD4+ cells |
485.5 [202–987] |
874 [415–1330] |
542 [72–1193] |
< 0.01 |
|
< 0.01a |
|
| Log(HIV-1 RNA) |
4.2 [3.4–5.6] |
3.5 [2.4–4.9] |
4.1 [2.9–5.5] |
0.06 |
|
0.02a |
|
| Log(HIV-1 DNA) |
3 [1.8–4] |
2.6 [1.7–3.2] |
2.7 [1.7–3.5] |
0.17 |
|
0.16a |
|
|
At NKp30 status assessment |
|||||||
| CD4+ cells |
457.5 [19–987] |
578 [305–940] |
488 [72–1193] |
0.16 |
|
0.09b |
|
| Log(HIV-1 RNA) |
4 [3–5.6] |
3.7 [3.1–5] |
4 [2.7–5.4] |
0.62 |
|
0.33b |
|
| Log(HIV-1 DNA) | 3.2 [1.6–5.2] | 2.6 [1.7–3.5] | 2.9 [1.9–3.7] | 0.14 | 0.10b | ||
# Ratio cluster groups; *number (%); ** median [min-max]; 1Fisher’s exact test; 2Kruskal-Wallis rank sum test; 3Linear regression adjusted for: amean time from contamination to inclusion in the study, bmean time from contamination to NKp30 status assessment.
In each of these three groups, we assessed the percentage of NKp30+ or NKp46+ NK cells (by flow cytometry). The three groups of patients showed a decrease in the percentage of NKp30+ NK cells as compared with HDs (p < 0.0001, p = 0.0064 and p = 0.0031 for the Low C, High B and High C groups, respectively) but no difference was detected in the percentage of NKp30+ or NKp46+ NK cells between the three ratio cluster groups (Fig. 4B).
We finally searched for a potential influence of NKp30 isoform expression profile on disease evolution. The Kaplan-Meier curves shown in Figure 5 illustrate that there is no association between the NKp30 isoform profile and time-to-CD4+ T-cell loss (p = 0.58), time-to-clinical AIDS (p = 0.64) or survival (p = 0.59).
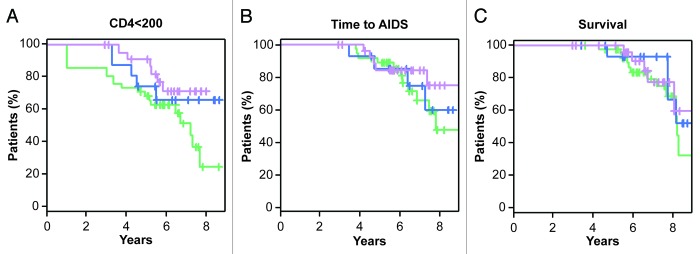
Figure 5. NKp30 isoform profiles do not correlate with the loss of CD4+ T cells and are not a prognostic factor for time-to-AIDS or survival. For each of the three groups of patients as identified by the ratio cluster (green = Low C, blue = High B, violet = High C), Kaplan-Meier curves for time-to-loss of CD4+ T cells (< 200/mm3), time-to-first AIDS-defining event and survival are shown.
Frequency of CD56neg NK cells and prognostic factors
As mentioned above, we found no significant expansion of the CD56neg NK-cell subset in HIV-1+ patients as compared with HDs (Fig. S1). Nevertheless, we evaluated whether the percentage of CD56neg NK cells would be correlated with the CD4+ cell count, plasma viral load or cell-associated proviral DNA at the time of our study. No association was found between the proportion of CD56neg NK cells and these parameters (Fig. 6) among the 89 patients included in the study (on average 6 mo post-infection), with a median serum viral load of 12,589 HIV-1 RNA copies/mL and a CD4+ cell count of 556 cells/mm3 (Table 1).
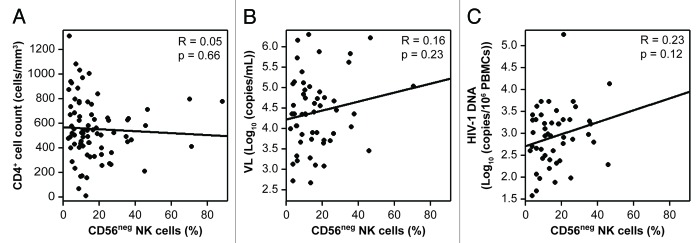
Figure 6. Lack of correlation between the percentages of CD56neg NK cells and clinical parameters. (A and C) Percentage of CD56neg cells among natural killer (NK) cells as a function of CD4+ T-cell counts (A), viral load (B) and proviral DNA levels (C) (Spearman correlation).
