Abstract
Tumor infiltration by lymphocytes has been linked to improved clinical outcome in children with neuroblastoma (NB) but T-cell activation has never been demonstrated to occur within the NB microenvironment. Here we show that tumor-associated lymphocytes (TALs) obtained from lesions representing all genetic subsets of NB and autologous peripheral blood lymphocytes (PBLs) analyzed on the day of tumor excision differed in composition, phenotype and functional characteristics. The NB microenvironment appeared to promote the accumulation of CD3+CD8+ T cells and contained a larger proportion of T cells expressing the interleukin-2 receptor α chain (CD25) and manifesting an effector memory (CCR7−CD45RA−) phenotype. Accordingly, the stimulation of PBLs with autologous tumor cells in short-term cultures increased the proportion of effector memory T cells, upregulated CD25, stimulated the expression of the TH1 cytokines interferon γ and tumor necrosis factor α, and reduced the expression of transforming growth factor β. In situ proliferation as well as a characteristic pattern of T-cell receptor aggregation at the contact sites with malignant cells was revealed by the immunohistochemical staining of TALs in primary tumors, indicating that the NB milieu is compatible with the activation of the immune system. Our results are compatible with the hypothesis that CD8+ T cells are specifically activated within the NB microenvironment, which appears to be permissive for effector memory responses.
Keywords: PBL, T lymphocyte, T-cell phenotype, TAL, cytokine, immunity, neuroblastoma
Introduction
Human neuroblastoma (NB), the most common extracranial solid malignancy in children,1 is frequently described as non-immunogenic due to a low/absent expression of MHC class I molecules, general lack of MHC class II molecules and reduced expression of adhesion molecules (reviewed in refs. 2 and 3). However, these defects can be corrected by the exposure of NB cells to pro-inflammatory cytokines, such as interferon γ (IFNγ) and tumor necrosis factor α (TNFα). Moreover, activated T cells can kill NB cells in an MHC-unrestricted fashion. We have previously shown that (1) activated cytotoxic T lymphocytes (CTLs) can induce the MHC-independent demise of NB cells not only in a caspase-dependent but also in a caspase-independent manner4 and that (2) soluble factors released by activated CTLs sensitize NB cells to death receptor-mediated killing by T cells.5 The latter observation is of special importance since the epigenetic silencing of caspase-8 has frequently been observed in NB cells (reviewed in ref. 6). Collectively, these data imply that the recruitment of activated CTLs into the tumor site may constitute a viable approach for the treatment of human NB, even in the absence of a specific recognition of tumor-associated antigens. However, it remains unclear whether immunotherapy can promote a sufficient infiltration of T lymphocytes into these tumors.
Despite the fact that lymphocytic infiltration has been indicated as a favorable prognostic factor for NB more than four decades ago,7,8 the current knowledge on T cells that reside in the NB milieu is limited to a study published in 1996 by Facchetti et al., describing the presence of CD4+ and CD8+ T cells in within NB lesions and suggesting that a proportion of these cells may exhibit an activated (CD25+ and/or HLA-DR+) phenotype.9 Still, neither a detailed description of T-cell subsets resident within NB lesions nor a characterization of the cytokines produced in this microenvironment is currently available. Moreover, it has not yet been documented whether the subset composition and functional properties of intratumoral T cells correspond to those observed in the peripheral blood of NB patients. Finally, to the best of our knowledge, a systematic analysis of the phenotype and cytokine production pattern of peripheral blood lymphocytes (PBLs) from NB patients has never been performed. Altogether, such unanswered questions are important, considering that immunotherapy is arising as a new treatment option for high-risk NB patients (reviewed in refs. 2 and 10).
In the present study, we analyzed the activation status, phenotype and cytokine production pattern of T-cell populations from malignant lesions and the peripheral blood of NB patients. Our results demonstrate that the NB microenvironment is permissive for a continuous T-cell activation and promotes the selective expansion and/or accumulation of T-cell subsets exhibiting an effector phenotype. These data encourage the development of new immunotherapeutic approaches for the clinical management of NB.
Results
T cells infiltrate NBs, proliferate in situ and exhibit CD3 aggregation at the sites of contact with tumor cells
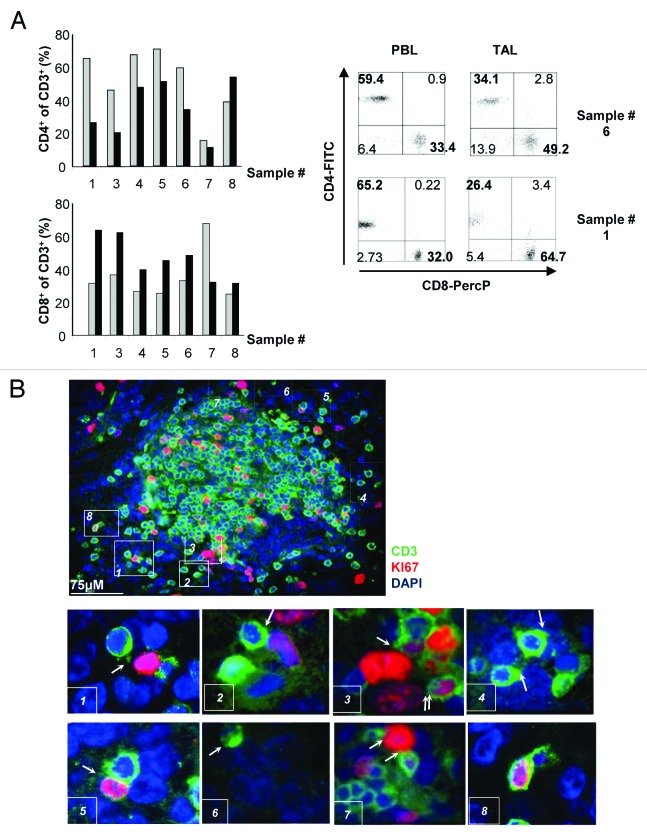
A direct ex vivo analysis of T-cell infiltration in eight primary NB samples representing all different genetic risk subsets (Table 1) was performed on the day of surgical intervention. All NB samples contained a detectable proportion of CD3+ cells, varying from 0.6% to 17.8% of the total cell mass. We did not observe any statistically significant correlation between the abundance of tumor-associated CD3+ cells and patient’s age, disease stage or pre-surgery therapeutic regimen (Table 1), which could be due to the relatively small number of samples analyzed. However, it is notable that the two non-survivors, patient 2 and patient 4 manifested the lowest levels of T-cell infiltration of all examined samples (Table 1). We next characterized the representation of different CD3+ T-cell populations expressing CD4 and/or CD8 molecules on their surface, both among tumor-associated lymphocytes (TALs) and PBLs collected from the patient at the day of tumor excision (Fig. 1A). The ratio between CD3+CD4+ and CD3+CD8+ cells in the peripheral blood varied from 1.2 to 2.7 in 5 out of 8 patients, and a relatively lower ratio was observed in TALs (Table 1). Notably, the drop in the CD4+/CD8+ T-cell ratio among TALs was prominent, 3-fold in average, and reached as high as 4- and 5-fold in tumors from patient 3 and 1, respectively. Using immunohistochemistry, we observed that T cells infiltrating the tumor mass (detected outside the lumen of blood vessels) are found in the close proximity of proliferating, Ki67-positive tumor cells (Fig. 1B, areas 1, 2, 3, 5 and 7) and non-proliferating tumor cells (Fig. 1B, areas 4 and 6). In some instances, we were able to observe a redistribution of the CD3 component of the T-cell receptor (TCR) toward the area of contact with the tumor cell (often referred to as “CD3 capping”) (Fig. 1B, areas 1, 2, 4 and 6). CD3+ T cells were also shown to proliferate within the tumor mass, as shown by Ki67 co-staining with (Fig. 1B, area 8). Though it is not practically feasible to demonstrate local T-cell degranulation, the pattern of CD3 distribution, reflecting TCR aggregation (Fig. 1B, areas 1, 2, 4 and 6) and the detection of the proliferation marker Ki67 (Fig. 1B, area 8; Fig. S1) in intratumoral CD3+ cells strongly indicate that tumor recognition takes place in situ, ultimately leading to T-cell activation.
