Abstract
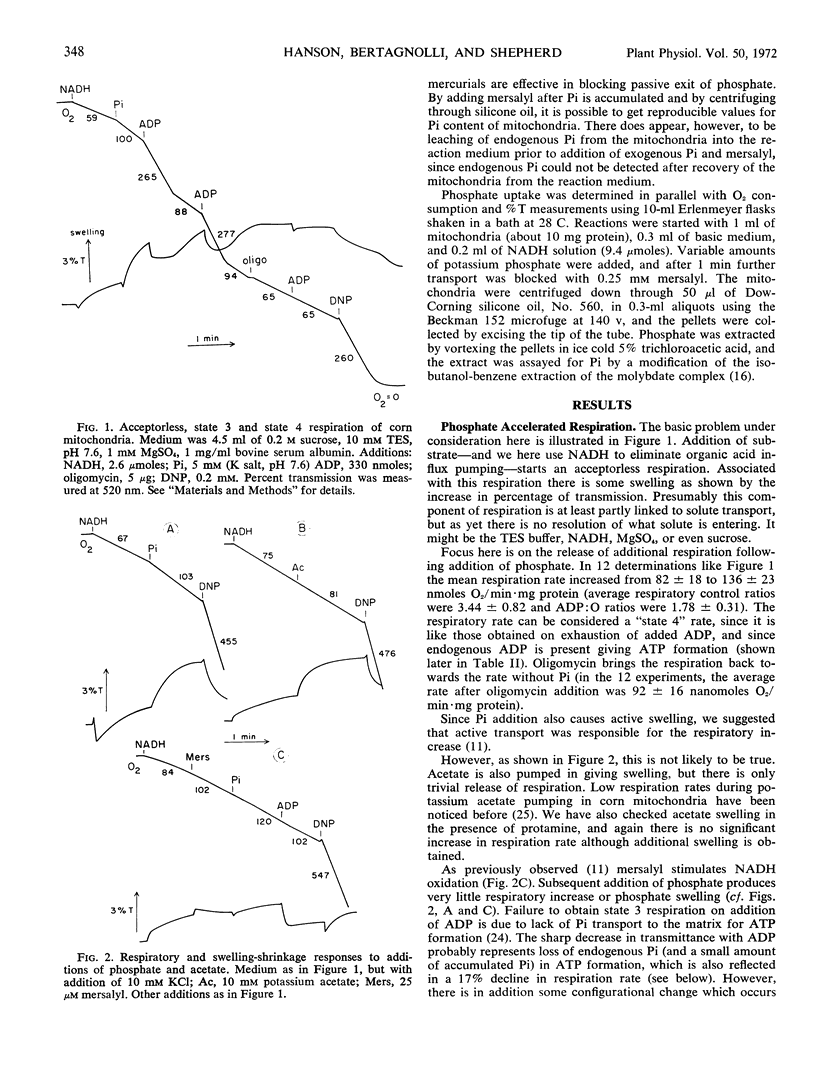
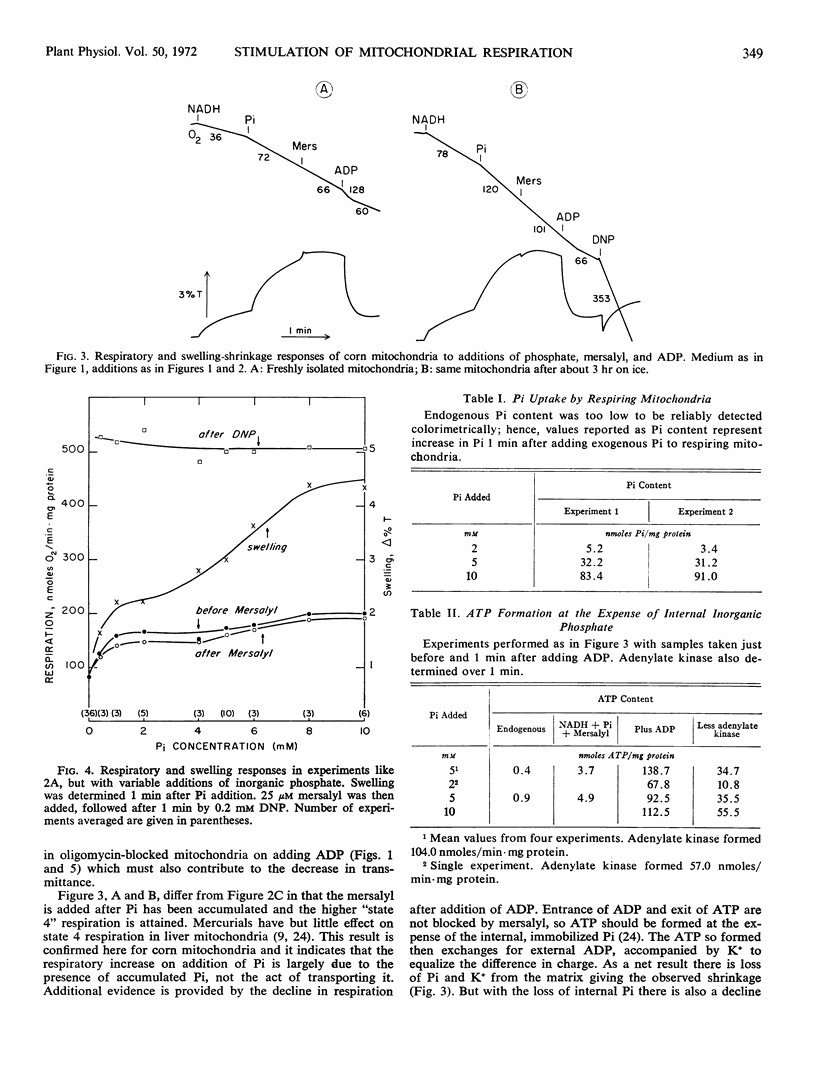
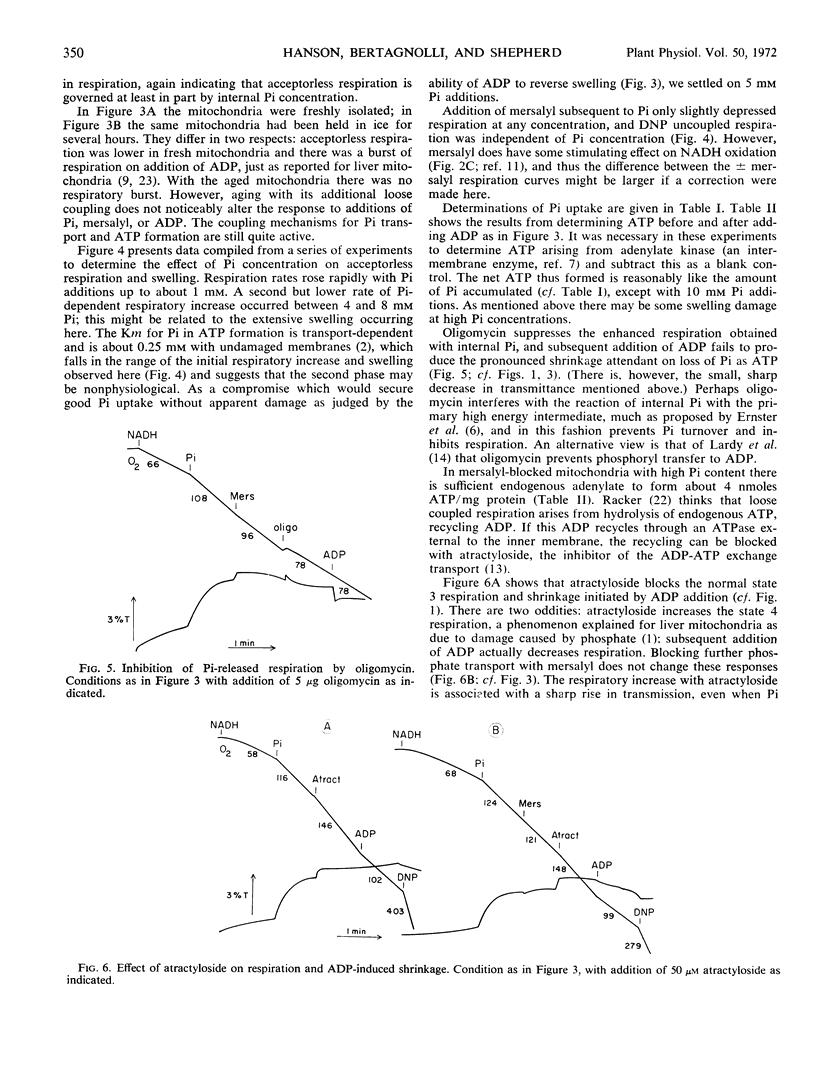
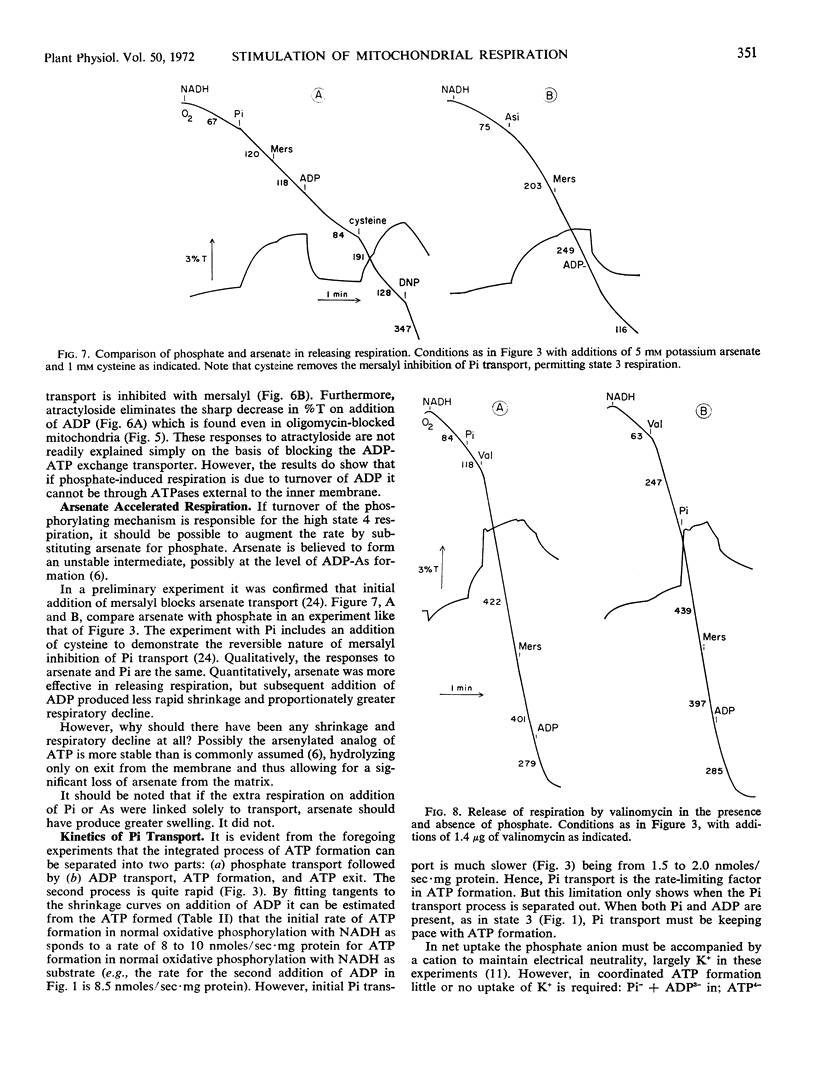
By use of the organic mercurial mersalyl to block phosphate transport, it has been shown that only a small fraction of the respiratory increase of corn mitochondria in response to additions of inorganic phosphate is due to energy expended in phosphate accumulation. Most of the respiratory release occurs from accelerated turnover of the coupling mechanism with internal phosphate in an oligomycin-sensitive reaction. Addition of ADP to mersalyl-blocked mitochondria depletes internal phosphate in ATP formation and respiration declines. Arsenate produces the same responses as phosphate but is more effective in respiratory release.