Discussion
The aim of our study was to assess the potential role of NKp30-related parameters (NKp30 surface expression, NKp30 transcriptional levels, NKp30 isoforms) on the progression of HIV-1 infection. The finding that the preferential expression of the immunosuppressive NKp30c isoform is associated with poor prognosis in GIST patients29 prompted us to perform a retrospective analysis of 89 HIV-1-infected individuals from the ANRS CO 02 SEROCO-HEMOCO cohort. This cohort included patients enrolled early after HIV seroconversion and followed from 1988 to 1995, before the introduction of highly active antiretroviral therapy (HAART). Thus, this cohort offered an extended follow-up of untreated patients, allowing us to determine the influence of the NKp30 isoform profile on spontaneous disease progression. We found that neither NKp30 expression levels nor the NKp30 isoform profile correlates with the virological and clinical parameters analyzed in this cohort of patients for whom the natural history of HIV-1 infection is available. Nevertheless, we noticed that—at the time of inclusion in the cohort—individuals belonging to the Low B group (exhibiting a reduced expression of NKp30b) were characterized by a significantly higher CD4+ T-cell count and a lower viral load as compared with the other groups of patients (Table 2). This might suggest an early effect of the NKp30 profile on the loss of CD4+ cells, which may become irrelevant with the progression of disease. Further studies are needed to test this hypothesis on a more homogeneous cohort of acutely infected patients.
Comparative flow cytometry analyses of NK-cell subsets on frozen PBMCs from HIV-1+ patients and HDs showed no difference in the percentage of NK cells among PBMCs nor in the proportion of CD56bright, CD56dim and CD56neg cells among NK cells (Fig. S1). This is in contrast with previous observations by Mavilio et al., who reported that in viremic patients with chronic HIV-1 infection, the proportion of CD56dim NK cells is sharply decreased and the CD56neg NK cells subpopulation is expanded as compared with HDs.30 This apparent discrepancy may be due to the differences in disease stage, i.e., recent infection vs. chronic late infection, across the two groups of patients studied, suggesting that alterations in the representation of NK-cell subsets require several years of infection to occur.
In HIV-1 infected individuals, we observed a decrease in the percentage of NK cells expressing NKp30, affecting both CD56dim and CD56neg NK cells (Fig. 1B). In line with this observation, Mavilio et al. reported that viremic, but not aviremic, chronically infected patients exhibit a significant downregulation of NCRs, including NKp30 and NKp46.30 In contrast, we observed no difference in the expression of NKp46 between HIV-1+ patients and HDs (Fig. 1B), suggesting that alterations in NKp46 expression appears later after primary infection. We did not find any association between the percentage of NKp30+ NK cells in HIV+ patients and the clinical parameters that we evaluated (loss of CD4+ T cells, time-to-AIDS-defining event or patient survival) (data not shown).
We subsequently quantified NCR3 mRNA from the PBMCs of both seroconverters and HDs, normalizing NKp30 expression levels to those of the housekeeping gene β2 microglobulin (B2M). The unsupervised hierarchical clustering of NCR3 mRNA expression data (ΔΔCt clustering) allowed us to classify patients into three groups expressing high, intermediate and low levels of NKp30 (Fig. 2A). The High NKp30 group showed NKp30 levels comparable to those observed among HDs (Fig. 2A). Interestingly, no correlation between NKp30 mRNA levels (ΔΔCt cluster groups) and surface NKp30 expression levels (assessed by flow cytometry) was found, in terms of both percentage of NKp30+ cells (Fig. 2B) and MFI on NKp30+ cells (data not shown), perhaps suggesting a consistent degree of post-transcriptional regulation of NKp30.
We then addressed the question as to whether the NKp30 isoform profile may influence the progression of HIV-1 infection. We performed qRT-PCR using primers specific for each of the three major NKp30 isoforms (NKp30a, NKp30b and NKp30c) and their relative expression level was calculated for all patients. These expression levels were comparable whether evaluated on the RNA from PBMCs or from purified NK cells, CD56bright, CD56dim or CD56neg cells (Fig. S2). NKp30 isoform profiles in HIV-1+ subjects were found to be stable over time (Fig. S3), similar to what has previously been shown for GIST patients,29 allowing us to analyze a single time point per patient. The unsupervised hierarchical clustering of the relative expression levels of the three isoforms (ratio clustering) resulted in the classification of patients into three groups: Low C, High B and High C (bearing low levels of the c isoform or high levels of the b or c isoforms, respectively) (Fig. 4A). These three groups of patients did not differ in terms of percentage of NKp30+ NK cells (Fig. 4B), nor in terms of NKp30 expression level on NKp30+ cells (data not shown). Therefore, our analysis of the influence of the NKp30 isoform profile on HIV-1 disease progression is unlikely to be biased by differences in surface expression levels of NKp30, yet suggest no prognostic significance for this parameters (at least in our cohort).