Table 1. Patient characteristics and T-cell subset composition of PBLs and TALs from NB patients.
| Sample ID | Stagea | Ageb | Geneticsc | Treatmentd | Sexe | Survivalf | %CD3g | CD4/CD8 PBLh | CD4/CD8 TALh |
|---|---|---|---|---|---|---|---|---|---|
| 1 |
1 |
13.5 |
Oth str |
|
M |
54+ |
2.4 |
2.0 |
0.4 |
| 2 |
4 |
35 |
11q- |
X |
M |
6 |
0.6 |
1.0 |
ND |
| 3 |
1 |
41.5 |
Num only |
|
M |
52+ |
2.36 |
1.2 |
0.3 |
| 4 |
2 |
14 |
17q+ |
X |
M |
10 |
0.39 |
2.5 |
1.2 |
| 5 |
1 |
4 |
Num only |
|
M |
50+ |
17.8 |
2.7 |
1.1 |
| 6 |
1 |
4.5 |
Oth str |
|
F |
49+ |
4.2 |
1.8 |
0.7 |
| 7 |
3 |
18 |
MNA |
X |
F |
44+ |
1.43 |
0.2 |
0.4 |
| 8 | 3 | 66 | Oth str | X | F | 42+ | 7.1 | 0.51 | 1.7 |
a Stage according to International Neuroblastoma Staging System, INSS.40bAge in months at the time of surgery
c According to Carén et al.29,30 (oth str, other structural abnormalities; 11q-, loss of chromosome 11q; num only, numerical only; 17q+, gain of chromosome 17q; MNA, MYCN amplification). dTreatment prior to surgery. em, male; f, female. fMonths after diagnosis (+, still alive). g% CD3+ cells of all cells in the tumor mass at the day of surgery, based on flow cytometry data on the whole cell mass as judged by forward/side scatter discrimination.
h Ratio between CD4+ and CD8+ cells within the CD3+ compartment. ND, not defined.
Figure 1. Infiltration and distribution of T-cell subsets in tumor-associated lymphocytes compared with peripheral blood lymphocytes in neuroblastoma patients. (A) Flow cytometry performed on the day of tumor excision. Graphs depict the percentage of CD4+ (upper panel) and CD8+ (lower panel) cells among CD3+ peripheral blood lymphocytes (PBLs, gray bars) and CD3+ tumor-associated lymphocytes (TALs, black bars). Examples of flow cytometry staining for cell-surface CD4 and CD8 expression in CD3+ cells from patient 1 and 6 are shown. (B) Staining of neuroblastoma (NB) tissue (from patient 8) with an anti-CD3 antibody (green) and an antibody specific to the cell proliferation marker Ki67 (red). Nuclei were counterstained with DAPI (blue). Areas indicated in the figure by numbers from 1 to 8 were selected to emphasize different events (marked by arrows) that characterize the tumor cell:T cell interaction.
T lymphocytes infiltrating NB lesions upregulate CD25 but not FOXP3
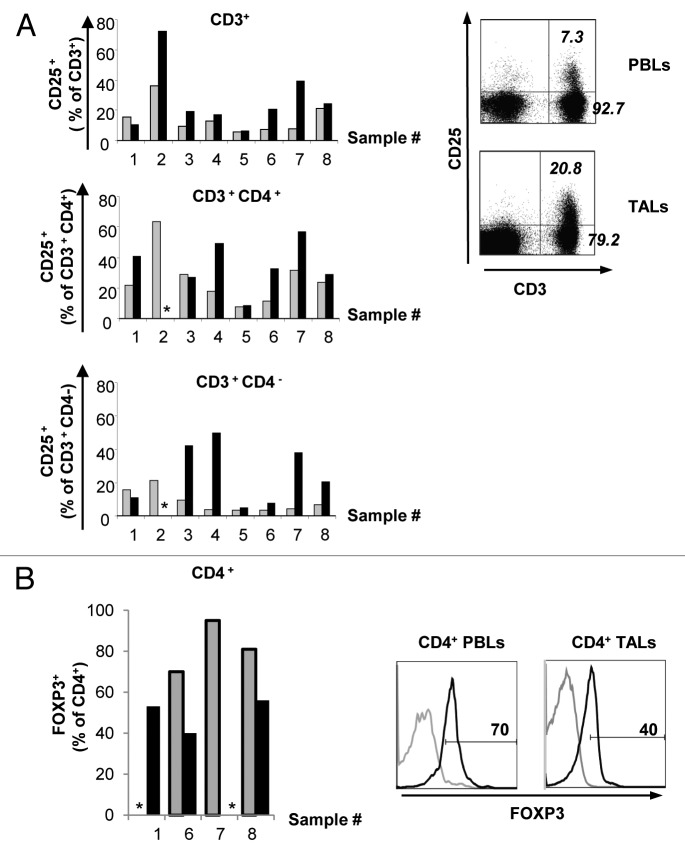
T lymphocytes infiltrating tumors of different origins often acquire an anergic state and cannot be activated, unless re-stimulated in vitro in a tumor-free environment. Therefore, we next compared the surface levels of CD25, the interleukin (IL)-2 receptor α chain, on PBLs and TALs from NB patients, as the upregulation of this molecule reflects T-cell activation. A higher proportion of TALs expressed CD25 on the cell surface as compared with autologous PBLs on the day of tumor excision (Fig. 2A). This observation held true for CD3+CD4+ and CD3+CD4− cell populations from most of the patients analyzed in this study (Fig. 2A), suggesting that both helper and cytotoxic T lymphocytes may either undergo activation in the tumor milieu, or maintain an activated state following homing to the tumor site.
