Abstract
We will review information about and present hypotheses as to the anatomy of brown adipose tissue (BAT). Why is it located where it is in humans? Its anatomical distribution is likely to confer survival value by protecting critical organs from hypothermia by adaptive thermogenesis. Ultimately, the location and function will be important when considering therapeutic strategies for preventing and treating obesity and type 2 diabetes, in which case successful interventions will need to have a significant effect on BAT function in subjects living in a thermoneutral environment. In view of the diverse locations and potential differences in responsiveness between BAT depots, it is likely that BAT will be shown to have much more subtle and thus previously overlooked functions and regulatory control mechanisms.
Until ~10 years ago, brown adipose tissue (BAT) was considered to be biologically active in neonates and young children generating heat during cold exposure by adaptive thermogenesis to maintain normal body temperature (1). BAT regressed with aging by transforming into white adipose tissue (WAT) (2), and BAT in adults was not considered important in energy metabolism (1,3). At that time, reports in the nuclear medicine literature surfaced that 18F-fluorodeoxyglucose (FDG), an intravenously administered radioactive glucose analog taken up but not metabolized by neoplasms and used to delineate metastatic cancers in positron emission tomography (PET) scans, also localized in adipose tissues pinpointed by concomitant computed tomography (CT) (PET-CT fusion) scans to be commensurate with BAT and in most instances not with tumor tissue (4,5). It was therefore demonstrated that the main BAT depot was within the supraclavicular region, although as detailed below a number of perhaps less important depots were identified (6–11). Because more attention has been given to the physiology (6–9), pathophysiology (6), and clinical characteristics (10,11) of human BAT rather than its anatomy, the purpose of this Perspective is to review information about and to consider hypotheses why BAT is located where it is in humans as well as the functional relevance and therapeutic implications of its locations.
Locations of BAT in Humans
Table 1 shows the sites of BAT identified in Heaton’s 1972 series of human autopsies (2) corroborated and supplemented by FDG PET-CT fusion scans performed in healthy volunteers exposed to cold under well-defined controlled clinical experimental conditions (6–8) or in patients referred for cancer scans (4,5,10) without prior controlled warming in the scanning room to neutralize the effect of environmental cold (12). We have used Heaton’s definition of BAT as fat containing multilocular adipocytes stained by hematoxylin-eosin on light microscopy (2) and have designated BAT as being visceral or subcutaneous, subdividing each category into separate depots according to their contiguous organ or tissue as depicted by Nedergaard, Bengtsson, and Cannon (1). It should be noted that, overall, the listed categories show similarities in BAT locations between humans (1) and rodents (13).
TABLE 1.
Distribution of human BAT
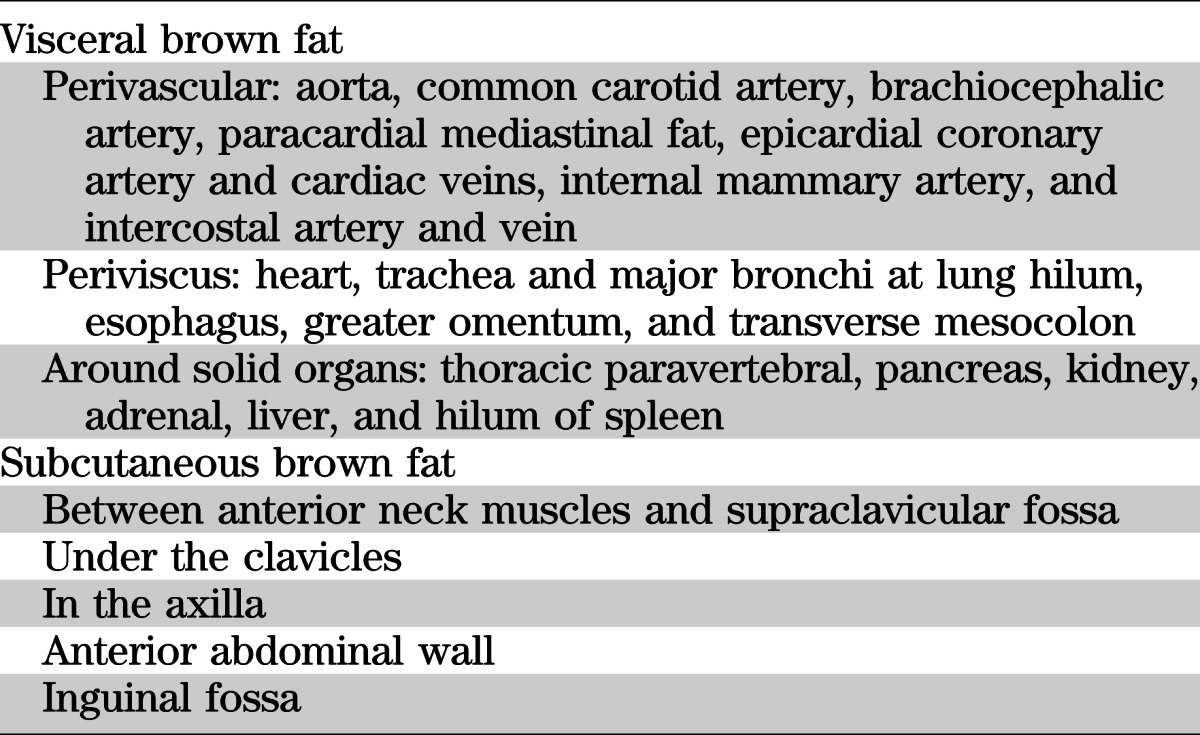
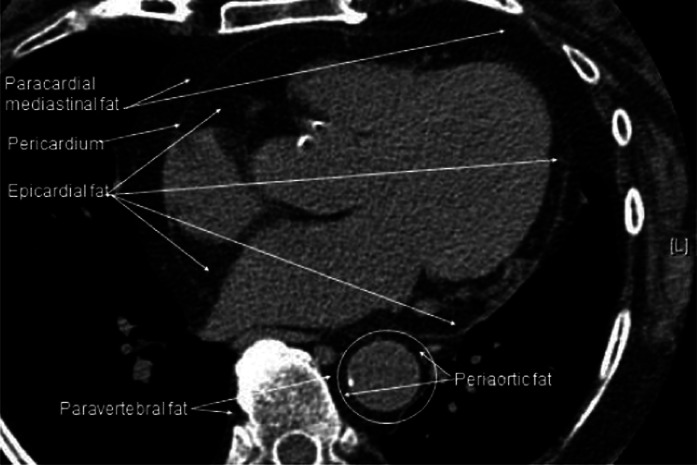
