Abstract
Objective
To identify factors associated with ovarian reserve (OR) impairment during and immediately after chemotherapy.
Design
Prospective cohort study.
Setting
Four university hospitals.
Patients
Adolescent and young adult females with a new diagnosis of cancer requiring chemotherapy.
Interventions
None.
Main Outcome Measures
Participants were followed to assess measures of OR (serum follicle-stimulating hormone, luteinizing hormone, estradiol, inhibin B, anti-mullerian hormone (AMH), and antral follicle counts and mean ovarian volume) at 3 month intervals. Changes in OR were quantified for both the acute impact of treatment using linear regression and the longitudinal recovery after therapy using mixed effects models adjusted for baseline OR, use of alkylating agent, and hormone use.
Results
46 women with at least 1 pretreatment and 2 post-treatment study visits were included (mean follow-up 12 months). All measures of OR demonstrated significant changes during chemotherapy. Alkylating agent exposure and baseline OR were associated with the magnitude of impairment acutely, and pretreatment AMH levels were associated with the rate of recovery of AMH post-treatment. In adjusted models, participants with a pretreatment AMH level >2 ng/mL recovered at a rate of 11.9% per month after chemotherapy, whereas participants with pretreatment AMH levels ≤2 ng/mL recovered at a rate of 2.6% per month after therapy (p=0.003).
Conclusion
Baseline OR and alkylating agent exposure effect the magnitude of acute changes in OR from chemotherapy. The rate of recovery of AMH is impacted by pretreatment levels. This should be considered during pretreatment fertility preservation counseling.
Keywords: fertility, gonadotoxicity, chemotherapy, ovarian reserve, young adults
Introduction
Cancer therapies have an adverse impact on reproductive function, increasing the risk of early menopause and infertility (1). One of the recognized challenges in studying the reproductive risks of cancer therapy is that outcomes such as fertility are difficult to capture and menstrual function is not a reliable predictor of fertility (2–4). Measures of ovarian reserve, including ultrasound measures (antral follicle count (AFC) and mean ovarian volume (MOV)), and serum measures (inhibin B (INH), anti-mullerian hormone (AMH), estradiol (E2), lutenizing hormone (LH) and follicle-stimulating hormone (FSH)) are useful surrogate markers for fertility potential (3, 5). AMH in particular has emerged as a valuable marker of ovarian reserve in women as it remains relatively stable throughout the menstrual cycle and may not be affected by hormone use (6, 7).
Limited data exist describing changes in measures of ovarian reserve during and shortly after completion of cancer treatment. While studies of older pre-menopausal women with breast cancer suggest that baseline AMH predicts resumption of menses after chemotherapy (8–10), no prospective study has examined changes in comprehensive measures of ovarian reserve in adolescent and young women undergoing chemotherapy and attempted to identify factors associated with these changes.
The primary objective of this study was to evaluate the effect of chemotherapy on longitudinal measures of ovarian reserve in post-pubescent adolescents and women ≤35 years with cancer. In addition, this study sought to identify factors associated with changes in ovarian reserve. We hypothesized that reduced baseline ovarian reserve, exposure to alkylating agents and pelvic radiotherapy would be associated with acute changes in measures of ovarian reserve during cancer therapy, and with a slower rate of recovery after cancer therapy.
Materials and Methods
This study is part of a collaboration between the University of Pennsylvania (Penn), Children’s Hospital of Philadelphia (CHOP), Children’s Memorial Hospital in Chicago (CMH) and the University of North Carolina-Chapel Hill (UNC). Institutional review board approval was obtained at each site and informed consent was obtained from participants. The study included a visit prior to initiation of cancer therapy and then visits every three months from chemotherapy initiation. Study visits included a questionnaire, physical examination, pelvic ultrasonography, and hormone analysis.
