Abstract
Oleanolic acid (OA), a natural component of many plant food and medicinal herbs, is endowed with a wide range of pharmacological properties whose therapeutic potential has only partly been exploited until now. Throughout complex and multifactorial mechanisms, OA exerts beneficial effects against diabetes and metabolic syndrome. It improves insulin response, preserves functionality and survival of β-cells, and protects against diabetes complications. OA may directly modulate enzymes connected to insulin biosynthesis, secretion, and signaling. However, its major contributions appear to be derived from the interaction with important transduction pathways, and many of its effects are consistently related to activation of the transcription factor Nrf2. Doing that, OA induces the expression of antioxidant enzymes and phase II response genes, blocks NF-κB, and represses the polyol pathway, AGEs production, and hyperlipidemia. The management of type 2 diabetes requires an integrated approach, which includes the early intervention to prevent or delay the disease progression, and the use of therapies to control glycemia and lipidemia in its late stages. In this sense, the use of functional foods or drugs containing OA is, undoubtedly, an interesting path.
Type 2 diabetes affects 220 million people worldwide. This number will be doubled by 2030 without urgent action. Diabetes prevalence has burst by the aging of population, socioeconomic disadvantages, and lifestyles that trend toward physical inactivity and overweight/obesity (1). Today, it is clear that insulin resistance plays an early role in diabetes pathogenesis and that failure in insulin secretion by pancreatic β-cells is instrumental in the progression to hyperglycemia.
Diabetes management requires an integrated approach that includes the early intervention to prevent or delay its appearance and the use of combined therapies to control glycemia and lipidemia in its late stages. Although many drugs with different modes of action are available, novel natural antidiabetic agents with insulin-sensitizing effects and preventive actions are highly desirable. The target is not only the reduction of hyperglycemia but also to address the metabolic syndrome as a whole.
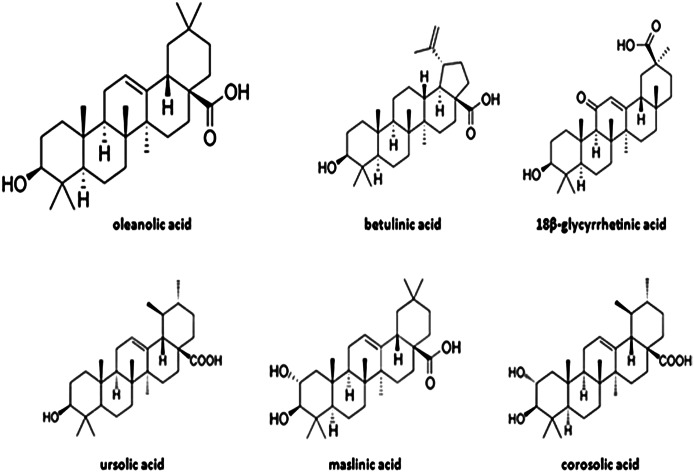
Different natural bioactive compounds have antidiabetic potential. Among them are triterpenoids, plant secondary metabolites biosynthesized by the acetate/mevalonate pathway and (3S)-2,3-oxidosqualene cyclization (2). Oleanolic acid (OA) (3β-hydroxy-olean-12-en-28-oic acid) (Fig. 1) is widely distributed in the plant kingdom as free acid or as aglycone of triterpenoid saponins. More than 120 plant species have been described by their relevant OA contents (3), but few of them are socioeconomically important crops as is olive (Olea europaea L.). OA is a component of the cuticle waxes that cover fruit and leaf epidermis. It is especially abundant in the olive leaf, where it represents up to 3.5% of the dry weight (4).
FIG. 1.
Chemical structures of OA and related natural triterpenes with antidiabetic effects.
OA and related triterpenes possess interesting pharmacological properties, including the antioxidant, microbicide, antidiabetic, anti-inflammatory, hypolipidemic, and antiatherosclerotic actions (5–7). They interfere in the development of different types of cancer (7) and neurodegenerative disorders (8). OA is therapeutically effective without apparent side effects (9–11). The aim of this review is to summarize the most significant knowledge existing to date on the molecular basis of the OA antidiabetic activity.
Reduction of postprandial hyperglycemia
Reducing postprandial hyperglycemia in diabetic people prevents glucose absorption after food intake. Carbohydrate digestion is facilitated by enteric enzymes, such as α-glucosidase and α-amilase, in the brush border of the small intestine cells. Their inhibition permits a better control of postprandial hyperglycemia and originates, at long term, a modest reduction of glycosylated proteins.
OA inhibits α-glucosidase in vitro in an uncompetitive and dose-dependent fashion (half-maximal inhibitory concentration [IC50] 10–15 μmol/L) (12–14). OA also inhibits the pancreatic and salivary α-amilase activity (IC50 0.1 mg/mL), producing a hypoglycemic effect in prediabetic individuals (patients having impaired fasting glucose) fed cooked rice. At a dose of 1 mg/kg, OA reduced blood glucose by 23% 30 min after the meal (15). A similar hypoglycemic effect was observed in diabetic GK/Jcl rats fed starch (15). Ursolic acid (UA) and lupeol also block α-amylase, and therefore it has been suggested that inhibition of this enzyme is a feature of the triterpenoid structure (16).
Improvement of pancreatic β-cell function and integrity
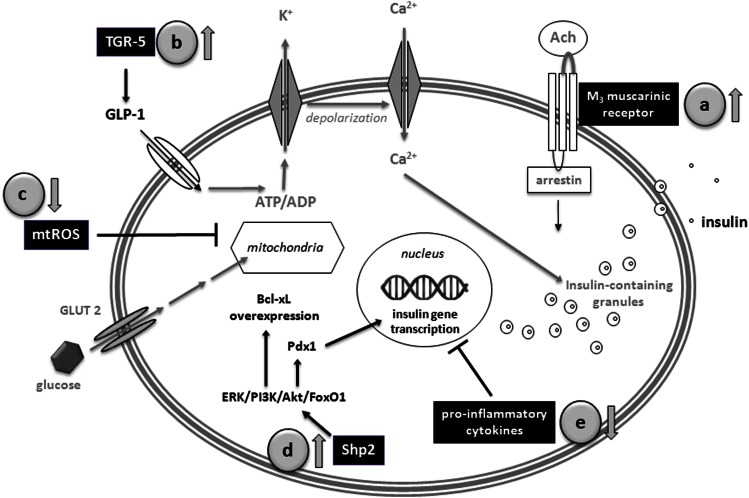
In type 2 diabetes, pancreatic β-cells fail to release insulin enough to compensate for hyperglycemia. This deficit involves morphological and functional β-cell alterations. Accumulated data indicate that OA increases biosynthesis and secretion of insulin and improves glucose tolerance through a multifactorial mechanism (Fig. 2).