Visceral BAT includes the following: 1) Perivascular BAT around the aorta, common carotid artery, and brachiocephalic artery; in anterior mediastinum (paracardial) fat (Fig. 1); and around epicardial coronary artery and cardiac veins as well as medium-sized muscular arteries and veins including the internal mammary and the intercostal artery branches from the subclavian and aorta. The intercostal veins drain blood from the chest and abdominal walls into the azygous veins, the left joining the main right azygous vein in the latter’s thoracic cephalad course closely adjacent to the inferior vena cava before emptying into the superior vena cava (14). 2) Viscus BAT, defined as BAT surrounding a hollow muscular organ other than blood vessels, situated in variable amounts in the epicardium around the heart (Fig. 1) and in the esophago-tracheal groove, as well as greater omentum and transverse mesocolon in the peritoneal cavity. 3) BAT around solid organs, namely, kidney, adrenal, pancreas, liver, and splenic hilum including paravertebral fat, which was not examined in Heaton’s series (2) but can be seen on CT scans of the thorax adjacent to periaortic fat (Fig. 1). It lies next to the intercostal artery from which a spinal branch supplies the spinal cord (14). To our knowledge, BAT has not been described in the meninges covering the brain and spinal cord or in the subcutaneous tissue of the scalp.
FIG. 1.
A view of the human heart on an axial CT scan showing the location of fat around the epicardium, paracardial mediastinum, descending aorta, and paravertebral region.
Subcutaneous BAT includes depots lying between the anterior neck muscles and in the supraclavicular fossa posterior to the brachial plexus (10); under the clavicles; in the axilla; in the anterior abdominal wall; and in the inguinal area. Importantly, BAT is present in all the aforementioned sites in infants and children under 10 years old and its distribution decreases in variable amounts with increasing age so that by 80 years old, few individuals have BAT at any site (2). Brown adipocytes defined by histology disappear from the interscapular fat pad at infancy and at 10 years old from inguinal and anterior abdominal wall areas.
In general, the most metabolically active BAT region based on calculations of maximal standard uptake values (SUVmax) of the FDG tracer is the supraclavicular fossa and axilla followed by the mediastinal, thoracic paravertebral, perinephric, and adrenal loci (1,5). The minimum threshold for uptake and therefore the detection limit for the tracer in BAT has been defined as 2 SD above the maximal SUV distribution in typical WAT (9,10) and varies between ≥1 (10) and ≥2 g/mL (9,10,15,16). These values serve as the criterion for the presence (PET+) or absence (PET−) of BAT in any given site. Its limitation is that a region of interest such as the neck can be PET− but still exhibit islands of brown adipocytes containing specific uncoupling protein (UCP)1 histologically (15) suggesting the PET-CT is not always detecting metabolically active BAT. This could relate in part to the radioactive FDG substrate adopted, as it is clear that although the radio density of 11C acetate, an alternative tracer of intermediary metabolism, increases with cold exposure, there is appreciable uptake under warm conditions (9). There are other limitations in studies using current imaging methodologies to delineate BAT and quantitate its function. These include 1) the need for exposure to significant amounts of radiation, 2) the need for tissue biopsy, and 3) as summarized in Table 2, the wide between-subject variability of FDG uptake and limited responsiveness to acute stimulation that could result from different individual temperature thresholds for switching on nonshivering thermogenesis. Like FDG PET-CT, thermal imaging (TI) (17) can detect incremental BAT activity as the heat released in the supraclavicular and related areas of both lean and obese subjects, as exemplified in Fig. 2, but cannot monitor heat emission from BAT inside thoracic and abdominal cavities because they are beyond the detection limit of the sensor.