We have previously reported that the activation of NK cells bearing different NKp30 isoforms results in different functional outcomes. The predominant expression of the immunosuppressive NKp30c isoform has indeed been associated with reduced survival of GIST patients, correlating with defective IFNγ, TNFα and IL-12 production in the NK-DC crosstalk, which could be restored by blocking IL-10.29 In spite of the important role played by NK cells during both acute and chronic HIV-1 infection,22 the crucial function of NKp30 in NK-cell activity,6,25 and the multiple effects exerted by IL-10 during HIV-1 infection,31 we were not able to detect in our cohort of 89 recently seroconverted HIV-1+ patients any association between the NCR3 mRNA expression levels or NKp30 isoform profiles and the clinical parameters that we evaluated, i.e., the loss of CD4+ T cells, the time-to-clinical AIDS and survival (Figs. 3 and 5).
Finally, we did not find any correlation between the percentage of CD56neg NK cells and CD4+ T-cell count, plasma viral load or proviral DNA levels (Fig. 6), in contrast with a previous report showing an association between the expansion of the CD56neg subpopulation and viremia.26 However, our study involves patients at an earlier stage of infection, leading us to speculate that alterations in the NK-cell subsets distribution, notably the expansion of the CD56neg subpopulation, are linked to persistent viral replication and hence constitute a late consequence of immune dysfunction.
Altogether, our observations do not support any correlation between NKp30 status and the clinical outcome of recently infected HIV-1+ patients that were left untreated for more than 3 y. However, we must acknowledge some potential limitations that might have undermined our study. First, although this cohort allowed us to follow the natural evolution of HIV-1 infection, the number of samples per patient was restricted. Second, given the general heterogeneity of HIV-1 infected patients, a greater number of patients may be needed to detect a correlation between NKp30 status and clinical outcome. Finally, due to the limited number of patient analyzed, we could not establish whether the differences among ratio cluster groups in CD4+ T-cell count and viremia at the time of inclusion (Table 2) have a biological meaning or reflect biases that may have compromised our analysis. It is time to reevaluate the influence of NKp30 status on the evolution of HIV-1 infection in the setting of primary infection or in long-term non-progressors.
Materials and Methods
Study population
Frozen PBMCs were obtained from patients enrolled in the ANRS CO 02 SEROCO-HEMOCO cohort, which includes individuals with a recent seroconversion or recent HIV-1 diagnosis enrolled from 1988 to 1995.32 Among these patients, we selected 89 individuals for whom frozen cells were available, who did not present hepatitis B virus or hepatitis C virus co-infection, who were not intravenous drugs users, hemophiliac, or pregnant, had neither autoimmune diseases nor malignancies within the 5 y preceding their enrolment, had no concomitant or previous treatment with interferon and other cytokines, steroids or other immunomodulators. The characteristics of these patients are reported in Table 1. Ten HDs served as controls for immunological parameters. A written informed consent was obtained from patients, in line with the guidelines formulated by local ethical committees.