FIG. 2.
OA increases insulin biosynthesis and secretion and improves glucose tolerance. It also promotes β-cell survival and proliferation. Actions of OA on pancreatic β-cells involve multitargeted mechanisms. a: Increase of acetylcholine release and activation of M3 muscarinic receptors. b: Enhancement of the glucagon-like peptide-1 (GLP)-1 release by agonist action on the TGR-5 receptors. c: Alleviation of the oxidative stress. d: Stimulation of the Src-homology phosphotyrosyl phosphatase 2 activity and PKB/Akt pathway. e: Improvement of the β-cell survival and proliferation.
Activation of the β-cell M3 muscarinic receptors.
Acetylcholine facilitates the glucose-dependent insulin release by activating the M3-subtype muscarinic receptors in the pancreatic β-cell membrane (17). In Wistar rats, the intraperitoneal injection of OA reduced fasting glycemia in parallel with the increase of plasma insulin (18). These actions were abolished by hemicholinium-3 and vesamicol, inhibitors of the choline uptake and acetylcholine transport, respectively, indicating that OA raises acetylcholine release from nerve terminals.
Agonist action on the TGR5 receptor.
Bile acids emerged as signaling molecules endowed with systemic endocrine functions. They induce production of glucagon-like peptide-1 by a TGR5-mediated mechanism that increases insulin secretion and β-cell regeneration (19). OA performs as a selective TGR5 agonist (EC50 1.42 μmol/L) (20), since a difference of bile acids does not activate the FXR receptor. Betulinic acid (BA) (EC50 1.04 μmol/L) and UA (EC50 1.43 μmol/L) also exhibit specific activity on TGR5. They are better TGR5 agonists than lithocholic acid, the most potent natural activator. TGR5 stimulation also increases cAMP production and the thyroid hormone-activating enzyme deiodinase type 2 activity in brown adipose tissue, enhances oxidative phosphorylation in muscle, and stimulates eNOS expression and improves the immune and inflammatory responses in enteroendocrine cells (21).
Different structure/activity studies have revealed the importance of C3-hydroxyl and C28-carboxylate groups in OA molecule for TGR5 activation. It has been postulated that triterpenes bind TGR5 through three binding motifs: 1) a narrow H-bonding site recognizing the hydroxyl, 2) other polar pocket anchoring the carboxylate, and 3) hydrophobic interactions with the pentacyclic skeleton that favor orientation of the polar groups (21).
Protective action on β-cell under oxidative stress.
Overproduction of mitochondrial reactive oxygen species (ROS) represent a common pathway of injury that ultimately results in β-cell failure. β-Cells have moderate catalytic capacity for conversion of superoxide anion into H2O2. However, their levels of H2O2-inactivating enzymes are extremely low, and therefore they are vulnerable to H2O2 accumulation and hydroxyl radical production (22). ROS injure mitochondria by promoting DNA fragmentation, protein cross-linking and membrane phospholipid peroxidation, and by activating stress-signaling pathways. In this regard, OA protects β-cells. It shows moderate free radical–scavenging activity but strongly reinforces cellular defenses. The antioxidant activity of OA will be detailed below.
Enhancement of the Shp-2 enzyme activity.
The Src-homology phosphotyrosyl phosphatase 2 (Shp-2) is implicated in receptor-activated pathways, including insulin biosynthesis and signaling. Cytoplasmic Shp-2 plays a critical role in insulin gene transcription, modulating signals that flow through PI3K/Akt/FoxO1 and extracellular signal–related kinase (ERK) pathways and culminate in control of the pancreatic and duodenal homeobox 1 (Pdx1) gene expression and activity on Ins1 and Ins2 promoters. OA acts as enhancer of the Shp-2 causing a hypoglycemic effect in streptozocin (STZ) diabetic mice (23). Its action is dose dependent and selective, since OA does not activate other phosphotyrosyl phosphatases such as Shp1, Vhr, and HePTP. The chronic administration of OA (30–50 μmol/L) to INS-1 rat β-cells stimulated insulin biosynthesis at the transcriptional level (24), enhancing the insulin protein level (~25%), with a parallel increase in proinsulin and a twofold rise in the insulin-2 mRNA. In addition, OA enhanced mitochondrial Shp-2 activity and repressed the caspase-3 apoptotic cascade (25,26).
Promotion of β-cell survival and proliferation.
β-Cells are extremely sensitive to cytokines, which lead to islet degeneration and cell death (27). In STZ diabetic mice, OA prolongs survival of transplanted islets by inhibiting the cytokine production by macrophages and antigen-presenting and other infiltrating cells (28). OA markedly reduced IP-10 and interleukin (IL)-4 cytokines in serum and declined the frequency of γ-interferon–, IL-4–, IL-7–, and IL-2–producing T cells. Likewise, asiatic acid, a natural OA analog, preserved functional β-cells in STZ diabetic rats as a consequence of both the improvement of β-cells survival and promotion of their proliferation (29). UA also preserved β-cell functionality and enhanced the immune system in diabetic mice. Triggering of protein kinase B (PKB)/Akt pathway was proposed as the mechanism that leads to Bcl-XL overexpression (30).
Improvement of the insulin response
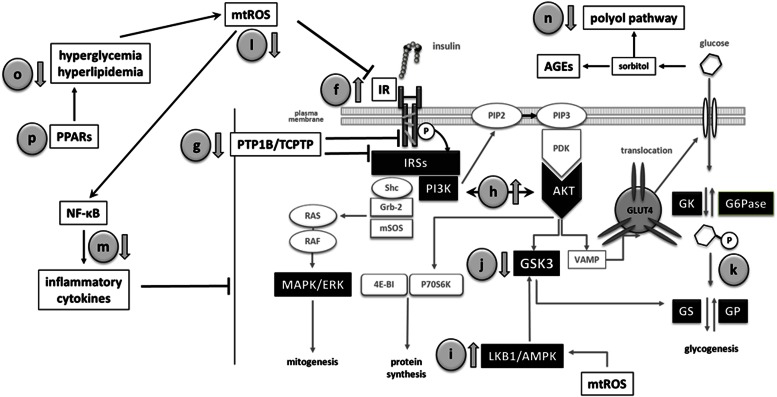
At the molecular level, insulin resistance, the pathogenic feature of type 2 diabetes, means impaired insulin signaling. Consistent evidence indicates that OA has beneficial effects on the insulin receptor (IR) and downstream signaling pathway (Fig. 3).
FIG. 3.