TABLE 2.
Main findings and limitations from prospective studies on BAT function in healthy humans
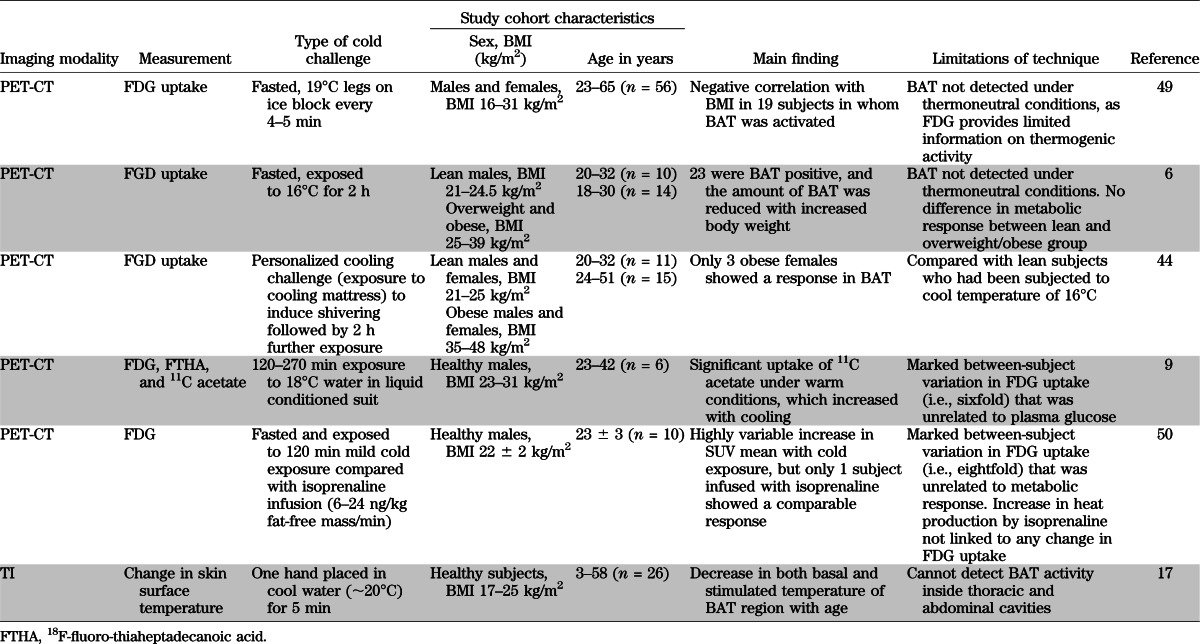
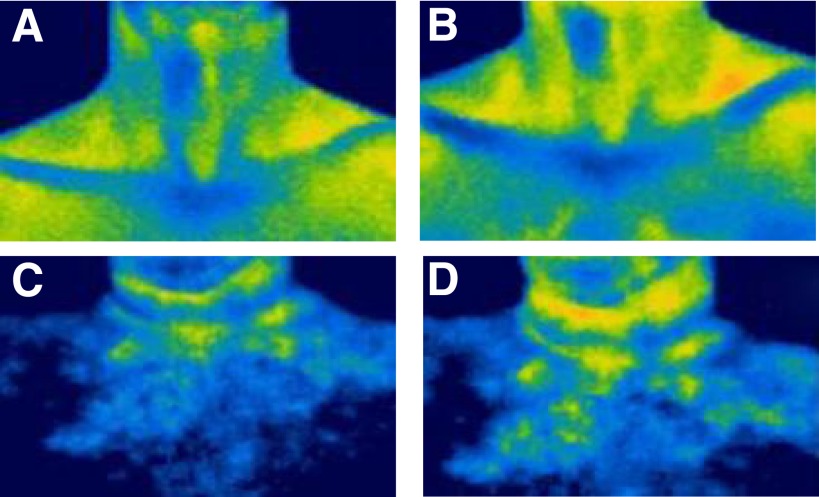
FIG. 2.
Representative examples of thermal images showing the difference in temperature of BAT located within the supraclavicular region in a lean 47-year-old adult male (A and B) (BMI 21 kg/m2) compared with an age-matched healthy obese male (C and D) (BMI 30 kg/m2) in a control period (A and C) and in response to cooling of the hand (B and D). Orange pixels: 36.0–36.5°C; yellow pixels: 35.5–36.0°C.