Figure 2. Expression of CD25 and FOXP3 in peripheral blood lymphocytes and tumor-associated lymphocytes from neuroblastoma patients. (A) Flow cytometry data demonstrate the expression of CD25 on both peripheral blood lymphocytes (PBLs) and tumor-associated lymphocytes (TALs) on the day of tumor excision in different CD3+ T-cell subsets. Gray bars represent PBLs and black bars represent TALs. One representative pattern of cell-surface CD25 expression in PBLs and TALs from patient 6 as detected by flow cytometry on the day of tumor excision is shown. Numbers indicate the percent of cells expressing or not expressing CD25 among CD4+ cells. (B) Expression of FOXP3 in CD4+ PBLs (gray bars) and CD4+ TALs (black bars) on the day of tumor excision, as analyzed by flow cytometry. One representative pattern of FOXP3 expression in PBLs and TALs of patient 6 is shown. Number indicates the percentage of positive cells. * = not performed.
High levels of CD25 in combination with the transcription factor FOXP3 coupled to the ability to suppress T-cell responses are used to define a subset of T cells with regulatory properties (regulatory T cells, Tregs), which are involved in the inhibition of T-cell immune responses (reviewed in ref. 11). Notably, 70 to 95% of CD3+CD4+ PBLs from three patients included in this study expressed FOXP3 (Fig. 2B). Surprisingly, the proportion of CD4+ T cells expressing FOXP3 was lower among TALs (Fig. 2B).
The NB microenvironment does not prevent the generation of T-cell memory
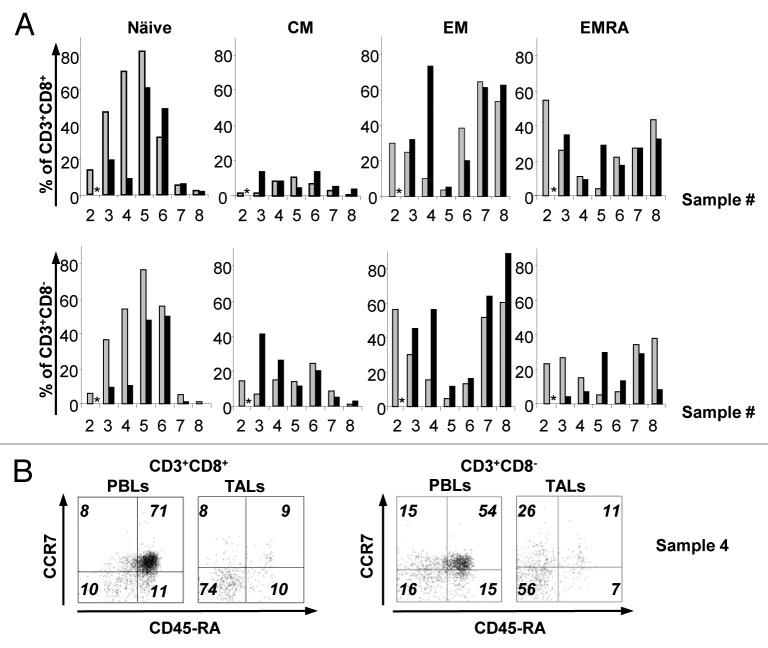
It is known that human effector memory (TEM) and CD45RA+ effector memory (TEMRA) T cells are more differentiated in terms of effector function than naïve (TN) and central memory (TCM) T cells.12 Therefore, we monitored the molecular signatures reflecting the T-cell memory status in the PBLs and TALs of NB patients, using the expression of CCR7 and CD45RA to define TN (CCR7+CD45RA+), TCM (CCR7+CD45RA−), TEM (CCR7−CD45RA−) and TEMRA (CCR7−CD45RA+) populations, as previously described.13 We observed that TN, TEM and TEMRA constitute the main CD8+ T-cell subsets in both PBLs and TALs of NB patients at the day of tumor excision, whereas CD8+ TCM cells represent a relatively minor cell compartment (Fig. 3A and B). The percentage of TN CD3+CD8+ PBLs was usually higher than or similar to that of TN CD3+CD8+ TALs. At the same time, different memory cell subsets were more frequent in the intratumoral T-cell pool. These differences were even more striking in the CD3+CD8− population, for which a prevalence of naïve T cells in the periphery was usually paralleled by a dominance of TEM T cells in the tumor environment (Fig. 3A and B).
Figure 3. Characterization of the T-cell phenotype of peripheral blood lymphocytes and tumor-associated lymphocytes from neuroblastoma patients. (A and B) T cells exhibiting the phenotype of naïve, central memory (TCM), effector memory (TEM) and CD45RA+ effector memory (TEMRA) cells were identified by flow cytometry. (A) The percentage of naïve, TCM, TEM and TEMRA cells within the CD3+CD8+ (upper row) and CD3+CD8− (lower row) cell populations was determined for the peripheral blood lymphocytes (PBLs, gray bars) and tumor-associated lymphocytes (TALs, black bars) of each patient on the day of tumor removal. (B) Example of CD45RA and CCR7 detection in samples from patient 4.