OA and related triterpenoids improve insulin sensitivity in peripheral tissues through a multiple mechanism. f: Stimulation of IR autophosphorylation. g: Inhibition of PTP1B/TCPTP activities. h: Stimulation of the PI3K/AKT pathway. i: Activation of LKB1/AMPK. j: Inhibition of GSK3. k: Stimulation of glycogenesis and inhibition of gluconeogenesis. l: Improvement of the antioxidant defenses. m: Inhibition of the proinflammatory cytokine production. n: Repression of polyol pathway and AGE formation. o: Amelioration of hyperlipidemia. GP, glycogen phosphorylase; GS, glycogen synthase; mtROS, mitochondrial ROS; VAMP, vesicle-associated membrane protein.
Insulin-mimetic effect as IR activator.
Small nonpeptide molecules known as IR activators are useful for treating type 2 diabetes because they restore IR autophosphorylation in insulin-resistant cells. OA and analogs act as activators that synergistically enhance the low-dose (1 nmol/L) insulin–mediated IR autophosphorylation in hamster ovary cells expressing the human receptor (31). In the absence of insulin, triterpenoids at 1 µg/mL could not activate IR, although at concentrations higher than 50 µg/mL they duplicated autophosphorylated receptors. These results suggest that triterpenes bind IR not at the insulin site but rather on the β-subunits.
Inhibition of protein-tyrosine phosphatases PTP1B and TCPTP.
Tyrosine phosphatases PTP1B and TCPTP negatively regulate insulin signaling in vivo. Inhibition of these proteins improves insulin sensitivity and stimulates glucose uptake (32). Oleanane-type triterpenes inhibit PTP1B in a potent, selective, and reversible way. The mechanism could be referred to as linear-mixed type (33). UA shows the greatest inhibition (IC50 3.1 μmol/L), followed by OA (IC50 3.4 μmol/L) and maslinic acid (MA) (IC50 5.93 μmol/L) (34). Molecular docking studies indicate that triterpenes bind not in the PTP1B catalytic site but in a secondary aryl phosphate binding site (35), where C28-carboxylate forms an extensive hydrogen bonds network with the enzyme. Other protein residues interact through Van der Waals contacts with the triterpene. The β-configuration of C3-OH also seems relevant because of the lower inhibitory potency of the α-epimer.
OA and UA discriminate other phosphatases involved in the insulin pathway but do not exhibit obvious selectivity among PTP1B and TCPTP. Since MA shows the highest selectivity (3.3-fold), a series of synthetic derivatives with modifications at C2 and C3 was assayed; all of them were stronger and more selective PTP1B inhibitors (36).
Activation of PI3K/Akt.
PKB/Akt is essential for insulin-stimulated events such as glucose uptake and glycogen synthesis. OA and 3β-taraxerol have been reported for stimulating Akt in vascular smooth muscle cells and 3T3-L1 adipocytes, respectively. Since wortmannin blocked these events, the requirement of PI3K activation seems obligatory (37,38). Recently, it was described that OA exerts a potent glucose-lowering effect in diabetic mice that is sustained well beyond the treatment period (39). The downregulation of glucose-6-phosphatase, the gate-keeping gluconeogenesis enzyme, induced by AKT and FoxO1, was proposed as the likely mechanism.
Activation of LKB1/AMPK.
The AMP kinase (AMPK) participates in many metabolic processes, including glucose uptake and fatty acid oxidation in muscle and fatty acid synthesis and gluconeogenesis in liver. AMPK is activated when ATP-to-AMP ratio decreases in response to nutrient deprivation and pathological stresses. OA, UA, and BA stimulate AMPK in human HepG2 hepatoma cells (40). Similarly, MA activates AMPK and improves insulin sensitization in KK-Ay mice (41). By using the synthetic OA derivative 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid methyl ester (CDDO-Me), we know that these events were mediated by phosphorylation of both liver kinase B1 (LKB1) and AMPK. Although the exact step that triggers LKB1/AMPK remains unclear, it seems that CDDO-Me targets the upstream kinase ERK1/2 (42).
Inhibition of glycogen synthase kinase-3β.
Glycogen synthase kinase (GSK)3β acts as negative modulator of insulin signal in absence of stimulus. OA, UA, and 3β-taraxerol inhibit GSK3β by phosphorylation (38,43,44), and molecular docking studies have indicated that triterpenes, like other GSK3β inhibitors, link to the ATP-binding site by hydrogen bounds (45).
Other effects on the glycogen pool.
Diabetes reduces glycogenesis and enhances glycogenolysis. UA has the ability to increase hepatic glycogen in STZ diabetic mice by stimulating glucokinase and inhibiting glucose-6-phosphatase (46). Inhibition of glycogen phosphorylase, the rate-limiting step of glycogenolysis, is another good target for treating diabetes. OA represses this activity in lung cancer A549 cells (IC50 5.98 μmol/L) (47) and rabbit muscle (IC50 14 μmol/L) (48)—very similar to the behavior of other natural OA analogs (49,50). Triterpenes bind at the AMP allosteric site (51), their C3 and C28 positions being important for the quaternary structure changes that cause enzyme inhibition (49,52).
Alleviation of the oxidative stress–induced insulin resistance.
ROS play a causal role in several types of insulin resistance. As we mentioned, OA exerts antioxidant activity through direct chemical response but mainly by indirect biological effects. OA moderately scavenges hydroxyl and superoxide radicals, although this effect is stronger than those of known antioxidants such as BHT or LD-α-lipoic acid (52). The presence of the phenolic OH at C3 is speculated to be responsible for this action (53). By contrast, OA yields solid protection against oxidative damage by indirect means. OA upregulated the expression of glutathione peroxidase (GSHPx) and superoxide dismutase (SOD) in hepatocytes treated with tert-butyl hydroperoxyde (52). In addition, OA and UA improved the viability of PC12 cells treated with H2O2 or 1-methyl-4-phenylpyridinium by increasing glutathione content and catalase and SOD activities. They also attenuated the lactate dehydrogenase release and malondialdehyde formation (54). Likewise, OA protected rat heart against myocardial ischemia reperfusion injury by enhancing the glutathione-mediated mitochondrial antioxidant mechanism (55). OA also increased GSHPx and SOD activities and diminished malondialdehyde level in liver and kidney of alloxan diabetic rats (56).
These OA effects appear mediated, to a great extent, through the activation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which is a pivotal regulator of endogenous antioxidants and phase II genes (53).
Effects on inflammatory mediators.