UCP1 immunohistochemical staining has been demonstrated in dense clusters of BAT from supraclavicular fat of healthy adults (7,10,15,18) and in omental fat from a patient with a pheochromocytoma (13) as well as in a biopsy from superficial subcutaneous periumbolical fat from a patient treated with chronic thyrotropin-suppressive doses of L-thyroxine for papillary thyroid cancer (19). Expression of UCP1 mRNA in supraclavicular fat (7) is several orders of magnitude higher than the low levels measured in neck subcutaneous WAT as well as upper manubrium sternum subcutaneous fat, which is significantly greater than its expression in superficial abdominal fat and lower-limb subcutaneous fat (20). The cellular source of the low expression of UCP1 mRNA in truncal and lower-extremity subcutaneous WAT remains to be defined but might stem from the same lineage as the beige, also known as brite (brown-in-white), adipocytes recently reported by Wu et al. (21) in human adult supraclavicular BAT and by Sharp et al. (22) in autopsy samples from infant, children, and teenager supraclavicular, posterior mediastinum, retroperitoneal, intra-abdominal, and mesenteric depots and that originate from a recruitable clone of precursors distinct from the adipo-myeloblasts found in classic BAT (21).
In healthy lean male and female adults (mean ± SD age 40 ± 9 years), mean weight of cold-activated human BAT calculated by PET-CT was 34 g (range 9–90) (8). This included the dominant supraclavicular area as well as paraspinal and para-aortic regions, the axillary depot, the mediastinum, and close to the adrenal glands. That estimate compares with the mean 151 g (range 29–296) in cold-exposed young men (9) and with 12–170 g in a cohort of PET+ patients without prior cold activation (10). Based on the calculation of 63 g cervical BAT reported in a different study by Virtanen et al. (7), it was estimated that activated BAT thermogenesis contributed 4.5% to whole-body energy expenditure, which was considered to be significant (23) and commensurate with the goal of exploiting BAT to burn excess calories stored in WAT for weight loss in obesity and type 2 diabetes (3). The ∼5% figure cited above could be an underestimate by a factor of two to three in some subjects because of their greater BAT mass.
Speculations on the functional relevance of the locations of human BAT
Blood vessel temperature regulation.
The distribution of BAT in warm-blooded animals provides the basis for understanding the functional relevance of the anatomic locations of human BAT. To quote Smith and Horwitz in their extensive 1969 review of brown fat and thermogenesis in mammals including rodents (24), “in brief, the brown fat provides an internal heating jacket that overlies parts of the systemic vasculature and on signal becomes an active metabolic heater applied directly to the flowing bloodstream as it passes to and from the cooler periphery.” Since blood vessel anatomy and topography in rodents (25) are similar to those of man (14), this explanation would account for the distribution of BAT in humans along the major arteries and veins functioning as a local heating jacket. Other than serving a vascular heating role, the absence of human data establishing the selective functional regulation of BAT depots or of models that assess the optimal locations of thermogenic sources for maintaining core temperature renders any teleological assignment of a “local” warming function based on the sites of BAT depots speculative and hypothesis generating.
Human BAT contains a rich plexus of blood vessels (18). Human cervical BAT nonshivering thermogenesis is coupled to a doubling of its blood perfusion from 7.5 to 15.9 mL/min/100 g fat tissue compared with no change in neck WAT perfusion (3.0 mL/min/100 g) (8). Its purpose is to supply more free fatty acids, glucose, and oxygen as substrates for augmented mitochondrial oxidation and uncoupling via UCP1 to generate heat released into the effluent blood reaching the systemic circulation (8). That this local mechanism would be supplemented by dissipation of heat outwardly from BAT to contiguous vessels and viscera in overall temperature homeostasis makes sense but is as yet unproven. Cannon and Nedergaard noted that the physiological significance of BAT would have been much simpler if it were only found in one place in the body rather than in defined but dispersed areas (26). Assuming a mass of ∼150 g human BAT (as discussed above), a cardiac output of 5 L/min, and a maximal activated blood flow of 15 mL/min/100 g in all BAT locations, the time taken to warm for the circulating blood volume to transverse BAT concentrated in one organ would be ~3.7 h. A more relevant functional calculation is the blood temperature gradient across the BAT depot between the vascular influx and efflux reflecting the release of heat generated by activated BAT. In the rodent’s dominant interscapular BAT depot, total heat production at 6°C increased to the order of seven times that of control values at 26°C (versus lesser fold increases in other BATs), which was enough to raise the temperature of its venous drainage by ∼1°C or more (25). Another estimate, which has not been reported in animal studies, is the fraction of generated heat that would diffuse outwardly to warm a contiguous organ. Those considerations aside from an evolutionary viewpoint and the biological necessity to heat organs quickly during acute cold stress, organizing BAT around the vasculature more diffusely but targeting warm blood directly to vital organs seems plausible.
