Abstract
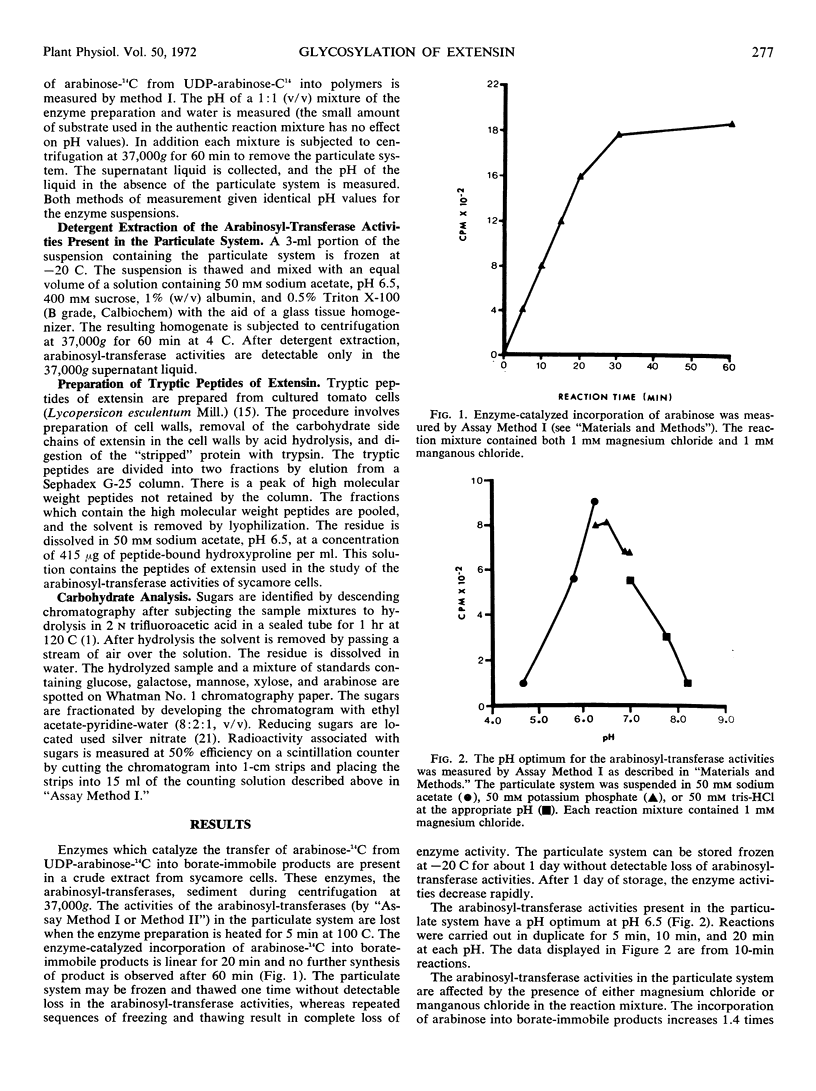
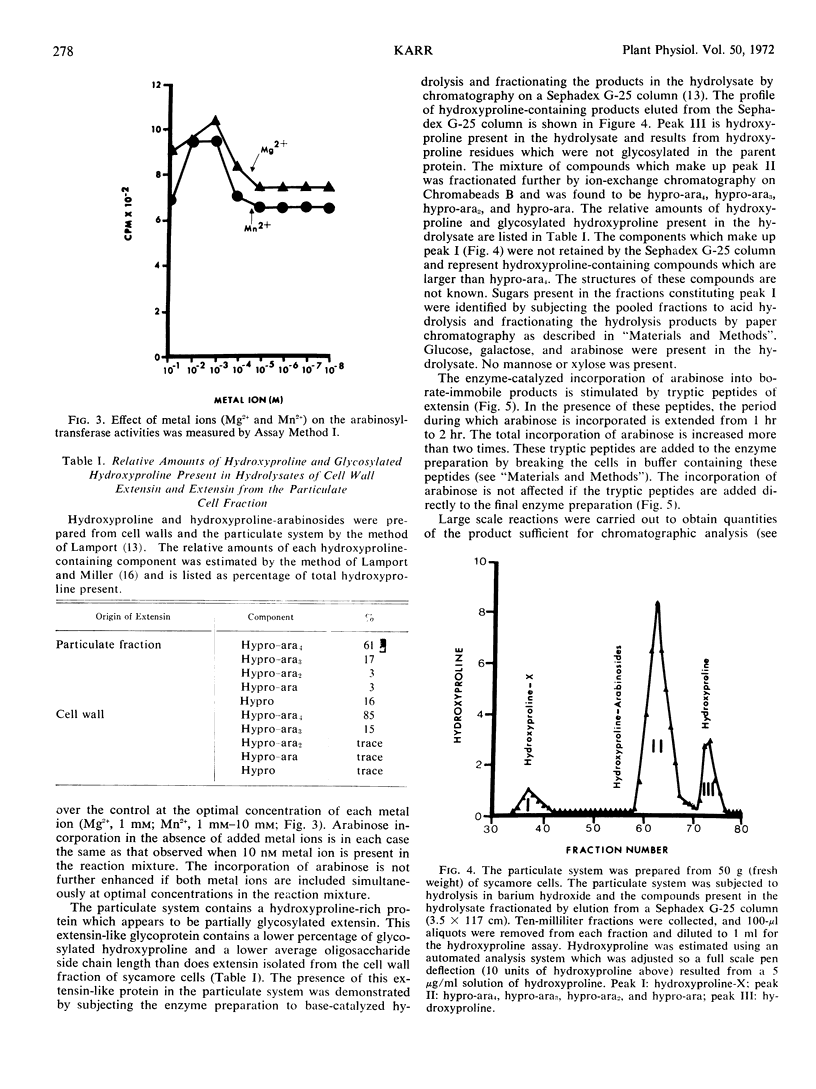
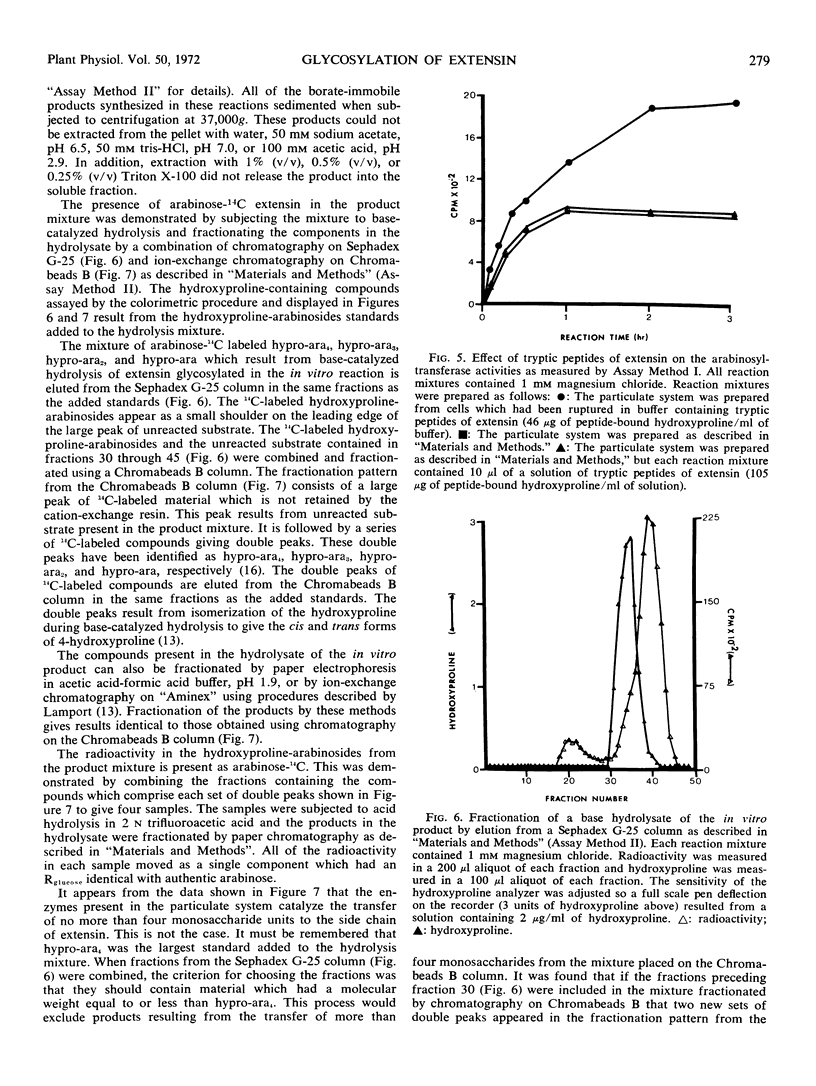
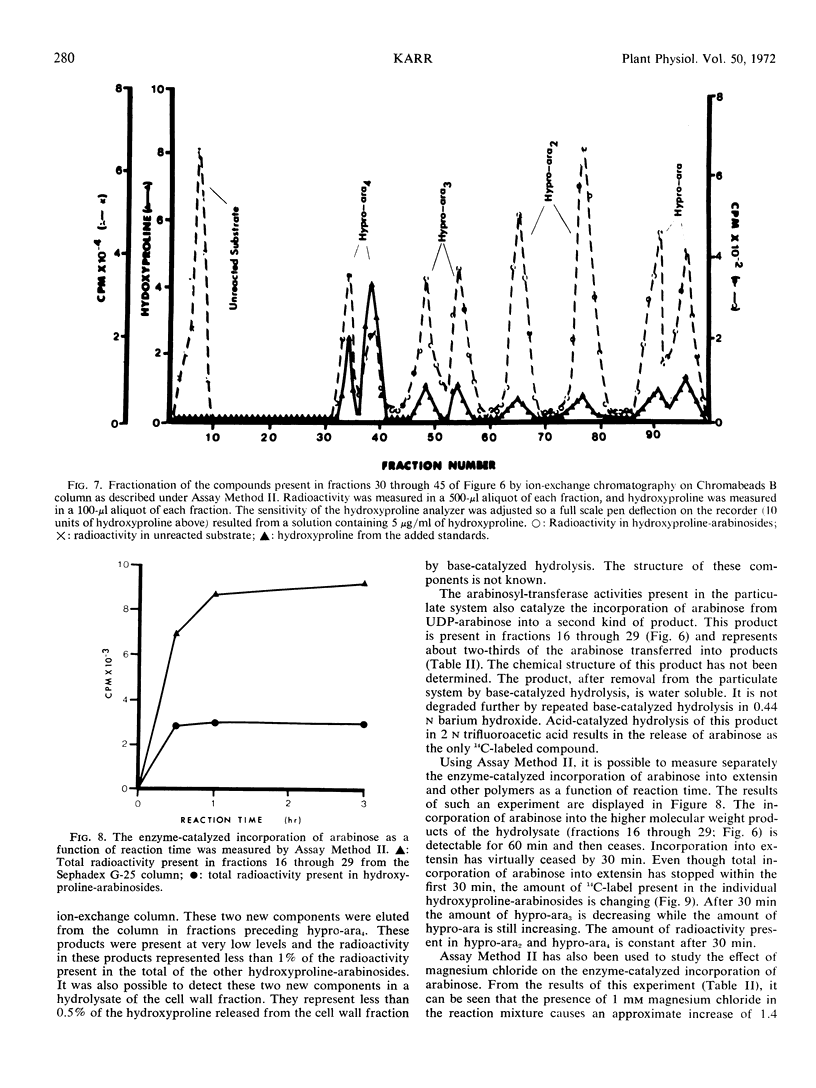
Enzymes which catalyze the glycosylation of the cell wall protein extensin using uridine diphosphate l-arabinose-14C as a substrate are present in a crude extract prepared from suspension cultured sycamore cells (Acer pseudoplatanus L.). This enzyme system sediments when the crude extract is subjected to centrifugation at 37000g. A base hydrolysate of the product contains a mixture of hydroxyproline-arabinosides which are electrophoretically and chromatographically identical to those obtained by hydrolysis of extensin isolated from the cell wall. The hydroxyproline-rich protein used as an acceptor in the glycosylation reactions is present in the particulate fraction. In addition, evidence is presented which indicates that hydroxyproline-rich tryptic peptides prepared from the cell wall can also be used as an acceptor by this enzyme system. The presence of Mg2+ or Mn2+ in the reaction mixture increases the enzyme-catalyzed incorporation of arabinose into extensin by about 1.4 times. About two-thirds of the product mixture is composed of arabinose-containing compounds which have not been identified. Some of these products appear to be hydroxyproline-glycosides which have not been previously reported.