The presence of NB cells affects the phenotype and activation status of autologous T cells
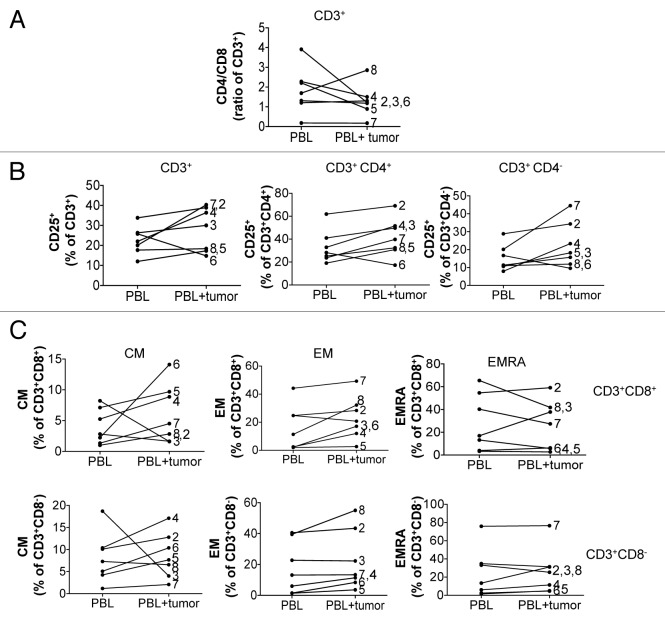
To test whether NB cells may create a microenvironment that promotes the expansion and/or differentiation of selected T-cell subsets from the peripheral blood, we cultivated PBLs of NB patients in the presence of autologous tumor cells in vitro. Albeit to a different extent, a drop in the CD4+/CD8+ T-cell ratio in the PBLs from 4 of 7 patients was observed upon co-incubation with autologous tumor cells (Fig. 4A). Of note, when PBLs from patient 8 were exposed to autologous tumor cells, the proportion of CD3+CD4+ T cells was considerably increased, consistent with an increased CD4+/CD8+ T-cell ratio in TALs vs. PBLs detected for the same patient.
Figure 4. Modulation of the phenotype of autologous peripheral blood lymphocytes upon exposure to tumor cells in vitro. (A–C) The phenotype of peripheral blood lymphocytes (PBLs) was monitored after 8 d of in vitro propagation in the presence or absence of autologous tumor cells. (A) The modulation of the CD4+/CD8+ T-cell ratio in CD3+ PBLs upon co-culture with autologous tumor cells (PBL+tumor), as compared with PBLs maintained alone, was monitored by flow cytometry. (B) The percentage of CD25-expressing CD3+, CD3+CD4+ and CD3+CD4− PBLs in the presence or absence of autologous tumor cells, as monitored by flow cytometry, is shown. (C) Modulation of the T-cell memory phenotype in PBLs co-cultured with autologous tumor cells for 8 d. The percentage of CD3+CD8+ (upper row) and CD3+CD8− (lower row) cells manifesting a central memory (TCM), effector memory (TEM) and CD45RA+ effector memory (TEMRA) phenotype upon culture in the presence or absence of autologous tumor cells is shown. * = not performed.
To investigate a possible role of NB in the expression of CD25 by T cells upon extravasation, we also measured CD25 levels on PBLs incubated in the presence or absence of autologous tumor cells. Consistent with the data on CD25 expression on PBLs and TALs on the day of tumor removal, we detected different degrees of CD25 upregulation in 5 out of 7 PBL cultures exposed to autologous tumor cells (Fig. 4B), suggesting that T-cell activation may take place in the NB microenvironment.
Furthermore, we investigated whether the patterns of T-cell differentiation associated with immunological memory can be acquired by T cells upon exposure to autologous tumor cells. We observed that memory T-cell pools, in particular TCM and TEM T cells, were increased in both the CD8+ and CD8− subsets of PBLs co-cultured with autologous tumor cells (Fig. 4C), suggesting that the NB microenvironment does not prevent, but rather contributes to the generation of immunological memory.
Magnitude and pattern of cytokine production differ between primary NB and PBL compartments
Cytokine production by T cells can serve both an immunoregulatory function (“signal three” in the T-cell activation process) and an effector function, along with the perforin/granzyme and death receptor-mediated elimination of antigen-expressing targets. Therefore, a comparison of the pattern and amount of cytokines produced within the tumor microenvironment and in peripheral blood of tumor-bearing patients can provide insights into the “quality” of T-cell immune responses, in particular relative to the ability of T cells to recognize and eliminate targets. We characterized the production of cytokines by NB resident cells as well as by autologous PBLs, the latter either in a steady-state setting or upon exposure to autologous tumor cells. Importantly, for the assessment of cytokine production by tumor-resident cells, tumor specimens were incubated in serum-free conditions (see Materials and Methods). We observed an elevated production of IL-8 by all primary tumors (for example, in 4 of 8 primary tumor samples, IL-8 levels varied from 2.3 ng/mL to 13.3 ng/mL, mean = 8.5 ng/mL). In contrast to IL-8, the levels of transforming growth factor β (TGFβ) were relatively low in 7 out of 8 primary tumors, whereas a significant production of this cytokine was observed among PBLs (Table 2).
Table 2. Cytokine expression by NB tumors and autologous PBLs.