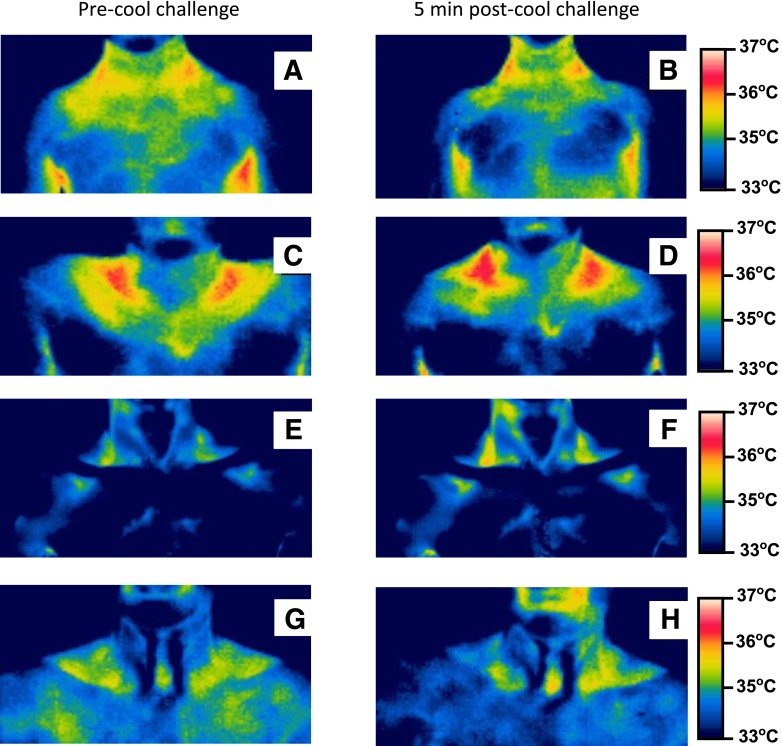
On the venous side of the human circulation, blood with lower-than-normal temperature in the subclavian and jugular veins resulting from cold-exposed upper extremities, head, and neck would be warmed by axillary, supra- and subclavicular, and superior mediastinal BAT. For individuals of normal body weight, the supraclavicular and axillary regions colocating to the primary areas in which BAT is located in humans can readily be detected using TI (17). Figure 3A and B shows the release of heat from bilateral cervical-axillary BAT upon its acute activation by immersion of an 7-year-old child’s left hand into cool water at 20°C. This response cannot be explained only by increased blood flow (17). TI demonstrates heat generation as the ultimate step in BAT activity in contrast to radioactive tracer uptake methods such as FDG or 18F-fluoro-thiaheptadecanoic acid (9) that show metabolic reactions at steps early in thermogenesis. The collection of BAT in the cervical-axillary region is strategically placed to offset the influx of cooled venous blood from the upper body into the right atrium, which confers survival value because the myocardium is susceptible to life-threatening arrhythmias resulting from a decrease in coronary blood temperature (27). In this respect, the age-dependent decline in overall BAT mass (2,10,11,16) and in epicardial UCP1 (20) could be one reason the elderly are prone to succumb to hypothermia (28). Venous blood from the abdominal and chest walls would be warmed by BAT around the intercostal veins and along the azygous venous system, which also drains blood from the interscapular fat pad, the primary BAT source of heating in rodents (25). Like BAT around intrathoracic viscera and the spine, the azygous system is not seen on TI because it is situated inside the chest wall beyond the limit of the infrared thermal sensor. Subcutaneous BAT in the inguinal fossa might contribute to warming the femoral vein blood from the lower limbs in young children. As inguinal BAT disappears in adults, it is assumed that lower-extremity blood could be warmed in the inferior vena cava in transit to the heart by the ambient intra-abdominal and intrathoracic cavity temperatures because there is a gradient of increasing temperature in the large veins as they approach the heart (29).
FIG. 3.
Anterior thermal images of the neck and upper thorax from four normal body weight males of the same family from three generations before and after placing one hand in cold water at 20°C. Seven-year-old (A and B) (on 25th BMI centile) and 13-year-old (C and D) (on 75th BMI centile) sons, 49-year-old father (E and F) (BMI 20 kg/m2), and 73-year-old grandfather (G and H) (BMI 24 kg/m2). Red pixels: 36.5–37.0°C; orange pixels: 36.0–36.5°C; yellow pixels: 35.5–36.0°C.