qRT-PCR
The levels of expression of the three major NKp30 isoforms were measured by qRT-PCR and normalized to the level of expression of the housekeeping gene β-2-microglobulin (B2M), as previously described.29 Total cellular RNA was isolated, by means of the RNeasy Mini kit (Qiagen, 74106), from frozen PBMCs, purified NK cells (isolated from PBMCs by magnetic sorting using the EasySep Human NK Cell Enrichment Kit, from Stem Cell, 19055) or purified NK-cell subpopulations (isolated from total NK cells using the a FACSAria cell sorter, from BD Biosciences). cDNA was synthesized from total RNA using the SuperScript™ III Reverse Transcriptase (Invitrogen, 18080-044) and random primers (Promega, C1181), according to the manufacturer’s instructions. The following primers and probes (Applied Biosystems) were used for qRT-PCR: NKp30-EC (Fwd): 5′-TTTCCTCCATGACCACCAGG-3′; NKp30-EX4I (Rev): 5′-TTCCCATGTGACAGTGGCATT-3′; NKp30-EX4II (Rev): 5′-CGGAGAGAGTAGATTTGGCATATT-3′; NKP30-EX4III (Rev): 5′-GGACCTTTCCAGGTCAGACATT-3′; NKp30-Probe (6-FAM/TAMRA): 5′-TGGTGGAGAAAGAACATCCTCAGCTAGGG-3′; B2M-F (Fwd): 5′- GATGAGTATGCCTGCCGTGT 3′; B2M-R (Rev): 5′-AATTCATCCAATCCAAATGCG-3′; B2M-Probe (6-FAM/TAMRA): 5′-AACCATGTGACTTTGTCACAGCCCAA-3′. First-strand cDNA was amplified using TaqMan Gene Expression Master Mix (Applied Biosystems, 4369016) and NKp30 or B2M primers (10 µM) and probes (5 µM) in a final volume of 25 µL. One initial incubation at 50°C for 2 min was followed by one cycle of denaturation (95°C for 10 min) and 45 cycles of amplification (95°C for 15 sec and 60°C for 1 min). qRT-PCR was performed in a StepOnePlus System (Applied Biosystems), samples were amplified in triplicate and the qRT-PCR data were analyzed using the 2-ΔCt method.
Unsupervised hierarchical clustering
The level of expression of NKp30 isoforms in HIV-1+ patients compared with HDs was determined using the ΔΔCt method: −ΔΔCT = − [(HIV-1NKp30 − HIV-1B2M) − (HDNKp30 − HDB2M)]. The level of expression of the distinct NKp30 isoforms compared with each other in each patient (ratio) was determined using the following formula: NKp30x / NKp30y = 2^(ΔΔCt NKp30y − ΔΔCt NKp30x). Unsupervised hierarchical clustering was applied to log-transformed and median-centered data using the Cluster and TreeView programs (average linkage clustering using Pearson’s centered correlation as similarity metric). Two clusters were created: the first one based on the different levels of expression of NKp30 in HIV-1+ patients compared with HDs (ΔΔCt cluster) and the second based on the levels of expression of each NKp30 isoform as compared with the others in HIV-1+ patients (ratio cluster).
Flow cytometry
The following mouse anti-human fluorescent monoclonal antibodies were used: CD3-APC-Cya7 (Beckman Coulter, A94680), CD16-Pacific Blue (Beckman Coulter, A82792), CD56-PE-Cy7a (Beckman Coulter, A21692), γδTCR-FITC (Beckman Coulter,), NKp30-PE (clone AF29-4D12) (Miltenyi, 130-092-483), NKp46-APC (Miltenyi,130-092-609). In addition, the dead-cell removal reagent LIVE/DEAD Fixable Yellow Dead Cell Stain Kit (Invitrogen, L34959) was employed. Frozen cells were thawed, washed and stained with the abovementioned reagents for 15 min at 4°C, washed and fixed with 1% PFA. Cells were acquired on a FACSAria cell sorter immediately following staining and analyses were performed using the FlowJo software (Tree Star).
Statistical analyses
The Fisher’s exact test and the non-parametric Kruskal-Wallis rank sum test were used for the comparison of different groups. Survival curves were plotted according to the Kaplan-Meier method. A Cox model was employed to take into account time from infection to NKp30 status assessment (left-entry model) and was used to compare the survival according to NKp30 status. All analyses were performed with the R package version 2.14.2.
Supplementary Material
Acknowledgments
This work was supported by Institut National du Cancer (INCa), la Ligue contre le cancer (LIGUE labelisée, L.Z.), l’Association pour la Recherche sur le Cancer (ARC); Fondation pour la Recherche Médicale, and Fondation de France. NP was supported by Ligue Natioanle Contre le Cancer. The ANRS SEROCO Cohort is funded by the ANRS.