| Cytokine | Sample | Patient number |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|
IFNγ |
Tumor |
0.0 |
- |
0.9 |
0.0 |
38.6 |
86.0 |
0.0 |
0.0 |
|
PBL |
- |
58.2 |
46.6 |
3.2 |
11.7 |
9.5 |
330.2 |
0.6 |
|
|
PBL+tumor |
- |
31.7 |
22.7 |
19.5 |
89.9 |
103.4 |
651.9 |
0.6 |
|
|
TNFα |
Tumor |
98.5 |
- |
8.1 |
6.7 |
130.1 |
509.7 |
1.2 |
0.3 |
|
PBL |
- |
82.3 |
151.0 |
9.0 |
18.1 |
72.8 |
148.5 |
28.2 |
|
|
PBL+tumor |
- |
149.4 |
61.9 |
105.9 |
160.5 |
289.4 |
167.4 |
14.2 |
|
|
IL-4 |
Tumor |
5.52 |
- |
3.2 |
1.5 |
1.5 |
2.1 |
0.0 |
0.0 |
|
PBL |
- |
0.9 |
3.2 |
2.1 |
0.0 |
2.1 |
2.1 |
5.5 |
|
|
PBL+tumor |
- |
3.2 |
4.4 |
5.5 |
4.4 |
2.1 |
0.0 |
2.1 |
|
|
IL-5 |
Tumor |
1.2 |
|
0.1 |
0.1 |
0.8 |
15.5 |
0.1 |
0.1 |
|
PBL |
- |
3.3 |
3.1 |
0.6 |
1.1 |
0.4 |
0.9 |
0.6 |
|
|
PBL+tumor |
- |
1.8 |
0.8 |
6.8 |
1.9 |
2.9 |
1.7 |
0.5 |
|
|
IL-6 |
Tumor |
1.5 |
- |
8.6 |
602.8 |
7.1 |
51.1 |
0.7 |
0.2 |
|
PBL |
- |
40.1 |
50.9 |
70.6 |
4.4 |
4.1 |
2.9 |
2.7 |
|
|
PBL+tumor |
- |
131.2 |
106.8 |
396.0 |
16.5 |
50.9 |
4.0 |
1.6 |
|
|
IL-8 |
Tumor |
1969 |
- |
12064.7 |
2285.6 |
7286.5 |
13290.7 |
277.8 |
51.4 |
|
PBL |
- |
13563.2 |
11339.4 |
14734.0 |
6043.4 |
7348.8 |
2810.8 |
7707.5 |
|
|
PBL+tumor |
- |
13920.3 |
17255.9 |
11291.4 |
12716.9 |
13416.1 |
3186.1 |
3837.1 |
|
|
IL-10 |
Tumor |
4.5 |
- |
5.3 |
1.8 |
45.4 |
8.1 |
0.5 |
0.5 |
|
PBL |
- |
23.4 |
19.7 |
1.2 |
1.8 |
1.5 |
3.9 |
0.5 |
|
|
PBL+tumor |
- |
27.2 |
14.6 |
8.8 |
18.2 |
12.4 |
67.9 |
1.2 |
|
|
IL-13 |
Tumor |
2.5 |
- |
0.8 |
0.4 |
124.6 |
692.9 |
0.5 |
0.1 |
|
PBL |
- |
809.7 |
1206.3 |
11.1 |
124.6 |
67.3 |
881.6 |
36.2 |
|
|
PBL+tumor |
- |
2034.6 |
1397.3 |
92.4 |
359.1 |
422.3 |
730.2 |
12.4 |
|
|
TGFβ |
Tumor |
238.2 |
- |
0.0 |
258.8 |
14.4 |
135.3 |
1195.1 |
0.0 |
|
PBL |
- |
7617.6 |
2036.8 |
3337.8 |
894.4 |
987.1 |
277.7 |
238.5 |
|
|
PBL+tumor |
- |
5673.1 |
1895.3 |
1857.7 |
1245.8 |
641.0 |
0.0 |
56.4 |
|
| GM-CSF |
Tumor |
6 |
- |
1.7 |
4.5 |
191.2 |
897.8 |
0.3 |
0.1 |
|
PBL |
- |
811.4 |
314.2 |
22.8 |
110.7 |
47.9 |
209.4 |
16.6 |
|
| PBL+tumor | - | 1191.8 | 325.0 | 88.9 | 477.2 | 636.4 | 334.5 | 10.9 | |
Table contains data on the concentration of cytokines (pg/ml) detected in supernatants of primary tumor medium. The concentrations of indicated cytokines were measured using human cytokine 13-Plex premixed kit. The concentration of TGFβ was measured by ELISA as immune detection of this cytokine requires additional low pH treatment which allows conversion of latent TGFβ to its immune reactive form; data shown as mean of triplicates.
When incubated in serum-free medium containing IL-2, IL-7 and IL-15 (the three cytokines that are essential for the homeostatic maintenance of T cells ex vivo in the absence of antigenic stimulation), PBLs from most of the patients produced relatively high but variable levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), TNFα, IL-8, IL-13 and TGFβ, relatively low levels of IFNγ, IL-6 and IL-10, as well as barely detectable levels of IL-4 and IL-5 (Table 2). Interestingly, the exposure of PBLs to autologous tumor cells enhanced the secretion of most cytokines including the TH1 factor IFNγ and TNFα, whereas the expression of TGFβ was downregulated in 5 of 7 patients (Table 2). Of note, the increased levels of some cytokines detected in PBL:tumor cell co-cultures as compared with PBLs alone (such as TNFα, GM-CSF and IL-13 in patient 6, IL-10 in patient 5 and IL-6 in patient 4) may have originated from the tumor compartment, which—in these instances—was characterized by a relatively elevated production of these cytokines (Table 2). This was not the case for the remaining co-cultures, as primary tumor cells cultured alone generated low levels of the cytokines under consideration. Collectively, these data confirm that PBLs from NB patients can undergo activation upon exposure to autologous tumor cells, which results in an altered pattern of cytokine secretion.
Discussion
Tumor infiltration by T lymphocytes is commonly observed in cancers of various histological origin and anatomical localization. Recently, a large body of evidence has uncovered a correlation between the presence of lymphoid infiltration and the survival of patients affected by many types of cancer. In 58 of 60 published studies, the presence of CD8+ memory T cells was associated with a favorable prognosis.14 In colon and breast cancer, the presence of a favorable immunological signature coincides with a good subsequent response to chemotherapy.15-17 Furthermore, although this still requires further verification in Phase III clinical studies, the use of an “immune score” based on the presence of CTLs and CD45RO+ memory cells has proven of an outstanding value in predicting the risk of relapse among patients affected by localized colon cancer without lymph node involvement or distant metastases.18 In fact, the ability of cancer cells to avoid immune recognition and elimination has recently suggested as a common hallmark of progressive cancer.19 Still, the immune system has also been proposed to contribute to tumor growth as it promotes tumor-associated inflammation.20 Therefore, when introducing immunotherapy into treatment protocols, the possible interactions between therapy-induced immunity and naturally occurring inflammatory responses in the tumor milieu should carefully be considered. Current clinical protocols for the management of NB include immunotherapeutic strategies based on the anti-GD2 monoclonal antibody ch14.18 and alternating the administration of GM-CSF and IL-2. This approach combined with high-dose isotretionin resulted in improved event-free survival in children affected by high-risk disease.10 A smaller clinical study has also reported that the infusion of CTLs bearing chimeric antigen receptors (CARs) specific for GD2 and a native TCR specific for the Epstein-Barr virus promoted promising clinical responses in 50% of enrolled patients.21 However, limited data are available describing the immunological signature of primary NBs, which is needed to define the prerequisites for immunotherapy and which will help to identify the patients that may truly benefit from immunotherapy.