On the arterial side, cardiac output should be optimally warmed to ensure normal function of the myocardium and other vital organs such as brain, kidney, adrenal, and liver supplied by the aorta and its respective major branches. With the exception of the brain, which is devoid of BAT in the cranial cavity, BAT in immediate contiguity with the other organs could act as local back-up systems. Thus, hypothetically, the presence of substantial amounts of BAT around the common carotid and vertebral arteries in the neck could provide an “insurance policy” to deal with any drop in cerebral arterial blood temperature en route to the brain when the head and neck might be exposed to very cold temperatures. There is evidence that in patients undergoing pentothal-nitrous oxide general anesthesia during carotid arteriography, intracarotid blood temperature measured by a thermistor probe decreased 0.2–0.5°C when ice packs were applied to the same side of the anterior parts of the face and forehead (30). The authors suggested that the probable mechanism for this cooling was heat exchange between the artery and cooler blood in the internal jugular vein derived from the facial veins. However, in humans the sympathetic neural outflow is inhibited by pentothal administration (31), and any compensatory warming by cervical BAT would have been negated. However, as demonstrated by TI, cooling the hand of children and adults can increase the temperature of subcutaneous cervical BAT by 0.3–0.7°C (17), which might be sufficient to attenuate this 0.2–0.5°C drop in intracarotid temperature by convectional heat transfer across the carotid sheath. In conscious healthy men, head and neck cooling that dropped forehead skin and external auditory canal (tympanic) temperature by ∼12 and ∼5°C, respectively, did not reduce core temperature and by inference carotid temperature but caused peripheral vasoconstriction and a rise in blood pressure (32). This showed that sympathetic nervous system activation did occur in a manner consistent with activation of central efferent sympathetic outflows to BAT and, consequently, by BAT thermogenesis as demonstrated by skin cooling experiments in rodents (33). Thus, protection of the extracranial arterial blood temperature against cold by cervical BAT thermogenesis seems plausible.
In rodents, the anatomical distribution of BAT and the use of vascular countercurrent heat exchange during the cold-induced thermogenic response also protects the animal by contributing heat to the vital organs of the thorax, the cervical and thoracic segments of the spinal cord, and the sympathetic chain (25). In man, local warming of the thoracic spinal cord and sympathetic ganglia would be done by paravertebral BAT and putatively by nearby intercostal arteries as they give off spinal branches that anastomose with the anterior spinal cord artery (14). Likewise, the proximity of supraclavicular BAT to the brachial plexus (9) might facilitate normal nerve conduction between upper-limb skin thermal receptors via afferent sensory neuronal inputs to various parts of the brain and spinal cord that regulate the neurophysiological control of thermogenesis (34). The subclavian outflow to the upper limbs would be warmed by the supraclavicular fat. Hence, from an anatomical perspective, cervical and thoracic paravertebral BAT might safeguard central nervous and autonomic system functioning and upper-limb muscle power and coordination during hypothermia. BAT along the trachea and the major bronchi at the lung hilum might heat inhaled cold air on its way through the airways to the alveolar-capillary units responsible for gaseous exchange, and the esophageal BAT might warm ingested food and fluids. Whether heat would be lost from lower temperatures in the alveolar-capillary network resulting in cooling of pulmonary vein blood is not clear because in a human who inspired 4°C air, common carotid (and by inference proximal aortic, left ventricular, and atrial as well as pulmonary venous blood) temperature was not substantially affected (30). In healthy humans who ingested cold water, esophageal motility markedly slowed down (35), so the function of activated esophageal BAT might be to maintain temperature necessary for normal esophageal peristalsis after ingestion of food and fluids, as has been shown with TI (36), which would be important as food consumption increases in the cold (26).
A putative functional role in vasomotor reactivity.
Perivascular fat around the adventitia of vessels (PVAT) can affect vasomotion in arteries by the release of pro- or anti- contractile adipokines (37). In rodents, aortic PVAT is composed of BAT and WAT (37), with BAT predominating in thoracic over abdominal aorta (38). Compared with isolated rodent aorta rings without PVAT, rings with intact PVAT exhibited anticontractile (vasodilatory) responses when incubated with norepinephrine (39).Whether vasoreactivity in rodent aorta originates from BAT-derived adipokines (“bradipokines”) (26) and whether UCP1 itself is involved are not known. BAT is present in some strains of UCP1 gene knockout mice (26), so UCP1-independent responses of aortic rings to bradykinin or other agonists with and without PVAT in vitro could be tested in this model. In other species, epicardial fat impaired bradykinin-mediated endothelial (i.e., nitric oxide)-dependent coronary vasodilation when tested in normal adult dogs with no effect in normal pigs (40). Adult dogs, unlike young puppies, have undetectable epicardial UCP1 protein and mRNA, which can be induced by administering a β3-adrenergic receptor agonist drug (41). Pigs lack BAT, and their UCP1 gene is nonfunctional, having been disrupted by several mutations (42), so that the absence of pig epicardial BAT is associated with normal coronary responses to bradykinin. Human epicardial fat in type 2 diabetic and metabolic syndrome patients with coronary atherosclerosis (CAD) expressed amounts of UCP1 similar to those expressed in control subjects without diabetes, metabolic syndrome, and CAD (20). Brown adipocytes have been observed in histology sections of human CAD plaques at autopsy (43). The role and significance of PVAT and perivascular BAT per se in vascular biology and pathophysiology remain to be clarified.
How could location of BAT affect therapeutic strategies for obesity and type 2 Diabetes?