Many inflammatory cytokines that adversely affect insulin signaling are regulated by the transcription factor nuclear factor (NF)-κB. NF-κB is activated by endogenous and exogenous stimuli via phosphorylation of the inhibitory subunit IκB by IKK. Several natural triterpenes inhibit IKK and block NF-κB activation. In this way, OA and UA reduce tumor necrosis factor-α–induced E-selectin expression in human endothelial cells (57), inhibit IL-6 release in lipopolysaccharide-activated Mono Mac 6 cells (58), and suppress the endothelin-1 pathway in Zucker diabetic rats (59). BA also represses NF-κB in human colorectal carcinoma HCT116, colon carcinoma Caco-2, and lung carcinoma H1299 cells, with these effects being correlated with its ability to inhibit IKK (60). Suppression of the NF-κB pathway is also the mechanism by which BA plays an antifibrotic role in ethanol-activated hepatic stellate cells (61).
Lastly, OA is also reported as an irreversible inhibitor of the inflammatory enzyme phospholipase A2 at micromolar concentrations (62).
Inhibition of polyol pathway and AGEs.
Chronic hyperglycemia significantly increases the polyol pathway in tissues that are not insulin sensitive. Aldose reductase (AR), the rate-limiting enzyme in this pathway, reduces glucose to sorbitol, which is further metabolized to fructose by sorbitol dehydrogenase (SDH). High levels of sorbitol and fructose promote the synthesis of advanced glycation end products (AGEs) and stimulate stress-sensitive signaling pathways. OA and UA inhibit both AR and sorbitol dehydrogenase in kidney and liver of STZ diabetic mice (46). In addition, OA enhances glyoxalase-I (GLI) and reduces methylglyoxal concentration. In vitro, OA dose dependently inhibits the formation of pentosidine and Nε-(carboxymethyl)lysine (CML), whereas UA suppressed CML but not pentosidine (63). In alloxan diabetic mice, OA and UA decreased the levels of plasma HbA1c, the renal pentosidine and CML, and urinary glycated albumin, with the inhibitory potency of OA greater than that of UA at equal concentration (56). With these data considered together, it seems that methyl position in the E ring that differentiates OA from UA determines their antiglycative strength.
Hypolipidemic effects.
Hypolipidemic and antiatherosclerotic abilities of OA are well-known since the nineties (5). In Sprague-Dawley rats fed a high-cholesterol diet, OA and MA reduced plasma concentrations of total cholesterol, triglyceride, HDL cholesterol, and LDL cholesterol and downregulated the expression of lipogenic genes (acetyl-CoA carboxylase [ACC], stearoyl-CoA desaturase 2 [SCD2], glycerol-3-phosphate acyltransferase [Gpam], and acyl-CoA cholesterol acyltransferase [ACAT]) (64). Likewise, OA and BA significantly reduced visceral fat in obese Swiss mice, with an increase of leptin and a decline of plasma lipids and ghrelin. The histological study of liver indicated that triterpenes markedly reduced microvesicular steatosis and lipid droplets caused by the diet (65). 18β-Glycyrrhetinic acid also decreased total cholesterol, triglyceride, free fatty acids, phospholipids, LDL cholesterol, and VLDL cholesterol in plasma, kidney, liver, and heart of STZ diabetic rats (66).
Transactivation of peroxisome proliferator–activated receptors.
The peroxisome proliferator–activated receptors (PPARs) are transcriptional regulators of genes involved in lipid metabolism and glucose homeostasis. Accumulated evidence indicates that OA may modulate PPAR activity. Acting as a PPAR-α agonist, OA improves cardiac lipid metabolism in the ZDF diabetic rat (59) and stimulates differentiation in keratinocytes HaCaT and African green monkey kidney fibroblast (CV-1) cells (67). In parallel, transactivation of PPAR-γ by OA produces a hypoglycemic effect in diabetic KK-Ay mice (68). By contrast, the attenuated lipid accumulation and decreased visfatin levels in differentiated 3T3-L1 adipocytes resulted from the OA-induced PPAR-γ downregulation (69). Two glycosylated OA derivatives from Kalopanax pictus achieve the transactivation of the three PPAR subtypes (70). Conceptually, these pan-PPAR activators are very interesting for the treatment of metabolic diseases because they could target simultaneously insulin resistance, atherogenic dyslipemia, and obesity/overweight.
Unifying hypothesis of the OA antidiabetic activity
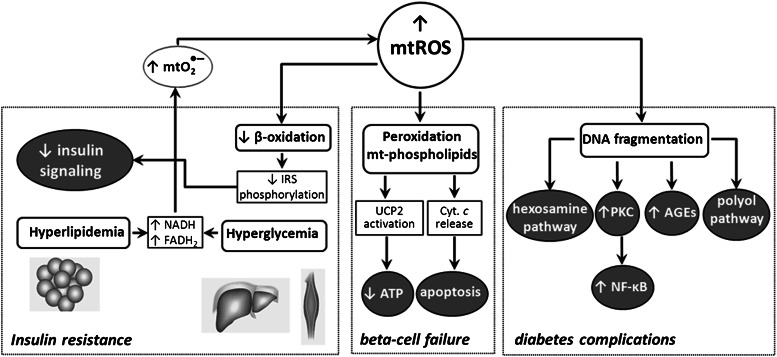
Hyperglycemia, hyperlipidemia, AGEs, and inflammatory cytokines all contribute to the progression of diabetes. Although many mechanisms have been proposed to underlie their effects, a unifying theme is that supraphysiological levels of ROS represent a common pathway of injury (22) (Fig. 4). Type 2 diabetes is now recognized as a phenotype of mitochondrial dysfunction, where impaired oxidative activity accumulates superoxide and other ROS (22,71). These radical species injure mitochondria by promoting DNA fragmentation, protein cross-linking, membrane phospholipid peroxidation, and activation of stress pathways, which result in insulin resistance, β-cell failure, and diabetes complications (71). Regarding this, much of the OA antidiabetic potential could be better explained if we understood the molecular basis of its protection against mitochondrial ROS overproduction and oxidative stress.
FIG. 4.
Diabetes is now considered a phenotype of mitochondrial dysfunction. Hyperglycemia and hyperlipidemia provoke overproduction of superoxide and ROS, which contribute to impair insulin signaling, β-cell failure, and other pathologies associated with diabetes.