The characterization of tumor-infiltrating lymphocytes (TILs) prior to the unspecific and/or antigen-specific triggering ex vivo represents a serious technical challenge. Multiple studies have reported a phenotypic analysis of TILs following rapid T-cell expansion protocols that included the exposure of T cells to a variety of strong unspecific stimuli including fetal calf serum, the exogenous supply of recombinant cytokines and irradiated allogeneic feeders.22-24 Here, we report the ex vivo phenotypic analysis of intratumoral and circulating T cells simultaneously obtained from NB patients. Such a comparison is essential for the rational design of novel immunotherapeutics against NB. To date, the characterization of TILs in pediatric neoplasms in respect to phenotype, clonality, specificity, frequency and response to expansion protocols in vitro is sparse, as compared with other oncological settings such as melanoma. In this latter case, a bulk of detailed studies have contributed to TIL therapy becoming a successful story for the treatment of metastatic melanoma patients, with some clinical trials reporting > 50% response rates.25,26 Interestingly, several features of isolated, non-manipulated TILs from NB patients, such as a skewing toward CD8+ vs. CD4+ T cells, the presence of a large proportion of effector memory-like T cells and the retention of reactivity against autologous tumor cells have also been observed after the application of rapid expansion protocols to TILs from melanoma patients.27,28 It still needs to be elucidated whether such immunologically favorable features are characteristic of TILs invading neural crest-derived tumors, such as melanoma and NB, or if they extend to TILs of different origin.
The analysis of immune activation and/or suppression in human tumors is hampered by logistical and ethical limitations. Some insights into this process can be gained by comparing the composition, phenotype and functional characteristics of circulating and intratumoral T cells. Here, we present a comparative analysis of T-cell populations from two different compartments, the peripheral blood and the neoplastic lesions of NB patients, performed within a minimal period of time after tumor excision and without exposure to any mitogenic stimulus ex vivo. Importantly, the genetic analysis of tumor specimens demonstrated that patients of all genetic subsets, as defined by Carén et al.,29,30 were included into our study (Table 1).
The analysis of the whole cell content of NBs by flow cytometry revealed CD3+ cell populations in all examined specimens, which in some cases represented up to 18% of the total cell number (Table 1; Fig. 1). These findings are in striking contrast with some previous reports, which failed to detect lymphoid infiltration in NBs.31 This surprisingly high degree of tumor-associated lymphocytic infiltration is unlikely to be an artifact due to the contamination of tumor samples with peripheral blood lymphocytes, as our subsequent analysis demonstrated that these two compartments significantly differ in their composition. First, we observed that CD8+ T cells prevail over CD4+ T cells in the tumor lesions derived from most patients, where in some cases the CD4+/CD8+ T-cell ratio was 4- to 5-fold lower than in the peripheral blood. CD4+/CD8+ T-cell ratios in the PBLs of NB patients were similar to those previously observed in a large cohort of healthy donors.32 Different molecular events may lead to the skewing of the CD4+/CD8+ T-cell ratio at the NB site, including a differential homing efficacy, a differential proliferative capacity and/or a differential viability at the tumor site of CD4+ and CD8+ subsets. We found that the physiological CD4+/CD8+ T-cell ratio in PBLs could be altered by exposure to autologous tumor cells in vitro, notably toward the ratio that we observed in TALs. The actual reason for the altered CD4+/CD8+ T-cell observed within NB lesions is currently not clear. It has previously been reported that human NB cells induce T-cell apoptosis via FAS-FASL interactions.33 However, we failed to detect FASL expression in the primary NB cells analyzed in this study (data not shown). Although we cannot formally exclude that NB cells possess other factors causing the demise or blocking the proliferation of CD4+ T cells, this appears unlikely as we repeatedly detected the expression of activation markers on CD3+CD4+ T cells at levels that were comparable to those observed on CD3+CD8+ cells (Fig. 2A). Similar results demonstrating a preferential presence of CD8+ T cells in various solid tumors and their correlation with prognosis have been reported in various settings during the last decade.34-36
The presence of T cells with potential regulatory properties (Tregs) was investigated using the intracellular marker FOXP3. Though the ectopic expression of FOXP3 confers regulatory capacities to CD4+ cells, FOXP3 may also be expressed transiently upon T-cell activation.11 Therefore, the regulatory potential of FOXP3+ cells should be measured directly, as their ability to suppress the activation of effector T cells. Due to the limited amount of material that was available for our study, we could not accomplish this. Notably, we detected decreased levels of FOXP3 in TALs as compared with PBLs in two patients (Fig. 2B). It was recently shown that patients affected by Stage 4 non-MYCN amplified NB exhibiting FOXP3 expression levels above the median have a reduced 5-year event-free survival,37 suggesting that the expression of FOXP3 in NB samples is relevant for disease outcome. A previous attempt to define immune responses in high-risk vs. low-risk NBs concluded that adaptive immune responses would play a role in disease progression. However, this study was limited by the fact that the authors did not investigate tumor tissues by flow cytometry, but relied only upon a few peripheral blood samples.38 These data from our cohort suggest that the NB milieu favors the specific activation of T cells at the tumor site rather than the accumulation of immunosuppressive Tregs. This hypothesis is supported by a number of additional lines of experimental evidence. First, a selective enrichment of CD8+ T cells bearing a memory phenotype was detected at the tumor site as compared with the peripheral blood (Fig. 3). Second, a higher proportion of cells expressing the surface IL-2 receptor α chain was observed among TALs as compared with autologous PBLs (Fig. 2A). Third, a variable but significant upregulation of CD25 was observed in 5 out of 7 PBL samples exposed to autologous tumor cells in vitro (Fig. 4B). Fourth, the exposure of PBLs to tumor cells stimulated the production of various cytokines including IFNγ and TNFα (Table 2). Fifth, a decreased production of TGFβ was detected in 6 out of 7 PBL specimens co-cultured with autologous tumors (Table 2). Of note, an elevated expression of TGFβ, one of the cytokines believed to mediate the immunosuppressive activity of Tregs,11 has been correlated with a reduced 5-year event-free survival in non-MYCN amplified NBs.37 Sixth, the memory T-cell pools, central and effector memory T cells in particular, were significantly increased in both the CD8+ and CD8− cell subsets of CD3+ PBLs exposed to autologous tumor cells (Fig. 4). Finally, we were able to detect a substantial number of proliferating T cells in situ and TCR aggregation at the sites of interaction between T lymphoctyes and tumor cells (Fig. 1B).
The exposure of PBLs to autologous tumor resulted in a prominent alteration in cytokine secretion. As mentioned above, a tendency toward the enhanced secretion of TH1 cytokines such as IFNγ and TNFα was observed, whereas the immunosuppressive factor TGFβ was decreased in most cases. The expression of TH2 cytokines such as IL-4, IL-5 and IL-10 was low in all primary tumor and PBL specimens, and was not significantly increased upon the exposure of lymphocytes to tumor cells (Table 2). IL-8, which is often defined as an angiogenic and metastasis-promoting factor,39 was the dominant cytokine produced by primary tumor cells, which is in agreement with previous data.9 The high levels of IL-8 produced by PBLs indicate that the cellular source of IL-8 in NB patients may reside outside of the tumor microenvironment.