Subjects
Post-menarchal, pre-menopausal participants with a new cancer diagnosis were enrolled from four sites (Penn, CHOP, CMH, and UNC). Participants were required to be between 11–35 years of age, have a uterus and two ovaries and anticipate treatment with chemotherapy (with or without radiation). Participants were excluded if they had been pregnant or lactating within three months or if they had received any chemotherapy or radiation treatment prior to enrollment.
Questionnaires
A structured interview included detailed information on demographics, medical history, menstrual characteristics, pregnancies, infertility history, contraception, medications, and substance use. Use of exogenous hormones was recorded at each visit, including hormonal contraceptives, tamoxifen, gonadotropin-releasing hormone (GnRH) agonists, and hormone replacement therapy.
Physical Examination
Height and weight were measured for calculating body mass index (BMI).
Pelvic Ultrasonography
MOV and AFC were determined by ultrasonography for participants at 3 sites with ultrasound capability. Transvaginal ultrasonography was preferred, though transabdominal ultrasound was performed in participants uncomfortable with the transvaginal approach. MOV was calculated using the ellipse formula (A × B × C × 0.52). AFC was determined as the number of follicles 2–10 mm in average diameter for subjects undergoing transvaginal ultrasonography when both ovaries were visualized.
Hormone Analysis
Blood samples were obtained at each study visit for determining levels of FSH, LH, E2, INH, and AMH. In regularly menstruating participants an attempt was made to collect hormone measures during the early follicular phase of the menstrual cycle (days 1–4), particularly for follow-up visits.
All hormone assays were measured at Penn’s Clinical Translational Research Center using FSH and E2 Coat-A-Count kits (Diagnostic Products Corporation) and INH and AMH ELISA kits (Diagnostic Systems). The FSH immunoradiometric assay’s range is 1.5–100 mIU/mL, with a sensitivity of 0.7 mIU/mL and inter- and intraassay coefficients of variation (cov) <6% and 4%, respectively. The E2 RIA’s range is 20–3,600 pg/mL, with a sensitivity of 7 pg/mL and inter- and intra-assay cov <8.1% and 7%, respectively. The INH’s ELISA’s range is 10–531 pg/mL, with a sensitivity of 7 pg/mL and inter- and intra-assay cov <8% and <6%, respectively. The AMH ELISA’s range is 0.050–10.0 ng/mL, with a sensitivity of 0.025 ng/mL and inter- and intra-assay cov <8% and 5%, respectively.
Menstrual Function
Subjects were given a menstrual diary and asked to provide dates for their two most recent menstrual cycles during the interview at each study visit. Self-reported cycle length at baseline was recorded for participants not taking exogenous hormones. At the first post-treatment study visit, presence of menstrual function was defined as having any menses since cancer therapy started. For subsequent visits the time period was since the previous visit.
Cancer Therapy
Diagnosis and treatment exposure data were obtained by abstracting medical records. Treatment was summarized for chemotherapeutic type, duration, cumulative dose, radiation dose and location. Alkylating agent dose scores (AAD) were determined by assigning a score ranging from 1 to 3 for each agent received and summing scores over all agents (11).
Data Analysis
Analysis included participants who had at least two post-treatment study visits. Baseline characteristics and log-transformed hormone levels and ultrasound data were summarized over the course of cancer therapy and recovery. Separate models were developed to focus on the acute impact of cancer treatment (acute phase) and ovarian recovery (recovery phase). A priori power calculations determined that 72 participants were required to be able to detect a difference of 1/3 of the standard deviation in the within-woman change of log-transformed AMH from baseline to post-treatment with a power of 80% and type I error of 0.05. This report represents a preliminary analysis of the data of this ongoing cohort study.
Acute Phase
The acute change (difference) in ovarian reserve measures over treatment course was summarized by subtracting baseline from the first post-treatment value. This metric was chosen to describe cumulative changes during treatment since participants had varying lengths of treatment. Percent change was computed as the ratio of geometric mean hormone levels (unadjusted), or back-transformed from multivariable linear regression models of log-transformed hormone values. Risk factors of interest included age, baseline measures of ovarian reserve, duration of cancer therapy, alkylator exposure, pelvic/abdominal radiation exposure, presence of menstrual cycles, and exogenous hormone use. Continuous variables were analyzed dichotomously with median cut points, with the exception of BMI which was assessed as a continuous measure and age which was categorized in 5 year intervals.