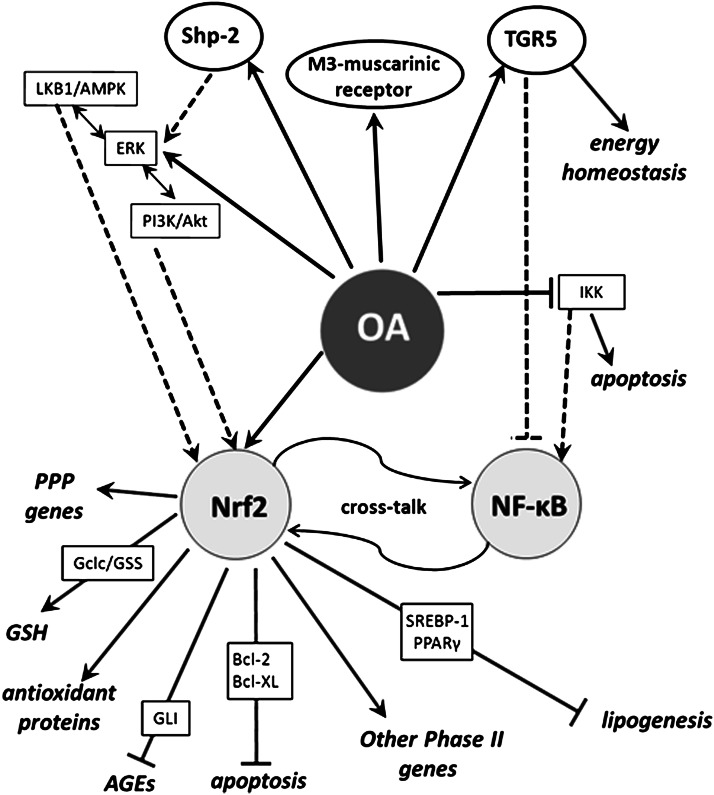
In vitro and in silico studies have revealed that OA is a versatile molecule. Its stereochemical structure, a hydrophobic pentacyclic skeleton with a β-phenolic hydroxyl at C3 and a carboxylate at C28, is appropriated to interact directly with single proteins involved in carbohydrate digestion, insulin secretion, and signaling. However, the major contributions of OA against diabetes seem to be derived from its interaction with transduction pathways modulating the expression of key defensive genes (Fig. 5). A significant number of OA effects might be consistently related to activation of Nrf2 (52). Nrf2 binds antioxidant response elements (AREs) in the gene promoter regions and stimulates transcription of cytoprotective genes in response to oxidative/electrophilic stresses. Nrf2 increases transcription of antioxidant enzymes (SOD, CAT, Heme Oxygenase 1 [HO-1], GSHPx, or peroxiredoxin [prdx]), as well as of genes involved in GSH biosynthesis (glutamate-cysteine ligase [Gclc] and glutathione synthase [GSS]) and regeneration (glutathione reductase [GSHR]). Nrf2 is also involved in the expression of NADPH-producing genes, such as malic enzyme and many of the pentose phosphate pathway ones (72).
FIG. 5.
A unifying hypothesis of the OA antidiabetic action. OA exerts its activity mainly through indirect mechanisms, stimulating stress-induced pathways in which the transcription factor Nrf2 plays a protagonist role. In this scheme, OA is a potent inducer of antioxidant enzymes and other phase II response genes, as well as a repressor of NF-κB.
OA-type triterpenes are extremely potent enhancers of phase 2 responses, with potential to activate Nrf2 at protein level via Michael addition reaction with Keap1, the primary sensor that retains Nrf2 for ubiquitin-dependent degradation (73). In addition, OA may activate Nrf2 through stress-induced signaling pathways.
ERK is thought to be part of the defensive mechanisms against H2O2. OA stimulates ERK and Jun NH2-terminal kinase phosphorylation in response to oxidative stress and inhibits the disruption of the mitochondrial membrane potential (74). However, these mitogen-activated protein kinases (MAPKs) appear to operate indirectly, since significant evidence demonstrates that direct phosphorylation by MAPKs has a limited contribution in modulating Nrf2 in vivo (74).
AMPK is activated by elevated mitochondrial ROS or a decline in the ATP-to-AMP ratio. AMPK stimulates HO-1 expression in human endothelial cells through activation of Nrf2 (75). As we aforementioned, OA induces phosphorylation of LKB1 and AMPK (40), which is consistent with activation of the upstream kinase ERK1/2 (42). AMPK could operate on Nrf2 through phosphatidylinositol 3-kinase (PI3K) (35), which is a necessary condition for events signaled by the PKB-Akt pathway, such as repression of β-cell apoptosis (30) and glycogen metabolism (36–38). We have also shown that OA is a potent and selective activator of Shp-2, which participates in PI3K activation in the β-cell (23) (Fig. 2). By triggering cytoplasmic Shp-2, OA stimulates insulin biosynthesis at the transcriptional level (24), whereas by enhancing mitochondrial Shp-2 it contributes to the preservation of β-cell mass (29,38).
On the other hand, oxidative stress in diabetes excites damaging pathways, such as NF-κB, leading to cell death. Since several anti-inflammatory agents that suppress NF-κB signaling also activate the Nfr2-ARE cascade, the existence of a cross-talk with each other was postulated (60). OA upregulates cytoprotective genes through Nrf2 activation, but it also blocks NF-κB via IKK inhibition (57–59). Hence, it seems clear that OA damps both oxidative stress and inflammatory response acting on these two opposite pathways.
Mitochondrial membrane phospholipid oxidation by ROS generates a mixture of oxidized lipid derivatives, including 4-hydroxynonenal (4-HNE). 4-HNE triggers β-cell apoptosis by disrupting the cytosolic Bcl-2/Bax protein interaction and translocation of Bax to mitochondrial membrane. This process involves Bcl-2 phosphorylation by IKK, giving a novel activity for this kinase (76), and is inhibited by substrate excess. Very recently (77), it was reported that Nfr2 controls cell apoptosis via regulation of Bcl-2 transcription. Therefore, the OA competence to inhibit IKK and stimulate PI3K/PKB/Akt pathway and Bcl-XL overexpression (34) supports both antioxidant and anti-inflammatory effects and endorses the existence of the Nrf2/NF-κB cross-talk. In consequence, the OA-induced improvement of β-cell functionality and enhancement of the immune system in diabetic mice could progress, at least partially, via Nrf2 transactivation.
Hyperglycemia increases glucose flux through the polyol pathway, where AR produces sorbitol with consumption of NADPH. Because NADPH is a critical cofactor for GSH regeneration, this pathway increases susceptibility to oxidative damage. Consequently, the inhibition of AR expression by OA (46) contributes to maintain the mitochondrial redox status and limits generation of AGEs. Nevertheless, AR has been postulated as an antioxidant enzyme that detoxifies cytotoxic aldehydes, including 4-HNE. In different cancerous cells, AR is upregulated by ROS and antioxidants, with putative participation of Nrf2 (78). Therefore, further investigation of regulation of AR expression is required to clarify its physiological role.
