Abstract
Light intensity during growth affects the proportion of carbon dioxide fixed by the reductive pentose phosphate cycle relative to that incorporated via C4 acids in acetate phototrophs of Rhodospirillum rubrum. With cells grown at high light intensity (9000 lux) the specific activities of ribulose-1, 5-diphosphate and propionyl CoA carboxylases were increased compared with cells grown at low light intensity (1500 lux), although pyruvate carboxylase activity was unaltered.
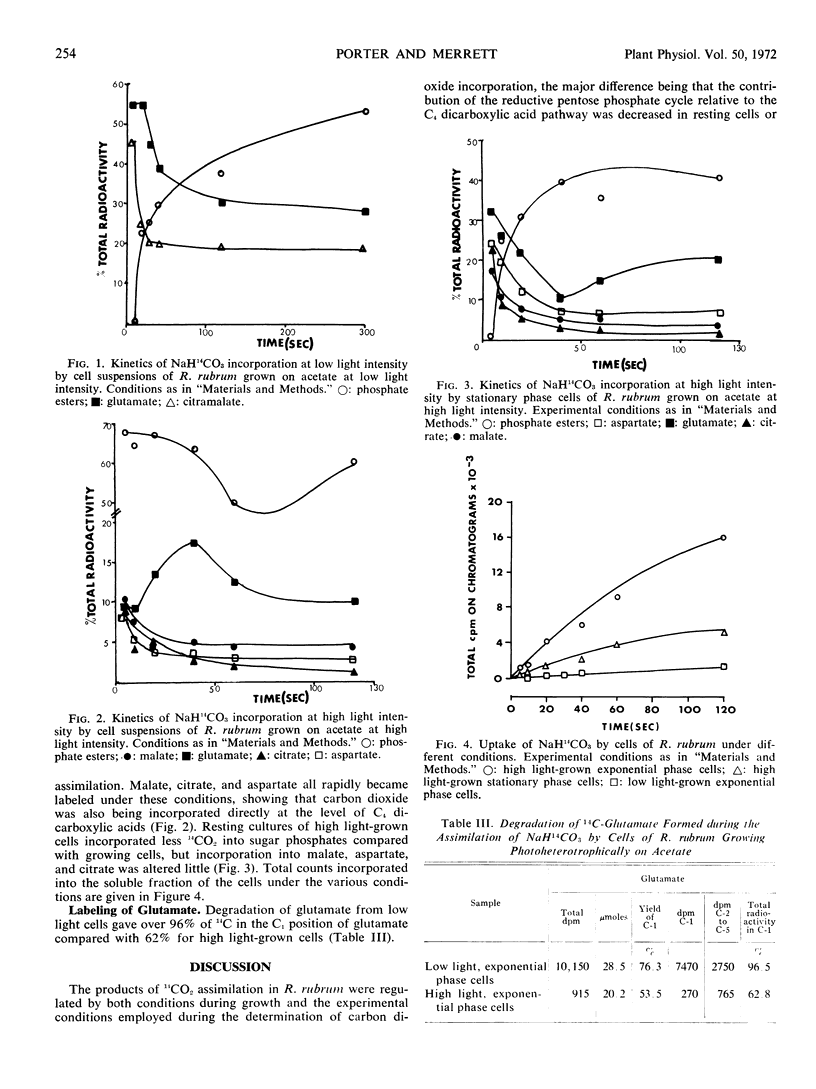
Kinetic experiments with cells assimilating acetate at high light intensity showed that when the cells had been grown at high light intensity there was a rapid incorporation of 14CO2 into phosphate esters compared with cells grown at low light intensity and fixing 14CO2 while assimilating acetate at low light intensity. The percentage of the total radioactivity present in phosphate esters plotted against time gave a negative slope for high light conditions compared with a positive slope for low light conditions. High light-grown cells assimilating acetate at high light intensity showed the greatest combined rate of 14CO2 fixation via the reductive pentose phosphate cycle and C4 acids, and this corresponded to the shortest mean generation time. When cells were grown at high light intensity and allowed to assimilate 14CO2 at high light intensity but in the stationary phase, the pattern of 14CO2 fixation resembled that for low light-grown cells assimilating acetate and fixing 14CO2 at low light intensity, showing that both acetate assimilation and high light intensity were necessary for the rapid incorporation of 14CO2 via the reductive pentose phosphate cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Fuller R. C. Photosynthesis in Rhodospirillum rubrum. II. Photoheterotrophic carbon dioxide fixation. Plant Physiol. 1967 Apr;42(4):491–496. doi: 10.1104/pp.42.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Evans M. C., Arnon D. I. Ferredoxin-dependent carbon assimilation in Rhodospirillum rubrum. Arch Mikrobiol. 1967;59(1):32–40. doi: 10.1007/BF00406314. [DOI] [PubMed] [Google Scholar]
- FULLER R. C., SMILLIE R. M., SISLER E. C., KORNBERG H. L. Carbon metabolism in Chromatium. J Biol Chem. 1961 Jul;236:2140–2149. [PubMed] [Google Scholar]
- HURLBERT R. E., LASCELLES J. RIBULOSE DIPHOSPHATE CARBOXYLASE IN THIORHODACEAE. J Gen Microbiol. 1963 Dec;33:445–458. doi: 10.1099/00221287-33-3-445. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B., BRUMMOND D. O., OCHOA S. Formation of 3-phosphoglyceric acid by carbon dioxide fixation with spinach leaf enzymes. J Biol Chem. 1956 Feb;218(2):811–822. [PubMed] [Google Scholar]
- LASCELLES J. The formation of ribulose 1:5-diphosphate carboxylase by growing cultures of Athiorhodaceae. J Gen Microbiol. 1960 Dec;23:499–510. doi: 10.1099/00221287-23-3-499. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Olsen I., Merrick J. M. Identification of propionate as an endogenous CO2 acceptor in Rhodospirillum rubrum and properties of purified propionyl-coenzyme A carboxylase. J Bacteriol. 1968 May;95(5):1774–1778. doi: 10.1128/jb.95.5.1774-1778.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J., Morris J. G. Acetate utilisation by Rhodopseudomonas spheroides. FEBS Lett. 1969 Jul;4(1):52–54. doi: 10.1016/0014-5793(69)80194-8. [DOI] [PubMed] [Google Scholar]
- Payne J., Morris J. G. Pyruvate carboxylase in Rhodopseudomonas spheroides. J Gen Microbiol. 1969 Nov;59(1):97–101. doi: 10.1099/00221287-59-1-97. [DOI] [PubMed] [Google Scholar]
- Porter J., Merrett M. J. Formation of glyoxylate from alpha-hydroxyglutarate by Rhodospirillum rubrum. FEBS Lett. 1970 Apr 16;7(3):271–273. doi: 10.1016/0014-5793(70)80178-8. [DOI] [PubMed] [Google Scholar]
- Rinne R. W., Buckman R. W., Benedict C. R. Acetate and bicarbonate metabolism in photosynthetic bacteria. Plant Physiol. 1965 Nov;40(6):1066–1073. doi: 10.1104/pp.40.6.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMILLIE R. M., RIGOPOULOS N., KELLY H. Enzymes of the reductive pentose phosphate cycle in the purple and in the green photosynthetic sulphur bacteria. Biochim Biophys Acta. 1962 Jan 29;56:612–614. doi: 10.1016/0006-3002(62)90618-2. [DOI] [PubMed] [Google Scholar]