Recovery phase
To investigate changes in ovarian reserve after the completion of therapy, log-transformed hormone levels and ultrasound data were modeled using general linear or random effects regression models over post-treatment visits. These models describe within-person change over time while also accounting for correlations within individuals in addition to averaging data across women. Various model assumptions were examined and a simple model assuming a common linear trend for recovery fit the data adequately. We assumed that risk factors could influence the recovery of ovarian reserve in two distinct ways. First, risk factors may influence the level of ovarian reserve at the start of the recovery period. In addition, risk factors may affect the rate of recovery over time. Examination of this later influence was performed using statistical tests of interaction between time and each risk factor.
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Pennsylvania. Statistical analysis was performed using STATA v12.0 (StataCorp, College Station, TX).
Results
Eighty-one women between the ages of 15 and 35 have been enrolled in this study. Forty-six women had sufficient visits to be included in this report. Thirty eligible women were recruited from Penn, 7 from CMH, 6 from CHOP, and 3 from UNC.
Baseline characteristics are presented in Table 1. Most participants were Caucasian, unmarried, normal weight, nulligravid, and college graduates. Forty-one percent (19/46) of participants were diagnosed with breast cancer and 37% with hematologic malignancies. Chemotherapy regimens for breast cancer and lymphoma participants were heterogeneous. Seventy-two percent of participants received an alkylating agent and the median alkylator score for all participants was 1 (range 0–4). The median length of cancer therapy was 91 days (range 30–257 days). Twelve women underwent radiation: 9 chest, 2 pelvic, and 1 upper abdominal. One participant underwent ovarian transposition prior to pelvic radiotherapy. Five women had ovarian biopsy and one had oophorectomy for tissue banking for fertility preservation. One participant with leukemia was initially treated with chemotherapy and then relapsed during the follow-up period. Data collected post-bone marrow transplant was censored.
Table 1.
Demographics
| N=46 | |
|---|---|
| Age (y), mean (range) | 26.1 (15.0 – 35.9) |
| BMI, mean (range) | 24.0 (16.9 – 39.6) |
| Race-Caucasian, % (n) | 74 (34/46) |
| Education>College Graduate, % (n) | 59 (27/46) |
| Income>75k, % (n) | 37 (17/46) |
| Marital status-single, % (n) | 70 (32/46) |
| Previous pregnancy, % (n) | 24 (11/46) |
| Cancer Type and Regimen | |
| Breast, % (n) | 41 (19/46) |
| TCH | 26 (5/19) |
| TC | 21 (4/19) |
| ACT | 47 (9/19) |
| ECT | 5 (1/19) |
| Leukemia, % (n) | 9 (4/46) |
| Lymphoma, % (n) | 28 (13/46) |
| BEACOPP | 31 (4/13) |
| ABVD | 46 (6/13) |
| ABVE | 8 (1/13) |
| R-CHOP | 8 (1/13) |
| ICE | 8 (1/13) |
| Sarcoma, % (n) | 9 (4/46) |
| Brain, % (n) | 2 (1/46) |
| Wilm’s, % (n) | 2 (1/46) |
| Germ Cell, % (n) | 2 (1/46) |
| Other, % (n) | 4 (2/46) |
| Alkylating agent use, % (n) | 72 (33/46) |
| Alkylator score, mean (range) | 1.4 (0 – 4) |
| Using exogenous hormones, % (n) | 54 (25/46) |
| Combined oral contraceptives | 80 (20/25) |
| Vaginal ring (Nuvaring) | 12 (3/25) |
| Progestin IUD | 4 (1/25) |
| Lupron Depot | 4 (1/25) |
| Seen in early follicular phase, % (n) | 54 (25/46) |
| Self-reported cycle length (d), mean (range)* | 28 (21–32) |
For participants not taking exogenous hormones
The total number of study visits for each participant ranged from 3–7, with a median of 4 visits. The average length of follow-up was 12.4 months including 9.3 months (range 1–17.3 months) of follow-up after completion of cancer therapy. Forty-three percent (20/46) and 29% reported amenorrhea at their first and second post-therapy visits, respectively. Observed changes in ovarian reserve over the study are summarized in Supplemental Figure 1, demonstrating that ovarian reserve became impaired acutely and then began to recover post-treatment.