Accumulation of methylglyoxal and lipid peroxidation products also provokes DNA fragmentation and mitochondrial dysfunction. These effects are countered by GLI. Since OA upregulates GLI mRNA (46), which carries a functional ARE in exon 1 with the ability to bind Nrf2 (79), this action looks to be coherent with the OA-induced Nrf2 activation.
OA reduces plasma lipids (65,66) and downregulates lipogenic genes (64). The repression of transcriptional regulators SREBP-1 (72) and PPAR-γ (74) was proposed as the underlying mechanism. These actions may be now observed as mediated by Nrf2, since both SREBP-1 (64) and PPAR-γ (69) are direct targets of the transcription factor. In fact, most of lipogenic genes are downregulated in Nrf2-null mutant mice (72).
In summary, we have shown that many endogenous antioxidant enzymes, most of pentose phosphate pathway genes and those for glutathione biosynthesis and regeneration, are upregulated by Nrf-2, whereas lipogenic enzymes consuming NADPH are downregulated. Thus, in a scenario of ROS overproduction, the physiological consideration of the OA-induced Nrf2 activation is very interesting, since it would prime the use of NADPH to sustain the level of reduced glutathione and to assure the intracellular redox status.
Conclusions and perspectives
OA, a natural component of many plant food and medicinal herbs, is endowed with a wide range of pharmacological properties. Throughout complex and multifactorial mechanisms, OA exerts beneficial effects against diabetes and the metabolic syndrome. Although it directly modulates enzymes connected to carbohydrate metabolism and insulin signaling, the main OA contributions appear to be derived from its interaction with critical transduction pathways. Many effects are consistently related to activation of the transcription factor Nrf2. OA induces the expression of genes regulating the intracellular redox status, blocks NF-κB, and represses the polyol pathway, AGE production, and hyperlipidemia.
In spite of the well-contrasted scientific evidence, very few clinical trials using triterpenoids as antidiabetic agents have been developed up to today. Animal and human assays have demonstrated that OA and natural analogs are therapeutically effective without apparent side effects (9–11). OA is under commercialization in the People’s Republic of China as a drug against acute and chronic liver diseases (9,10), and its use is authorized in Japanese hair tonics and sport drinks. By contrast, a significant number of trials have used the synthetic derivative CDDO-Me (Bardoxolone) in the treatment of different types of cancer and chronic renal diseases in diabetic individuals (clinicaltrials.gov/show/NCT00811889 or www.clinicaltrials.gov/ct2/show/NCT01563562). Unfortunately, this deeply modified molecule is under serious debate as a result of findings of adverse events and mortality.
The prevalence of type 2 diabetes has burst by the aging of the population and lifestyles that trends toward physical inactivity and overweight/obesity. Therefore, it is of capital importance for the National Health Systems to introduce urgent preventive measures that delay or avoid the appearance of the disease. The use of functional foods containing natural triterpenoids is, undoubtedly, an interesting alternative. In this vein, our research group designed the PREDIABOLE Study (www.controlled-trials.com/ISRCTN003372660), a 2009–2013 phase 2 trial that pretends to demonstrate that a dietary intervention based in the regular consumption of olive oil enriched in OA may prevent the development of type 2 diabetes in prediabetic patients with impaired fasting glucose and impaired glucose tolerance. Further investigations like this will be necessary to extend the use of natural triterpenes in the design of new drugs and foods, which allow personalized diets and nutrigenomic approaches for the prevention of high-prevalence chronic disorders such as neurodegenerative and cardiovascular diseases, cancer, or diabetes.
ACKNOWLEDGMENTS
The Spanish Ministry of Economy and Competitiveness (Carlos III Institute of Health) and the Andalusian Ministry of Health and Social Well-being financed the research projects PI10/01415 and PI-0037/2008, respectively.
No potential conflicts of interest relevant to this article were reported.
J.M.C. researched data and wrote the manuscript. A.G. and T.D. contributed to discussion and edited the manuscript. M.R. and J.A.C. participated in the critical reading of the text. J.M.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the Andalusian Public System of Health (Spain) and the ACESUR Group for making possible the PREDIABOLE Study.
REFERENCES
- 1.WHO. Media Centre. Diabetes. Fact sheet N°312. Updated March 2013. Available from http://www.who.int/mediacentre/factsheets/fs312/en/index.html
- 2.Humphrey AJ, Beale MH. Terpenes. In Plant Secondary Metabolites. Crozier A, Clifford MN, Ashihara H, Eds. Oxford, U.K., Blackwell Publishing Ltd., 2006, p. 47–101 [Google Scholar]
- 3.Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules 2009;14:2016–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guinda A, Rada M, Delgado T, Gutiérrez-Adánez P, Castellano JM. Pentacyclic triterpenoids from olive fruit and leaf. J Agric Food Chem 2010;58:9685–9691 [DOI] [PubMed] [Google Scholar]
- 5.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 1995;49:57–68 [DOI] [PubMed] [Google Scholar]
- 6.Liu J. Oleanolic acid and ursolic acid: research perspectives. J Ethnopharmacol 2005;100:92–94 [DOI] [PubMed] [Google Scholar]
- 7.Dzubak P, Hajduch M, Vydra D, et al. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat Prod Rep 2006;23:394–411 [DOI] [PubMed] [Google Scholar]
- 8.Martín R, Carvalho-Tavares J, Hernández M, Arnés M, Ruiz-Gutiérrez V, Nieto ML. Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: a potential therapeutic role. Biochem Pharmacol 2010;79:198–208 [DOI] [PubMed] [Google Scholar]
- 9.Xu LZ, Wan ZX. The effect of oleanolic acid on acute hepatitis (70 cases). Humane Med 1980;7:50–52 [Google Scholar]
- 10.Xu SL. Effects of oleanolic acid on chronic hepatitis: 188 case reports. 1985. Symposium on oleanolic acid pp. 23-25 [Google Scholar]
- 11.Minich DM, Bland JS, Katke J, et al. Clinical safety and efficacy of NG440: a novel combination of rho iso-alpha acids from hops, rosemary, and oleanolic acid for inflammatory conditions. Can J Physiol Pharmacol 2007;85:872–883 [DOI] [PubMed] [Google Scholar]