It is important to stress that the interpretation of changes in cytokine production by PBLs exposed to NB in co-culture experiments described herein must consider the presence of TALs that contribute to cytokine secretion. We have noted, however, that in all control tumor samples incubated in parallel with “co-culture” samples, T cells gradually declined in number and were barely detectable at day 8, when the analysis was usually performed. This may possibly be explained by the low dose of IL-2 (10 IU/mL) used in our experiments. Therefore, the contribution of TALs to cytokine production in these experiments is expected to be minor and the observed changes most probably reflect the alterations inflicted by NB cells to PBLs.
The activated phenotype of NB-infiltrating lymphocytes appears paradoxical in the light of obvious tumor progression. Current methodologies are not sufficiently advanced to directly demonstrate the specific recognition of malignant cells by TILs in patients. It is also difficult to assess the impact of T-cell activation on tumor progression in situ. However, several observations discussed above support an ongoing interaction between TALs and tumor cells within NB lesions. Furthermore, our previously published data suggest that even unspecific T-cell activation could result in strong suppressive effects on NB cells.4 One must conclude that the extent of naturally occurring TAL activation in NBs is not sufficient for mediating antitumor effects. This may result from the suppression of CTL activation by factors produced within the tumor microenvironment. Alternatively, an intense proliferation of tumor cells may overcome the antineoplastic effects of local immune responses. In this light, we suggest that the adoptive transfer of activated CTLs capable of resisting an immunosuppressive microenvironment may add up to the TAL pool and limit tumor progression.
In conclusion, our study demonstrates the presence of activated T cells within primary NBs and the possibility for autologous T cells to become activated in the presence of NB cells. Further studies on the crosstalk between TALs and NB cells may help to exploit the immune system for the treatment of children affected by this dreadful disease.
Materials and Methods
Patient material
Primary samples from NB lesions were isolated from eight patients with NB diagnosed and treated at Astrid Lindgren Children’s Hospital, Stockholm, Sweden. Informed consent was obtained from all patients/parents and all experiments were performed in accordance with permission obtained from the ethical committee. Diagnosis and staging were defined according to international criteria.40 A single-nucleotide polymorphism (SNP)-based CGH array revealed that the tumor samples included in the study represented all different genetic subsets, as recently defined29,30 (Table 1). Two of eight patients died from progressive tumors, whereas the other six survived for more than three years following diagnosis. Freshly isolated single cell suspensions were kept in FCS-free AIM-V culture medium containing 100 IU/mL penicillin, 100 μg/mL streptomycin and 20 μg/mL ciproxofloxacin (complete AIM-V) throughout the experimental procedures. PBLs were isolated from fresh blood drawn obtained on the day of surgery and purified by density gradient separation through Ficoll-Paque (Amersham Biosciences).
Antibodies and recombinant cytokines
Allophycocyanin (APC)-conjugated anti-CD3 and anti-CD45RA (561811 and 550855, respectively), fluorescein isothiocyanate (FITC)-conjugated anti-CD4, anti-CD25 and anti-CD3 (561842, 555431 and 561806, respectively), peridinin chlorophyll protein (PerCP)-conjugated anti-CD8 and anti-CD4 (347314 and 550631, respectively), R-phycoerythrin (RPE)-conjugated anti-CCR7 (150503) and the respective fluorochrome-conjugated isotype control antibodies were purchased from BD Pharmingen. The human regulatory T-cell staining kit and buffer were purchased from eBioscience (12-4777-41 and 00-5523, respectively). Recombinant human IL-15 and IL-7 were from R&D Systems (247-IL-005 and 317-ILB-050 respectively) and recombinant human IL-2 was from PeproTech (200-02).
Flow cytometry
The analysis of surface molecules was performed either on the day of tumor excision or, in co-incubation experiments, as indicated. Single-cell suspensions generated from a piece of tumor were incubated with specific fluorochrome-conjugated primary antibodies or with relevant isotype control antibodies for 30 min on ice, washed in 0.1% BSA (in PBS) and analyzed on a FACScan flow cytometer (Becton Dickinson,). The extent of T-cell infiltration in each NB was determined on the day of tumor excision and defined as a percentage of CD3+ cells within the given tumor specimen detected in a broad forward/side scatter gate that includes all events with a size and granularity compatible with live cells (cell debris were excluded from the analysis). Since the CD3 marker was used in multiple staining sets on the same tumor (CD3/CD4/CD25, CD3/CD4/CD8 and CD3/CD8/CD45RA/CCR7), the percentage of CD3+ cells was calculated as average of values obtained from at least three different stainings. The detection of FOXP3+ T cells was performed using the Human Regulatory T-cell Staining Kit (eBioscience), according to manufacturer’s instructions. Briefly, 5 × 105 cells obtained from the total NB mass were first stained with anti-CD4-FITC antibodies for 30 min on ice and washed three times in 0.1% BSA (in PBS) followed by fixation and permeabilization. Next, after extensive washing in permeabilization buffer and pre-incubation with 2% rat serum for 15 min on ice, total cell suspensions were exposed to either a FOXP3-specific antibody or the relevant isotype control. All flow cytometry data were analyzed using the Flow Jo software (TreeStar).
Co-culture experiments
A total number of 5 × 104 PBLs was incubated with autologous tumor cells at a 1:5 ratio in 200 μL complete AIM-V medium in 96-well U-bottom shaped culture plates. As control conditions, PBLs and tumor cells were incubated separately at corresponding cell numbers and cell density. Cultures were maintained at 37°C 5% CO2 in complete AIM-V containing 10 U/mL IL-2, 50 ng/mL IL-7 and 50 ng/mL IL-15. At day 8 of incubation, culture supernatants were collected and stored at −20°C for cytokine analysis, whereas cells were analyzed by flow cytometry as described above.
Quantification of cytokines
Co-culture supernatants were analyzed for the presence of IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, GM-CSF, IFNγ and TNFα using the human cytokine 13-Plex premixed kit according to the manufacturer’s instruction. The concentration of TGFβ was assessed using an ELISA kit according to the manufacturer’s instructions.
Immunohistochemical analysis
Formalin-fixed paraffin-embedded tissue sections were deparaffinized in xylene and graded alcohols, hydrated and washed in PBS. Antigen retrieval was performed in sodium citrate buffer (pH 6) in a microwave oven. Sections were incubated overnight at 4°C with monoclonal mouse anti-human CD3 antibody (DAKO, M7254) and rabbit polyclonal anti-Ki67 (Thermo Scientific, RM-9106-S0). Secondary anti-mouse Alexa 488® and anti-rabbit Alexa 599® conjugates were employed. Matched isotype antibodies were used to control for nonspecific background staining. Sections were mounted in a DAPI-containing medium (Southern Biotech, 0101-20) and examined in an Axiophot photomicroscope (Zeiss).