Acute phase
For each ovarian reserve measure, the median difference between the baseline and the first post-treatment measure is summarized in Table 2, representing the raw change as a result of cancer therapy. The mean number of months elapsed from the last day of chemotherapy to the first post-treatment study visit was 2.4 (SD=2.3). All measures of ovarian reserve had statistically significant changes from baseline to the first post-treatment visit in unadjusted models (p<0.001). As a result of cancer therapy, FSH rose 321%, LH rose 183%, AMH fell 89%, E2 fell 56%, INH fell 55%, MOV fell 46% and AFC fell 75% (Table 2).
Table 2.
Acute Changes in Ovarian Reserve
| Measure of Ovarian Reserve (N=46) | Baseline Levels Median (interquartile range) | Unadjusted Median Change During Treatment | Unadjusted Percent Change During Treatment n (95%CI) | P value | Adjusted Percent Change During Treatment* n (95% CI) | P value |
|---|---|---|---|---|---|---|
| FSH** | 5.2 mIU/ml (3.3–8.6) | 13.8 mIU/mL | 321 (167 to 565) | <0.0001 | ||
| Treatment < 90 days | 5.0 mlU/mL | 96 (−11 to 332) | 0.088 | 37 (−90 to 1765) | 0.27 | |
| Treatment ≥ 90 days | 29.8 mIU/mL | 554 (225 to 1216) | <0.0001 | 1544 (300 to 5779) | 0.001 | |
| LH | 4.0 mIU/mL (2.0–7.3) | 5.2 mIU/mL | 183 (69 to 373) | <0.0001 | 315 (46 to 1080) | 0.009 |
| AMH | 2.0 ng/mL (1.0–3.5) | −1.7 ng/mL | −89 (−84 to −93) | <0.0001 | −61 (−82 to −6) | 0.018 |
| Estradiol | 41.0 pg/mL (25.3–61.8) | −27.2 pg/mL | −56 (−31 to −71) | .001 | −25 (−69 to 81) | 0.64 |
| Inhibin | 39.7 pg/mL (14.1–84.6) | −19.0 pg/mL | −55 (−25 to −73) | .003 | 37 (−46 to 247) | 0.5 |
| MOV (n=26) | 9.8 cm3 (6.4–13.7) | −4.0 cm3 | −46 (−28 to −60) | <0.0001 | −18 (−55 to 49) | 0.48 |
| AFC (n=26) | 20 follicles (11−41) | −27 follicles | −75 (−56 to −85) | <0.0001 | 24 (−57 to 258) | 0.67 |
Models adjusted for hormone levels at baseline and alkylator score. FSH and Estradiol also adjusted for exogenous hormone use
Overall adjusted change in FSH not reported due to presence of interaction with treatment length
Models adjusting for alkylator use and baseline measures of ovarian reserve (and exogenous hormone for FSH and E2) confirmed independent statistically significant changes for FSH, LH, and AMH (Table 2). Age of participants, menstrual function for those not on hormones, and use of abdominal/pelvic radiotherapy were not associated with acute changes (data not shown). For FSH, length of treatment was shown to impact the magnitude of change during the acute phase. Participants whose cancer therapy lasted ≥90 days had larger increases in FSH (554%) than those with treatment regimens lasting < 90 days (96%) (p<0.001). There was a trend towards length of therapy increasing the magnitude of change for the other measures of ovarian reserve, but this reached statistical significance only for FSH.