- 12.Ortiz-Andrade RR, García-Jiménez S, Castillo-España P, Ramírez-Avila G, Villalobos-Molina R, Estrada-Soto S. alpha-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: an anti-hyperglycemic agent. J Ethnopharmacol 2007;109:48–53 [DOI] [PubMed] [Google Scholar]
- 13.Ali MS, Jahangir M, Hussan SS, Choudhary MI. Inhibition of alpha-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry 2002;60:295–299 [DOI] [PubMed] [Google Scholar]
- 14.Hou W, Li Y, Zhang Q, et al. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother Res 2009;23:614–618 [DOI] [PubMed] [Google Scholar]
- 15.Komaki E, Yamaguchi S, Maru I, et al. Identification of Anti-Amylase Components from Olive Leaf Extracts. Food Sci Technol Res 2003;9:35–39 [Google Scholar]
- 16.Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 2006;107:449–455 [DOI] [PubMed] [Google Scholar]
- 17.Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr Rev 2001;22:565–604 [DOI] [PubMed] [Google Scholar]
- 18.Hsu JH, Wu YC, Liu IM, Cheng JT. Release of acetylcholine to raise insulin secretion in Wistar rats by oleanolic acid, one of the active principles contained in Cornus officinalis. Neurosci Lett 2006;404:112–116 [DOI] [PubMed] [Google Scholar]
- 19.Whalley NM, Pritchard LE, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic α-cells: is this a paracrine mechanism enabling GLP-1 to act on β-cells? J Endocrinol 2011;211:99–106 [DOI] [PubMed] [Google Scholar]
- 20.Sato H, Genet C, Strehle A, et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 2007;362:793–798 [DOI] [PubMed] [Google Scholar]
- 21.Genet C, Strehle A, Schmidt C, et al. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J Med Chem 2010;53:178–190 [DOI] [PubMed] [Google Scholar]
- 22.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 2002;23:599–622 [DOI] [PubMed] [Google Scholar]
- 23.Bu Y, Shi T, Meng M, et al. A novel screening model for the molecular drug for diabetes and obesity based on tyrosine phosphatase Shp2. Bioorg Med Chem Lett 2011;21:874–878 [DOI] [PubMed] [Google Scholar]
- 24.Teodoro T, Zhang L, Alexander T, Yue J, Vranic M, Volchuk A. Oleanolic acid enhances insulin secretion in pancreatic β-cells. FEBS Lett 2008;582:1375–1380 [DOI] [PubMed] [Google Scholar]
- 25.Ivins Zito C, Kontaridis MI, Fornaro M, Feng G-S, Bennett AM. SHP-2 regulates the phosphatidylinositide 3′-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J Cell Physiol 2004;199:227–236 [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol 2003;160:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White SA, James RFL, Swift SM, Kimber RM, Nicholson ML. Human islet cell transplantation—future prospects. Diabet Med 2001;18:78–103 [DOI] [PubMed] [Google Scholar]
- 28.Nataraju A, Saini D, Ramachandran S, et al. Oleanolic acid, a plant triterpenoid, significantly improves survival and function of islet allograft. Transplantation 2009;88:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, He T, Lu Q, Shang J, Sun H, Zhang L. Asiatic acid preserves beta cell mass and mitigates hyperglycemia in streptozocin-induced diabetic rats. Diabetes Metab Res Rev 2010;26:448–454 [DOI] [PubMed] [Google Scholar]
- 30.Jang SM, Yee ST, Choi J, et al. Ursolic acid enhances the cellular immune system and pancreatic β-cell function in streptozotocin-induced diabetic mice fed a high-fat diet. Int Immunopharmacol 2009;9:113–119 [DOI] [PubMed] [Google Scholar]
- 31.Jung SH, Ha YJ, Shim EK, et al. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem J 2007;403:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galic S, Hauser C, Kahn BB, et al. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol Cell Biol 2005;25:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramírez-Espinosa JJ, Ríos MY, López-Martínez S, et al. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem 2011;46:2243–2251 [DOI] [PubMed] [Google Scholar]
- 34.Zhang YN, Zhang W, Hong D, et al. Oleanolic acid and its derivatives: new inhibitor of protein tyrosine phosphatase 1B with cellular activities. Bioorg Med Chem 2008;16:8697–8705 [DOI] [PubMed] [Google Scholar]
- 35.Puius YA, Zhao Y, Sullivan M, Lawrence DS, Almo SC, Zhang ZY. Identification of a second aryl phosphate-binding site in protein-tyrosine phosphatase 1B: a paradigm for inhibitor design. Proc Natl Acad Sci USA 1997;94:13420–13425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu W-W, Shen Q, Yang F, et al. Synthesis and biological evaluation of heterocyclic ring-substituted maslinic acid derivatives as novel inhibitors of protein tyrosine phosphatase 1B. Bioorg Med Chem Lett 2009;19:6618–6622 [DOI] [PubMed] [Google Scholar]
- 37.Feng J, Zhang P, Chen X, He G. PI3K and ERK/Nrf2 pathways are involved in oleanolic acid-induced heme oxygenase-1 expression in rat vascular smooth muscle cells. J Cell Biochem 2011;112:1524–1531 [DOI] [PubMed] [Google Scholar]
- 38.Sangeetha KN, Sujatha S, Muthusamy VS, et al. 3beta-taraxerol of Mangifera indica, a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochim Biophys Acta 2010;1880:359–366 [DOI] [PubMed]
- 39.Zeng X-Y, Wang Y-P, Cantley J, et al. Oleanolic acid reduces hyperglycemia beyond treatment period with Akt/FoxO1-induced suppression of hepatic gluconeogenesis in type-2 diabetic mice. PLoS ONE 2012;7:e42115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ha T, Tuan DT, Thu NB, et al. Palbinone and triterpenes from Moutan Cortex (Paeonia suffruticosa, Paeoniaceae) stimulate glucose uptake and glycogen synthesis via activation of AMPK in insulin-resistant human HepG2 Cells. Bioorg Med Chem Lett 2009;19:5556–5559 [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Sun H, Duan W, Mu D, Zhang L. Maslinic acid reduces blood glucose in KK-Ay mice. Biol Pharm Bull 2007;30:2075–2078 [DOI] [PubMed] [Google Scholar]
- 42.Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J Biol Chem 2010;285:40581–40592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang DL, Gu LJ, Liu L, et al. Effect of Wnt signaling pathway on wound healing. Biochem Biophys Res Commun 2009;378:149–151 [DOI] [PubMed] [Google Scholar]
- 44.Azevedo MF, Camsari C, Sá CM, Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Ursolic acid and luteolin-7-glucoside improve lipid profiles and increase liver glycogen content through glycogen synthase kinase-3. Phytother Res 2010;24(Suppl. 2):S220–S224 [DOI] [PubMed] [Google Scholar]