Supplementary Material
Acknowledgments
This work was supported by The Swedish Childhood Cancer Foundation, Karolinska Institutet and The Norwegian Cancer Society. Dr. Jelena Levitskaya is currently supported by NIH, NCI, grant R01-CA136663-03.
Glossary
Abbreviations:
- CTL
cytotoxic T lymphocyte
- NB
neuroblastoma
- TAL
tumor-associated lymphocyte
- PBL
peripheral blood lymphocyte
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
These authors equally contributed to this work.
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23618
References
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–11. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroesen M, Lindau D, Hoogerbrugge P, Adema GJ. Immunocombination therapy for high-risk neuroblastoma. Immunotherapy. 2012;4:163–74. doi: 10.2217/imt.11.169. [DOI] [PubMed] [Google Scholar]
- 3.Seeger RC. Immunology and immunotherapy of neuroblastoma. Semin Cancer Biol. 2011;21:229–37. doi: 10.1016/j.semcancer.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Geer A, Kiessling R, Levitsky V, Levitskaya J. Cytotoxic T lymphocytes induce caspase-dependent and -independent cell death in neuroblastomas in a MHC-nonrestricted fashion. J Immunol. 2006;177:7540–50. doi: 10.4049/jimmunol.177.11.7540. [DOI] [PubMed] [Google Scholar]
- 5.De Geer A, Carlson LM, Kogner P, Levitskaya J. Soluble factors released by activated cytotoxic T lymphocytes interfere with death receptor pathways in neuroblastoma. Cancer Immunol Immunother. 2008;57:731–43. doi: 10.1007/s00262-007-0412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decock A, Ongenaert M, Vandesompele J, Speleman F. Neuroblastoma epigenetics: from candidate gene approaches to genome-wide screenings. Epigenetics. 2011;6:962–70. doi: 10.4161/epi.6.8.16516. [DOI] [PubMed] [Google Scholar]
- 7.Martin RF, Beckwith JB. Lymphoid infiltrates in neuroblastomas: their occurrence and prognostic significance. J Pediatr Surg. 1968;3:161–4. doi: 10.1016/0022-3468(68)91005-1. [DOI] [PubMed] [Google Scholar]
- 8.Lauder I, Aherne W. The significance of lymphocytic infiltration in neuroblastoma. Br J Cancer. 1972;26:321–30. doi: 10.1038/bjc.1972.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facchetti P, Prigione I, Ghiotto F, Tasso P, Garaventa A, Pistoia V. Functional and molecular characterization of tumour-infiltrating lymphocytes and clones thereof from a major-histocompatibility-complex-negative human tumour: neuroblastoma. Cancer Immunol Immunother. 1996;42:170–8. doi: 10.1007/s002620050267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 12.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175:5895–903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 14.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto T, Bianchini G, Booser D, Qi Y, Coutant C, Shiang CY, et al. Gene pathways associated with prognosis and chemotherapy sensitivity in molecular subtypes of breast cancer. J Natl Cancer Inst. 2011;103:264–72. doi: 10.1093/jnci/djq524. [DOI] [PubMed] [Google Scholar]
- 16.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 17.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–7. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 18.Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–51. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 21.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klapper JA, Thomasian AA, Smith DM, Gorgas GC, Wunderlich JR, Smith FO, et al. Single-pass, closed-system rapid expansion of lymphocyte cultures for adoptive cell therapy. J Immunol Methods. 2009;345:90–9. doi: 10.1016/j.jim.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadeghi A, Pauler L, Annerén C, Friberg A, Brandhorst D, Korsgren O, et al. Large-scale bioreactor expansion of tumor-infiltrating lymphocytes. J Immunol Methods. 2011;364:94–100. doi: 10.1016/j.jim.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Powell DJ, Jr., Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–50. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep. 2012;14:468–74. doi: 10.1007/s11912-012-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junker N, Thor Straten P, Andersen MH, Svane IM. Characterization of ex vivo expanded tumor infiltrating lymphocytes from patients with malignant melanoma for clinical application. J Skin Cancer. 2011;2011:574695. doi: 10.1155/2011/574695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junker N, Kvistborg P, Køllgaard T, Straten Pt, Andersen MH, Svane IM. Tumor associated antigen specific T-cell populations identified in ex vivo expanded TIL cultures. Cell Immunol. 2012;273:1–9. doi: 10.1016/j.cellimm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Carén H, Kryh H, Nethander M, Sjöberg RM, Träger C, Nilsson S, et al. High-risk neuroblastoma tumors with 11q-deletion display a poor prognostic, chromosome instability phenotype with later onset. Proc Natl Acad Sci U S A. 2010;107:4323–8. doi: 10.1073/pnas.0910684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carén H, Erichsen J, Olsson L, Enerbäck C, Sjöberg RM, Abrahamsson J, et al. High-resolution array copy number analyses for detection of deletion, gain, amplification and copy-neutral LOH in primary neuroblastoma tumors: four cases of homozygous deletions of the CDKN2A gene. BMC Genomics. 2008;9:353. doi: 10.1186/1471-2164-9-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coughlin CM, Fleming MD, Carroll RG, Pawel BR, Hogarty MD, Shan X, et al. Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J Clin Oncol. 2006;24:5725–34. doi: 10.1200/JCO.2005.05.3314. [DOI] [PubMed] [Google Scholar]
- 32.Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–83. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 33.Shurin GV, Gerein V, Lotze MT, Barksdale EM., Jr. Apoptosis induced in T cells by human neuroblastoma cells: role of Fas ligand. Nat Immun. 1998;16:263–74. doi: 10.1159/000069452. [DOI] [PubMed] [Google Scholar]
- 34.Poschke I, De Boniface J, Mao Y, Kiessling R. Tumor-induced changes in the phenotype of blood-derived and tumor-associated T cells of early stage breast cancer patients. Int J Cancer. 2012;131:1611–20. doi: 10.1002/ijc.27410. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 36.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 37.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–36. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gowda M, Godder K, Kmieciak M, Worschech A, Ascierto ML, Wang E, et al. Distinct signatures of the immune responses in low risk versus high risk neuroblastoma. J Transl Med. 2011;9:170. doi: 10.1186/1479-5876-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 40.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.