Models were constructed to assess factors associated with the levels of ovarian reserve at the end of cancer therapy. Associations between post and pre-treatment levels (categorized as above or below the sample baseline median) were examined. For LH, E2, and MOV, relative impairment at baseline resulted in greater impairment after the end of cancer therapy. Participants with baseline LH levels ≥4.5mIU/mL, E2 levels <40.4pg/mL, and MOV measurements < 8.1cm3 had higher levels of LH (p=0.007), and lower levels of E2 (p=0.005) and MOV (p=0.027) after treatment compared to participants with hormone levels outside of this range. While data showed a trend for other measures of ovarian reserves, they did not reach statistical significance.
Exposure to alkylating agents was significantly associated with post-therapy impairment of AMH, E2, INH, and AFC. Participants exposed to alkylating agents had AMH levels 76% lower (p<0.001); E2 levels 13% lower (p=0.016); INH levels 54% lower (p<0.001); and AFC 59% lower (p=0.004) at the end of cancer therapy compared to unexposed participants. While the following associations did not reach statistical significance, alkylator exposure was associated with 34% higher FSH levels (p=0.09), 51% higher LH levels (p=0.24) and 29% lower MOV (p=0.06) compared to unexposed. Length of treatment, age, menstrual function, and pelvic/abdominal radiotherapy were not significantly associated with post-therapy measures.
Finally, as expected, use of exogenous hormones during cancer therapy or at the first post-treatment visit was associated with post-therapy levels of FSH and E2 (though not LH). Participants who used hormones had post-treatment FSH levels 40% lower than those who did not (p=0.001) and post-treatment E2 levels 77% higher than those who did not (p=0.018).
Recovery phase
Longitudinal changes in measures of ovarian reserve post- therapy are shown in Table 3. The average length of follow-up after the end of cancer therapy was 9.3 months (SD=2.9), and the number of visits for each participant in the recovery phase ranged from 2–6. At the end of follow-up, 9% (4/46) of participants had AMH levels that had returned to baseline levels and 26% (12/46) had FSH values that had returned to pre-treatment values. Twenty percent (9/46) showed no recovery in AMH levels.
Table 3.
Recovery of Ovarian Reserve
| Measure of Ovarian Reserve N=46 | Unadjusted Change Per Month % (CI) | P value | Adjusted Change Per Month* % (CI) | P value |
|---|---|---|---|---|
| FSH | −7.0 (−1.7 to −12.0) | 0.010 | −6.9 (−1.6 to −12.0) | 0.011 |
| LH | −7.7 (−2.2 to −12.9) | 0.007 | −5.0 (1.1 to −10.8) | 0.11 |
| AMH** | 6.9 (2.9 to 11.1) | 0.001 | ||
| Baseline AMH<2 | 0.2 (−3.6 to 4.3) | 0.93 | 2.6 (−2.7 to 6.2) | 0.47 |
| Baseline AMH≥2 | 11.9 (6.0 to 18.1) | <0.0001 | 11.9 (5.7 to 18.3) | <0.0001 |
| Estradiol | 6.2 (−0.4 to 13.3) | 0.065 | 5.4 (−1.2 to 12.5) | 0.11 |
| Inhibin | 1.2 (−3.8 to 6.5) | 0.64 | 2.3 (−2.8 to 7.7) | 0.38 |
| MOV | 3.2 (−1 to 7.7) | 0.12 | 4.3 (0.3 to 8.4) | 0.035 |
| AFC | 3.6 (−1.5 to 9) | 0.18 | 4.4 (−0.2 to 9.3) | 0.062 |
Models adjusted for hormone levels at baseline and alkylator score. FSH and Estradiol also adjusted for exogenous hormone use
Overall adjusted change in AMH not reported due to presence of interaction with baseline level
In the models of recovery, FSH recovered at a rate of −7.0 % per month (p=0.01); LH at −7.7% per month (p=0.007); E2 at 6.2% per month (p=.065); INH at 1.2% per month (p=.64); MOV at 3.2% per month (p=.12) and AFC at 3.6% per month (p=.18). Models adjusted for alkylator use, baseline measures of ovarian reserve, and exogenous hormone use for FSH and E2, confirmed statistically significant changes for FSH, AMH, and MOV.