- 45.Ahamed KBM, Gowdru HB, Rajashekarappa S, Malleshappa KS, Krishna V. Molecular docking of glycogen synthase kinase3-β inhibitor oleanolic acid and its wound-healing activity in rats. Med Chem Res 2013;22:156–164 [Google Scholar]
- 46.Jang S-M, Kim M-J, Choi M-S, Kwon E-Y, Lee M-K. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metabolism 2010;59:512–519 [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Wang X, Luo D, Sun H-B, Shang J, Zhang L-Y. Anti-proliferative effect of pentacyclic triterpenes associated with glycogen accumulation in A549 cells. Chinese Journal of New Drugs 2011;20:2350–2353 [Google Scholar]
- 48.Wen X, Sun H, Liu J, et al. Pentacyclic triterpenes. Part 1: the first examples of naturally occurring pentacyclic triterpenes as a new class of inhibitors of glycogen phosphorylases. Bioorg Med Chem Lett 2005;15:4944–4948 [DOI] [PubMed] [Google Scholar]
- 49.Wen X, Sun H, Liu J, et al. Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: synthesis, structure-activity relationships, and X-ray crystallographic studies. J Med Chem 2008;51:3540–3554 [DOI] [PubMed]
- 50.Chen J, Liu J, Zhang L, et al. Pentacyclic triterpenes. Part 3: Synthesis and biological evaluation of oleanolic acid derivatives as novel inhibitors of glycogen phosphorylase. Bioorg Med Chem Lett 2006;16:2915–2919 [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Ye XL, Liu R, et al. Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chem Biol Interact 2010;184:328–337 [DOI] [PubMed] [Google Scholar]
- 52.Cheng K, Liu J, Sun H, Xie J. Synthesis of oleanolic acid dimers as inhibitors of glycogen phosphorylase. Chem Biodivers 2010;7:690–697 [DOI] [PubMed] [Google Scholar]
- 53.Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res 2005;579:200–213 [DOI] [PubMed] [Google Scholar]
- 54.Tsai SJ, Yin MC. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J Food Sci 2008;73:H174–H178 [DOI] [PubMed] [Google Scholar]
- 55.Du Y, Ko KM. Oleanolic acid protects against myocardial ischemia-reperfusion injury by enhancing mitochondrial antioxidant mechanism mediated by glutathione and α-tocopherol in rats. Planta Med 2006;72:222–227 [DOI] [PubMed] [Google Scholar]
- 56.Gao D, Li Q, Li Y, et al. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytother Res 2009;23:1257–1262 [DOI] [PubMed] [Google Scholar]
- 57.Takada K, Nakane T, Masuda K, Ishii H. Ursolic acid and oleanolic acid, members of pentacyclic triterpenoid acids, suppress TNF-α-induced E-selectin expression by cultured umbilical vein endothelial cells. Phytomedicine 2010;17:1114–1119 [DOI] [PubMed] [Google Scholar]
- 58.Saaby L, Jäger AK, Moesby L, Hansen EW, Christensen SB. Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.). Phytother Res 2011;25:195–201 [DOI] [PubMed] [Google Scholar]
- 59.Huang TH-W, Yang Q, Harada M, et al. Pomegranate flower extract diminishes cardiac fibrosis in Zucker diabetic fatty rats: modulation of cardiac endothelin-1 and nuclear factor-kappaB pathways. J Cardiovasc Pharmacol 2005;46:856–862 [DOI] [PubMed] [Google Scholar]
- 60.Bellezza I, Mierla AL, Minelli A. Nfr2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers 2010;2:483–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szersze’n M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting recative oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxycology 2011;280:152–163 [DOI] [PubMed] [Google Scholar]
- 62.Dharmappa KK, Kumar RV, Nataraju A, Mohamed R, Shivaprasad HV, Vishwanath BS. Anti-inflammatory activity of oleanolic acid by inhibition of secretory phospholipase A2. Planta Med 2009;75:211–215 [DOI] [PubMed] [Google Scholar]
- 63.Yin MC, Chan KC. Nonenzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J Agric Food Chem 2007;55:7177–7181 [DOI] [PubMed] [Google Scholar]
- 64.Yunoki K, Sasaki G, Tokuji Y, et al. Effect of dietary wine pomace extract and oleanolic acid on plasma lipids in rats fed high-fat diet and its DNA microarray analysis. J Agric Food Chem 2008;56:12052–12058 [DOI] [PubMed] [Google Scholar]
- 65.de Melo CL, Queiroz MGR, Fonseca SGC, et al. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chem Biol Interact 2010;185:59–65 [DOI] [PubMed] [Google Scholar]
- 66.Kalaiarasi P, Kaviarasan K, Pugalendi KV. Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats. Eur J Pharmacol 2009;612:93–97 [DOI] [PubMed] [Google Scholar]
- 67.Lee WS, Im KR, Park YD, Sung ND, Jeong TS. Human ACAT-1 and ACAT-2 inhibitory activities of pentacyclic triterpenes from the leaves of Lycopus lucidus TURCZ. Biol Pharm Bull 2006;29:382–384 [DOI] [PubMed] [Google Scholar]
- 68.Kuroda M, Mimaki Y, Ohtomo T, et al. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-Ay mice and identification of the active ingredients. J Nat Med 2012;66:394–399 [DOI] [PubMed] [Google Scholar]
- 69.Sung HY, Kang SW, Kim JL, et al. Oleanolic acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutr Res 2010;30:831–839 [DOI] [PubMed] [Google Scholar]
- 70.Quang TH, Ngan NTT, Minh CV, et al. Effect of triterpenes and triterpene saponins from the stem bark of Kalopanax pictus on the transactivational activities of three PPAR subtypes. Carbohydr Res 2011;346:2567–2575 [DOI] [PubMed] [Google Scholar]
- 71.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 72.Wu KC, Cui JY, Klaassen CD. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 2011;123:590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinkova-Kostova AT, Liby KT, Stephenson KK, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA 2005;102:4584–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Z, Huang Z, Zhang DD. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 2009;4:e6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi X, Zhou B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci 2010;115:391–400 [DOI] [PubMed] [Google Scholar]
- 76.Bodur C, Kutuk O, Tezil T, Basaga H. Inactivation of Bcl-2 through IκB kinase (IKK)-dependent phosphorylation mediates apoptosis upon exposure to 4-hydroxynonenal (HNE). J Cell Physiol 2012;227:3556–3565 [DOI] [PubMed] [Google Scholar]
- 77.Niture SK, Jaiswal AK. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem 2012;287:9873–9886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishinaka T, Yabe-Nishimura C. Transcription factor Nrf2 regulates promoter activity of mouse aldose reductase (AKR1B3) gene. J Pharmacol Sci 2005;97:43–51 [DOI] [PubMed] [Google Scholar]
- 79.Xue M, Rabbani N, Momiji H, et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem J 2012;443:213–222 [DOI] [PubMed] [Google Scholar]