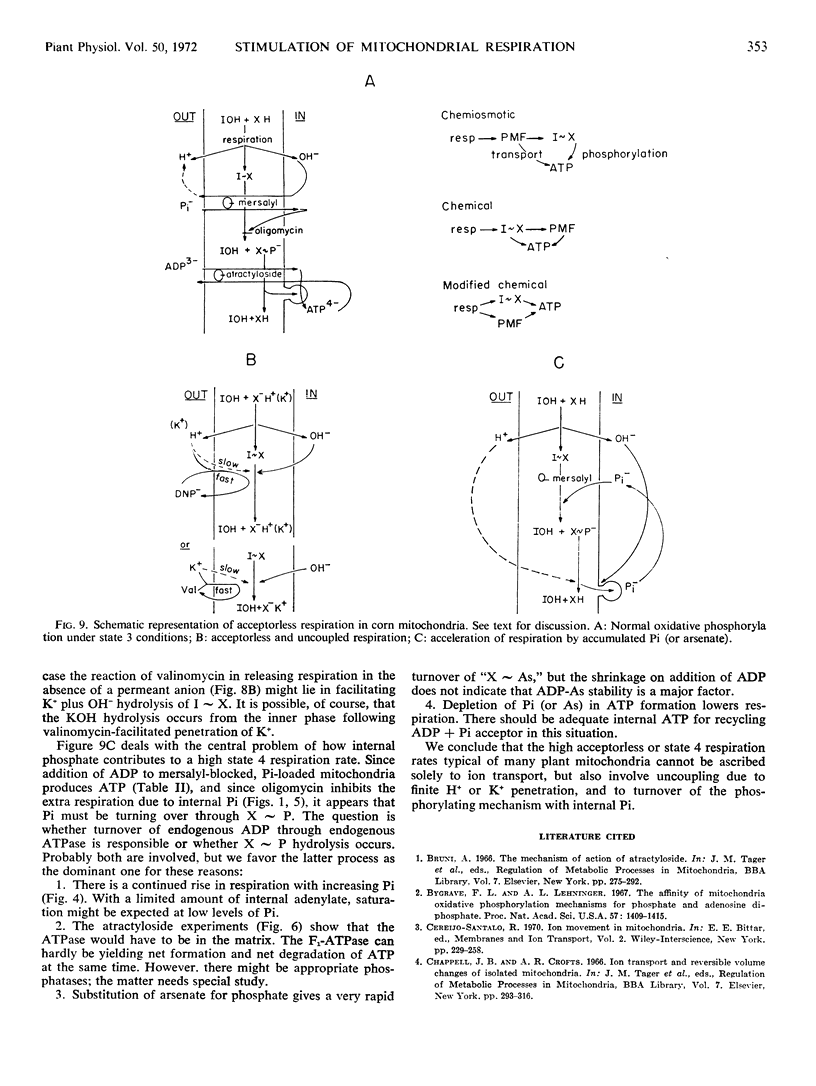
Inhibition of the ADP-ATP antiporter with atractyloside shows that the increased respiration with internal phosphate is not due to turnover of ADP acceptor through exogenous ATPase.
Use of valinomycin to facilitate movement of K+ greatly accelerates the rate of phosphate swelling, but there is no consistent correlation between respiration and swelling. In the absence of phosphate, valinomycin dramatically releases respiration with only trivial swelling.
The data indicate that loose coupling in due only fractionally to energy expenditure in ion transport. An explanation consistent with the observations can be derived by assuming that both a high energy intermediate (I ∼ X) and a proton motive force—or its electrochemical equivalent—arise at coupling sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bygrave F. L., Lehninger A. L. The affinity of mitochondrial oxidative phosphorylation mechanisms for phosphate and adenosine diphosphate. Proc Natl Acad Sci U S A. 1967 May;57(5):1409–1415. doi: 10.1073/pnas.57.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B. Ion transport induced by polycations and its relationship to loose coupling of corn mitochondria. Plant Physiol. 1972 May;49(5):707–715. doi: 10.1104/pp.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M., Pfaff E. Metabolic control in mitochondria by adenine nucleotide translocation. Biochem Soc Symp. 1968;27:105–122. [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- Lyman G. E., DeVincenzo J. P. Determination of picogram amounts of ATP using the luciferin-luciferase enzyme system. Anal Biochem. 1967 Dec;21(3):435–443. doi: 10.1016/0003-2697(67)90318-1. [DOI] [PubMed] [Google Scholar]
- Rossi C., Scarpa A., Azzone G. F. Ion transport in liver mitochondria. V. The effect of anions on the mechanism of aerobic K+ uptake. Biochemistry. 1967 Dec;6(12):3902–3910. doi: 10.1021/bi00864a036. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem J. 1969 Mar;111(5):665–678. doi: 10.1042/bj1110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. H., Hanson J. B., Mollenhauer H. H. Active swelling and acetate uptake in corn mitochondria. Biochemistry. 1969 Mar;8(3):1203–1213. doi: 10.1021/bi00831a055. [DOI] [PubMed] [Google Scholar]
- Young J. H., Blondin G. A., Green D. E. Conformational model of active transport: role of protons. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1364–1368. doi: 10.1073/pnas.68.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]