The rate of recovery for AMH was significantly impacted by pre-treatment AMH levels. For participants who began with pre-treatment AMH levels < 2.0ng/mL, rate of recovery was 2.6% per month, whereas for those starting with pre-treatment AMH levels ≥2.0ng/mL, recovery rate was 11.9% per month (interaction p-value=0.003). For other measures of ovarian reserve, recovery rate was not significantly modified by other factors. Figure 1 demonstrates differences in rate of recovery for AMH depending on pre-treatment levels (p=0.003) and differences in rate of recovery for AMH based on alkylator use (p=0.062). Age, use of pelvic/abdominal radiotherapy, treatment length, exogenous hormone use, and menstrual function were not significantly associated with recovery for any measure of ovarian reserve. A subgroup analysis in participants not using hormonal contraceptives had results of similar magnitude, as the coefficients in the restricted model differed from the original by <3%.
Figure 1.
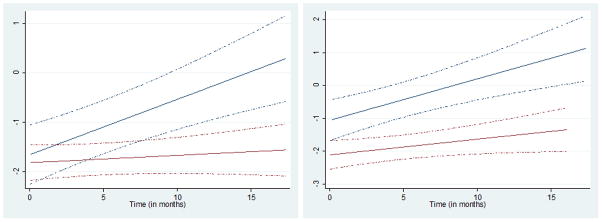
Rate of Recovery of AMH After Cancer Therapy
2a: Blue: Pre-Treatment AMH >2. Slope is 11.7% per month.
Red: Pre-Treatment AMH≤2. Slope is 1.7% per month.
(Interaction P= 0.003)
Dashed lines=95% CI
2b: Blue: No alkylator use. Slope is 13.4% per month.
Red: Alkylator use. Slope is 4.9% per month.
(Interaction P= 0.062)
Dashed lines=95% CI
Discussion
While cancer therapies have been shown to impair reproductive function in cancer survivors, the acute impact of therapies on ovarian function in post-pubertal adolescents and young women is not well documented. Future fertility is an important consideration for young women newly diagnosed with cancer, and better methods are needed to predict long-term reproductive potential in individual patients for counseling regarding fertility preservation strategies (12, 13).
In this study, a comprehensive longitudinal assessment of ovarian reserve was completed in young females with a new cancer diagnosis. Overall, we observed that ovarian reserve became impaired acutely during treatment and then began to recover post-treatment. The acute impairment of measures of ovarian reserve appears to occur as a result of chemotherapy-related destruction of ovarian follicles. The recovery in these measures reflects reinitiation of follicle growth after therapy. However, it must be emphasized that primordial follicles destroyed during cancer therapy are irreversibly lost, thereby leading to compromised ovarian reserve in survivors remote from therapy (5, 14).
In the current report, all measures of ovarian reserve demonstrated considerable acute impairment after chemotherapy. The magnitude of acute impairment was associated with a number of factors including baseline pre-treatment levels of ovarian reserve, use of alkylators, and treatment duration. Participants exposed to alkylating agents had higher post-treatment FSH and LH levels and lower AMH, E2, INH, and AFCs than those who did not receive alkylators. Cancer treatment lasting > 90 days was also independently associated with FSH impairment, and while a trend was seen for other hormone measures, no statistically significant difference was noted, most likely due to sample size. This is a novel finding and potentially has important implications for oncologists designing therapies to minimize ovarian toxicity.
Our study provides new information about the recovery of ovarian reserve post-treatment. Over approximately 9 months post-therapy, models demonstrate that recovery rates depend on baseline levels of ovarian reserve. Specifically, participants with baseline AMH levels <2ng/mL demonstrate a recovery in AMH of 2.6% per month whereas participants with baseline AMH levels ≥2ng/mL demonstrated a recovery rate of 11.9% per month.