Glossary
Abbreviations:
- DC
dendritic cell
- GIST
gastrointestinal stromal tumor
- HD
healthy donor
- KIR
killer-cell immunoglobulin-like receptor
- MFI
mean fluorescence intensity
- NCR
natural cytotoxicity receptors
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23472
References
- 1.Borrow P, Shattock RJ, Vyakarnam A, EUROPRISE Working Group Innate immunity against HIV: a priority target for HIV prevention research. Retrovirology. 2010;7:84. doi: 10.1186/1742-4690-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashirova AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu Rev Genomics Hum Genet. 2006;7:277–300. doi: 10.1146/annurev.genom.7.080505.115726. [DOI] [PubMed] [Google Scholar]
- 3.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Aricò M, Moretta L, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–71. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 5.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mavilio D, Lombardo G, Kinter A, Fogli M, La Sala A, Ortolano S, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203:2339–50. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutjens E, Mazza S, Biassoni R, Koopman G, Ugolotti E, Fogli M, et al. CD8+ NK cells are predominant in chimpanzees, characterized by high NCR expression and cytokine production, and preserved in chronic HIV-1 infection. Eur J Immunol. 2010;40:1440–50. doi: 10.1002/eji.200940062. [DOI] [PubMed] [Google Scholar]
- 8.Yutkin V, Pode D, Pikarsky E, Mandelboim O. The expression level of ligands for natural killer cell receptors predicts response to bacillus Calmette-Guerin therapy: a pilot study. J Urol. 2007;178:2660–4. doi: 10.1016/j.juro.2007.07.118. [DOI] [PubMed] [Google Scholar]
- 9.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–55. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 10.Chisholm SE, Howard K, Gómez MV, Reyburn HT. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J Infect Dis. 2007;195:1160–8. doi: 10.1086/512862. [DOI] [PubMed] [Google Scholar]
- 11.Fuller CL, Ruthel G, Warfield KL, Swenson DL, Bosio CM, Aman MJ, et al. NKp30-dependent cytolysis of filovirus-infected human dendritic cells. Cell Microbiol. 2007;9:962–76. doi: 10.1111/j.1462-5822.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 12.Mavoungou E, Held J, Mewono L, Kremsner PG. A Duffy binding-like domain is involved in the NKp30-mediated recognition of Plasmodium falciparum-parasitized erythrocytes by natural killer cells. J Infect Dis. 2007;195:1521–31. doi: 10.1086/515579. [DOI] [PubMed] [Google Scholar]
- 13.Neville MJ, Campbell RD. A new member of the Ig superfamily and a V-ATPase G subunit are among the predicted products of novel genes close to the TNF locus in the human MHC. J Immunol. 1999;162:4745–54. [PubMed] [Google Scholar]
- 14.Nalabolu SR, Shukla H, Nallur G, Parimoo S, Weissman SM. Genes in a 220-kb region spanning the TNF cluster in human MHC. Genomics. 1996;31:215–22. doi: 10.1006/geno.1996.0034. [DOI] [PubMed] [Google Scholar]
- 15.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–71. doi: 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 16.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88:242–50. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 17.Fausther-Bovendo H, Sol-Foulon N, Candotti D, Agut H, Schwartz O, Debré P, et al. HIV escape from natural killer cytotoxicity: nef inhibits NKp44L expression on CD4+ T cells. AIDS. 2009;23:1077–87. doi: 10.1097/QAD.0b013e32832cb26b. [DOI] [PubMed] [Google Scholar]
- 18.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelak K, Need AC, Fellay J, Shianna KV, Feng S, Urban TJ, et al. NIAID Center for HIV/AIDS Vaccine Immunology Copy number variation of KIR genes influences HIV-1 control. PLoS Biol. 2011;9:e1001208. doi: 10.1371/journal.pbio.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas R, Low HZ, Kniesch K, Jacobs R, Schmidt RE, Witte T. Nkg2c Deletion Is a Risk Factor of Hiv Infection. AIDS Res Hum Retroviruses. 2012;28:844–51. doi: 10.1089/aid.2011.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomescu C, Abdulhaqq S, Montaner LJ. Evidence for the innate immune response as a correlate of protection in human immunodeficiency virus (HIV)-1 highly exposed seronegative subjects (HESN) Clin Exp Immunol. 2011;164:158–69. doi: 10.1111/j.1365-2249.2011.04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–86. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reitano KN, Kottilil S, Gille CM, Zhang X, Yan M, O’Shea MA, et al. Defective plasmacytoid dendritic cell-NK cell cross-talk in HIV infection. AIDS Res Hum Retroviruses. 2009;25:1029–37. doi: 10.1089/aid.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melki MT, Saïdi H, Dufour A, Olivo-Marin JC, Gougeon ML. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLoS Pathog. 2010;6:e1000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–8. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 26.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–91. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–43. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 28.Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24:406–15. [PubMed] [Google Scholar]
- 29.Delahaye NF, Rusakiewicz S, Martins I, Ménard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17:700–7. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 30.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–6. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon DS, Kaufmann DE. Protective and detrimental roles of IL-10 in HIV pathogenesis. Eur Cytokine Netw. 2010;21:208–14. doi: 10.1684/ecn.2010.0201. [DOI] [PubMed] [Google Scholar]
- 32.Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, et al. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–7. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.