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J. Synthesis and Secretion of Hydroxyproline-containing Macromolecules in Carrots: II. In vivo Conversion of Peptidyl Proline to Peptidyl Hydroxyproline. Plant Physiol. 1970 Feb;45(2):223–227. doi: 10.1104/pp.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashek W. V. Synthesis and Transport of Hydroxyproline-rich Components in Suspension Cultures of Sycamore-Maple Cells. Plant Physiol. 1970 Dec;46(6):831–838. doi: 10.1104/pp.46.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleman J. Direct incorporation of hydroxyproline into protein of sycamore cells incubated at growth-inhibitory levels of hydroxyproline. Proc Natl Acad Sci U S A. 1967 Jan;57(1):50–54. doi: 10.1073/pnas.57.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLLAR S. J., JARAI M. Biochemical chlorination in Streptomvces aureofaciens. Nature. 1960 Nov 19;188:665–665. doi: 10.1038/188665a0. [DOI] [PubMed] [Google Scholar]
- Karr A. L., Albersheim P. Polysaccharide-degrading Enzymes are Unable to Attack Plant Cell Walls without Prior Action by a "Wall-modifying Enzyme". Plant Physiol. 1970 Jul;46(1):69–80. doi: 10.1104/pp.46.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H. A plant mannosyl-lipid acting in reversible transfer of mannose. FEBS Lett. 1969 Sep;5(1):81–84. doi: 10.1016/0014-5793(69)80298-x. [DOI] [PubMed] [Google Scholar]
- LAMPORT D. T. CELL SUSPENSION CULTURES OF HIGHER PLANTS: ISOLATION AND GROWTH ENERGETICS. Exp Cell Res. 1964 Jan;33:195–206. doi: 10.1016/s0014-4827(64)81026-0. [DOI] [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab J. M., Villemez C. L., Albersheim P. Biosynthesis of galactan by a particulate enzyme preparation from Phaseolus aureus seedlings. Biochem J. 1968 Jan;106(2):355–360. doi: 10.1042/bj1060355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Cell wall protein in plants: autoradiographic evidence. Science. 1969 Jul 18;165(3890):299–300. doi: 10.1126/science.165.3890.299. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Hassid W. Z. Solubilization and Separation of Uridine Diphospho-d-glucose: beta-(1 --> 4) Glucan and Uridine Diphospho-d-glucose:beta-(1 --> 3) Glucan Glucosyltransferases from Coleoptiles of Avena sativa. Plant Physiol. 1971 Jun;47(6):740–744. doi: 10.1104/pp.47.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]