Our findings are consistent with other studies assessing changes in reproductive function. Similar to our findings, most measures of ovarian reserve fell rapidly during chemotherapy in a study of 50 premenopausal breast cancer patients (median age 41 years) (8) and another study of 26 breast cancer patients (median age 37 years) (15). In the latter study, AMH levels remained suppressed over time while E2 and FSH levels returned to baseline by 6 months post-therapy. Similarly, we found that over nine months of post-therapy follow-up AMH completely recovered in only 9% of participants. However, only 26% of participants in the current study had complete recovery of FSH. Differences in age and length of follow-up may account for some of the discrepancies observed between studies.
Our findings may also be compared to a study assessing changes in AMH in 30 women treated for lymphoma using GnRH agonists. That study reported no recovery of AMH 12 months after therapy in participants receiving alkylator therapy (16). Unlike those findings, in our study, exposure to alkylating agents appeared to be associated with a slower rate of AMH recovery (though this did not meet statistical significance), but did not exclude the possibility of recovery.
Many prospective studies in breast cancer patients have focused on predicting chemotherapy-related amenorrhea as an outcome. For example, some studies have shown that pre-treatment measures of AMH predict menstrual function 4–5 years after the end of cancer therapy (9), while others do not (14). We found that menstrual function as an outcome measure was particularly limited in our study population since participants were younger than other cohorts and many used exogenous hormones. Indeed, we did not find an association between measures of ovarian reserve and menstrual function. We believe that measures of ovarian reserve provide a better assessment of reproductive function in this population.
Data in survivors remote from treatment suggests that measures of ovarian reserve are impaired when compared to similarly aged controls (5). Alkylating agents and pelvic radiotherapy exposure correlate with degree of impairment. Our results confirm the relationship of alkylating agents and decreased ovarian reserve. However, we failed to see an association with pelvic radiotherapy, likely due to the limited number of exposed participants, and because 1 of the 2 exposed to radiotherapy underwent ovarian transposition.
One challenge of studying the reproductive risks of cancer therapy is that outcomes such as fertility are difficult to capture. While other studies have demonstrated that AMH, and AFC are the most sensitive measures of ovarian reserve in survivors (5), we did not detect statistically significant associations with AFC likely because of the limited number of women undergoing vaginal ultrasound.
While measures of ovarian reserve are a proxy for fertility and do not consistently predict the ability to get pregnant, there is evidence in both natural conception and in vitro fertilization populations that abnormal AMH and FSH values are associated with lower fecundability and poorer response to fertility treatment (17, 18). All patients should be offered fertility preservation counseling prior to the onset of cancer therapy (13, 19). This study provides valuable information for fertility preservation counseling, as we have shown that women planning to receive alkylating agents, and especially those with low baseline AMH, can expect slower recovery in ovarian reserve.
This study has several strengths. Recall bias was minimized by prospective enrollment and confounding was reduced by restricting the study to non-pregnant, non-lactating females. Cancer diagnoses and treatments were validated with medical records to diminish misclassification bias. Unlike other prospective studies of hormone measurements, this study included women with all types of cancer and only those who were 35 years or younger. The statistical models used account for correlations within individuals and variability in the timing of study visits. This study has one of the longest follow-up times of any prospective study following measures of ovarian reserve.
Several limitations should be mentioned. A variety of diagnoses and treatments were included in this study and therefore it was not possible to compare the effect of specific chemotherapeutic regimens or fertility preservation techniques on ovarian reserve due to limited sample size. Because of timing of other cancer-related appointments some visits were not conducted during the early follicular phase, though unlike FSH, LH, and E2, AMH and AFC may be less prone to variation during menstrual cycle (6, 20, 21). While there is some evidence that AMH may be slightly lower in hormonal contraceptive users (22), other reports have not confirmed this finding (7, 23). The inclusion of women on hormonal contraceptives is an inherent limitation of any study assessing ovarian reserve in young women with cancer at risk for pregnancy, as a significant proportion of this population uses hormonal contraceptives for birth control and/or menstrual suppression. However, our analysis suggests that hormone use was not a significant factor in our analyses for AMH.