Obesity is associated with a reduction in BAT activity (6,44) but the extent to which obesity is cause or effect or involved in a vicious cycle or energy intake exceeding expenditure remains to be established. Moreover, subcutaneous fat provides insulation against heat loss and therefore has a role in the defense against sustained cold exposure; its depth would not be a major factor in the acute response to cold that is primarily driven by cool receptors in the skin (44). As a consequence, the retention of heat by insulation in obesity might be a factor that results in the individual’s decreased BAT responsiveness to cold. Despite these concerns, potential interventions that target BAT bioenergetics in order to treat obesity and its related metabolic disorders have been published (3,45,46). A clear challenge is to chronically upregulate a tissue that has a marked divergence in metabolic sensitivity between individuals, at least when functionally assessed using PET-CT (Table 2). They could, however, include 1) in vivo methods such as increasing BAT mass and thermogenesis in established depots or by differentiation (recruitment) from brite/beige progenitor cells located in WAT using systemically administered agents, 2) local injection of pharmacological compounds into BAT or WAT depots, 3) lowering ambient indoor temperature to values below thermoneutrality to trigger nonshivering thermogenesis (such as 16–17°C) if tolerated by people, 4) promoting skeletal muscle thermogenesis, and 5) increasing general mitochondrial uncoupling.
In a small cohort of morbidly obese patients (mean BMI 40 kg/m2, mean age 42 years), reduced BAT activity was restituted in sites normally present in lean individuals 1 year after bariatric surgically induced weight reduction in some, but not all, patients and with lower amounts of PET+ uptake (47). This recruitment of BAT needs to be studied in a larger number of patients and its cellular mechanism(s) ascertained, but if BAT remains deficient, BAT replacement should be considered. In this context, if precursors are harvested from WAT depots in obese BAT-deficient subjects and engineered ex vivo to produce mature brown adipocytes that are transplanted by injection in vivo (46), should those cells be transplanted in the neck where most BAT normally resides? Alternatively, should they be administered into subcutaneous abdominal or other superficial WAT and would those cells acquire the blood and nerve supply necessary for a process as tightly regulated as thermogenesis? The same consideration applies to choosing the (nonintravenous) site of injection of a therapeutic agent that can promote local brown adipogenesis. In either case, targeting the neck might be riskier than abdominal WAT given cervical and supraclavicular BAT’s anatomical relations to the brachial plexus, carotid sheath, and subclavian vessels. In addition to the obese, the question arises whether the elderly might be a cohort that benefits from BAT replacement therapy because of the elderly population’s low BAT mass and increased risk of death from accidental hypothermia. The ultimate choice of these and other approaches will depend on and be guided by comprehensive animal studies, safety considerations, and the refinement of accurate imaging modalities sufficiently sensitive to detect BAT not only in established sites in the neck and inside the thorax and abdomen but also in smaller clusters or islands in subcutaneous WAT (3,44,46).
Although the utility of TI in assessing BAT activation in obesity may be limited, TI could be used in physiological, genetic, and epidemiological investigations. TI technology is relatively inexpensive compared with PET, safe, and repeatable. From the physiological perspective, TI shows that the supraclavicular BAT depot responds to stimuli including extremity cooling as well as drinking liquid (36), and BAT activity measured by this technique decreases with age in accord with findings from PET-CT studies (10,11,16). As illustrated in Fig. 3A–H, there is a clear genetic component in determining the abundance and activity of BAT. Given the extensive genotyping data from GWAS studies that have previously relied on the relatively crude measurement of BMI to determine genetic traits for body weight regulation (48), TI can now be used to directly assess the relationship between genotype, BAT function, and body composition. That said, it remains to be established that BAT within the neck, as the single dominant location and with the relative ease with which it can be stimulated and recorded with TI, will be a valid technique in enabling these types of population-based as well as genetic studies. Indeed, as the cost of TI cameras starts to fall, then it could be part of routine biometric data collected on all individuals through their life span.