Continued follow-up and new recruitment to this cohort will enable future analysis that will overcome many of these limitations. Ultimately, more longitudinal data are needed to identify factors associated with reproductive dysfunction after cancer therapy so that clinically useful prediction tools can be developed. Such tools would improve patient counseling regarding the risks and benefits of cancer therapies and technologies available for fertility preservation.
Supplementary Material
Supplemental Figure 1: Mean Measures of Ovarian Reserve During and After Chemotherapy
Maroon= Standard Error
Post-Tx Visit 1= 3 months, SD 1.2 months
Post-Tx Visit 2= 6 months, SD 2.3 months
Post-Tx Visit 3= 9 months, SD 3.1 months
Acknowledgments
Funding/Support: Supported by NIH Grant K01 L:1-CA-133839-03 (CG); 1R01HD062797 (CG), and the Doris Duke Clinical Research Fellowship (KED).
Footnotes
Financial Disclosures: None
References
- 1.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 2.Rosen MP, Johnstone E, McCulloch CE, Schuh-Huerta SM, Sternfeld B, Reijo-Pera RA, et al. A characterization of the relationship of ovarian reserve markers with age. Fertil Steril. 2012;97:238–43. doi: 10.1016/j.fertnstert.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen EC, Muller J, Rechnitzer C, Schmiegelow K, Andersen AN. Diminished ovarian reserve in female childhood cancer survivors with regular menstrual cycles and basal FSH <10 IU/l. Hum Reprod. 2003;18:417–22. doi: 10.1093/humrep/deg073. [DOI] [PubMed] [Google Scholar]
- 4.Letourneau JM, Ebbel EE, Katz PP, Oktay KH, McCulloch CE, Ai WZ, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2011 doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134, 40.e1. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90:395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196–201. doi: 10.1016/j.ejogrb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–92. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 9.Anders C, Marcom PK, Peterson B, Gu L, Unruhe S, Welch R, et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest. 2008;26:286–95. doi: 10.1080/07357900701829777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RA, Cameron DA. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–43. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 11.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27:2677–85. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–8. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 14.Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18:117–21. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 15.Yu B, Douglas N, Ferin MJ, Nakhuda GS, Crew K, Lobo RA, et al. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer. 2010;116:2099–105. doi: 10.1002/cncr.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D. Anti-Mullerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20:280–5. doi: 10.1016/j.rbmo.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, et al. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–8. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Sowers M, McConnell D, Gast K, Zheng H, Nan B, McCarthy JD, et al. Anti-Mullerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94:1482–6. doi: 10.1016/j.fertnstert.2009.07.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts SA. Variability in anti-Mullerian hormone levels: a comment on Sowers et al., Anti-Mullerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94:e59. doi: 10.1016/j.fertnstert.2010.06.016. author reply e60. [DOI] [PubMed] [Google Scholar]
- 22.Kristensen SL, Ramlau-Hansen CH, Andersen CY, Ernst E, Olsen SF, Bonde JP, et al. The association between circulating levels of antimullerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertil Steril. 2012;97:779–85. doi: 10.1016/j.fertnstert.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Li HW, Wong CY, Yeung WS, Ho PC, Ng EH. Serum anti-mullerian hormone level is not altered in women using hormonal contraceptives. Contraception. 2011;83:582–5. doi: 10.1016/j.contraception.2010.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Mean Measures of Ovarian Reserve During and After Chemotherapy
Maroon= Standard Error
Post-Tx Visit 1= 3 months, SD 1.2 months
Post-Tx Visit 2= 6 months, SD 2.3 months
Post-Tx Visit 3= 9 months, SD 3.1 months