In conclusion, the distribution of human BAT along the vasculature and around critical organs putatively confers survival value by hypothermia-induced adaptive thermogenesis, thereby preserving normal body temperature and, in turn, vital functions (26). In particular, its role in the defense of the central and autonomic (sympathetic) nervous systems against cold may be important. Where BAT resides normally in humans should be considered in basic and clinical science investigations as well as in weight loss strategies for treating obesity and type 2 diabetes, but currently, the role of BAT-based therapy as it relates to BAT anatomy requires further study.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
H.S. developed and wrote the manuscript and prepared Fig. 1. M.E.S. developed and wrote the manuscript and prepared Figs. 2 and 3. H.S. and M.E.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293:E444–E452 [DOI] [PubMed] [Google Scholar]
- 2.Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972;112:35–39 [PMC free article] [PubMed] [Google Scholar]
- 3.Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 2010;17:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med 2003;44:170–176 [PubMed] [Google Scholar]
- 5.Yeung HW, Grewal RK, Gonen M, Schöder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med 2003;44:1789–1796 [PubMed] [Google Scholar]
- 6.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 7.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 8.Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011;14:272–279 [DOI] [PubMed] [Google Scholar]
- 9.Ouellet V, Labbé SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012;122:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011;96:192–199 [DOI] [PubMed] [Google Scholar]
- 12.Garcia CA, Van Nostrand D, Atkins F, et al. Reduction of brown fat 2-deoxy-2-[F-18]fluoro-D-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imaging Biol 2006;8:24–29 [DOI] [PubMed] [Google Scholar]
- 13.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab 2010;11:253–256 [DOI] [PubMed] [Google Scholar]
- 14.Standring S. (Ed.). Gray's Anatomy London, Elsevier, Churchill Livingstone, 2008
- 15.Lee P, Zhao JT, Swarbrick MM, et al. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 2011;96:2450–2455 [DOI] [PubMed] [Google Scholar]
- 16.Yoneshiro T, Aita S, Matsushita M, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011;19:1755–1760 [DOI] [PubMed] [Google Scholar]
- 17.Symonds ME, Henderson K, Elvidge L, et al. Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. J Pediatr 2012;161:892–898 [DOI] [PubMed] [Google Scholar]
- 18.Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009;23:3113–3120 [DOI] [PubMed] [Google Scholar]
- 19.Skarulis MC, Celi FS, Mueller E, et al. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J Clin Endocrinol Metab 2010;95:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 2009;94:3611–3615 [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol 2011;301:R285–R296 [DOI] [PubMed] [Google Scholar]
- 24.Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev 1969;49:330–425 [DOI] [PubMed] [Google Scholar]
- 25.Smith RE. Thermoregulatory and adaptive behavior of brown adipose tissue. Science 1964;146:1686–1689 [DOI] [PubMed] [Google Scholar]
- 26.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 27.Mattu A, Brady WJ, Perron AD. Electrocardiographic manifestations of hypothermia. Am J Emerg Med 2002;20:314–326 [DOI] [PubMed] [Google Scholar]
- 28.Rango N. Exposure-related hypothermia mortality in the United States, 1970-79. Am J Public Health 1984;74:1159–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson S. Physiological effects of heat and cold. Annu Rev Physiol 1952;14:73–96 [DOI] [PubMed] [Google Scholar]
- 30.Rubenstein E, Meub DW, Eldridge F. Common carotid blood temperature. J Appl Physiol 1960;15:603–604 [DOI] [PubMed] [Google Scholar]
- 31.Ebert TJ, Kanitz DD, Kampine JP. Inhibition of sympathetic neural outflow during thiopental anesthesia in humans. Anesth Analg 1990;71:319–326 [DOI] [PubMed] [Google Scholar]
- 32.Koehn J, Kollmar R, Cimpianu CL, et al. Head and neck cooling decreases tympanic and skin temperature, but significantly increases blood pressure. Stroke 2012;43:2142–2148 [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2007;292:R127–R136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 2011;16:74–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaye MD, Kilby AE, Harper PC. Changes in distal esophageal function in response to cooling. Dig Dis Sci 1987;32:22–27 [DOI] [PubMed] [Google Scholar]
- 36.Symonds ME, Pope M, Budge H. Adipose tissue development during early life: novel insights into energy balance from small and large mammals. Proc Nutr Soc 2012;71:363–370 [DOI] [PubMed] [Google Scholar]
- 37.Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des 2007;13:2185–2192 [DOI] [PubMed] [Google Scholar]
- 38.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 1991;13:277–296 [DOI] [PubMed] [Google Scholar]
- 39.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 2009;29:1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne GA, Borbouse L, Kumar S, et al. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol 2010;30:1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Champigny O, Ricquier D, Blondel O, Mayers RM, Briscoe MG, Holloway BR. Beta 3-adrenergic receptor stimulation restores message and expression of brown-fat mitochondrial uncoupling protein in adult dogs. Proc Natl Acad Sci USA 1991;88:10774–10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg F, Gustafson U, Andersson L. The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet 2006;2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salisbury E, Hipp J, Olmsted-Davis EA, Davis AR, Heggeness MH, Gannon FH. Histologic identification of brown adipose and peripheral nerve involvement in human atherosclerotic vessels. Hum Pathol 2012;43:2213–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS ONE 2011;6:e17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tam CS, Lecoultre V, Ravussin E. Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation 2012;125:2782–2791 [DOI] [PubMed] [Google Scholar]
- 46.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov 2010;9:465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vijgen GH, Bouvy ND, Teule GJ, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab 2012;97:E1229–E1233 [DOI] [PubMed] [Google Scholar]
- 48.Meyre D, Delplanque J, Chèvre JC, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 2009;41:157–159 [DOI] [PubMed] [Google Scholar]
- 49.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vosselman MJ, van der Lans AA, Brans B, et al. Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes 2012;61:3106–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]