Abstract
BTBR mice develop severe diabetes in response to genetically induced obesity due to a failure of the β-cells to compensate for peripheral insulin resistance. In analyzing BTBR islet gene expression patterns, we observed that Pgter3, the gene for the prostaglandin E receptor 3 (EP3), was upregulated with diabetes. The EP3 receptor is stimulated by prostaglandin E2 (PGE2) and couples to G-proteins of the Gi subfamily to decrease intracellular cAMP, blunting glucose-stimulated insulin secretion (GSIS). Also upregulated were several genes involved in the synthesis of PGE2. We hypothesized that increased signaling through EP3 might be coincident with the development of diabetes and contribute to β-cell dysfunction. We confirmed that the PGE2-to-EP3 signaling pathway was active in islets from confirmed diabetic BTBR mice and human cadaveric donors, with increased EP3 expression, PGE2 production, and function of EP3 agonists and antagonists to modulate cAMP production and GSIS. We also analyzed the impact of EP3 receptor activation on signaling through the glucagon-like peptide (GLP)-1 receptor. We demonstrated that EP3 agonists antagonize GLP-1 signaling, decreasing the maximal effect that GLP-1 can elicit on cAMP production and GSIS. Taken together, our results identify EP3 as a new therapeutic target for β-cell dysfunction in T2D.
Signaling through G-protein–coupled receptors (GPCRs) on β-cells modulates the effects of glucose and other nutrients on β-cell function. The most studied GPCR of relevance to type 2 diabetes (T2D) is the glucagon-like peptide 1 (GLP-1) receptor. It is coupled to a stimulatory G-protein, Gs, which activates adenylate cyclase, increasing cAMP production and potentiating glucose-stimulated insulin secretion (GSIS) (1). Agents that stabilize or mimic GLP-1 are now widely used in the treatment of T2D (2). Not all patients respond to these treatments (3–5), raising the possibility that endogenous negative regulatory pathways suppress full GLP-1 action.
The BTBR mouse strain, when made obese with the leptinob/ob mutation, is highly susceptible to diabetes (6). We previously profiled gene expression in multiple tissues, including pancreatic islets, of lean and obese BTBR mice before and after the onset of diabetes (7). Ptger3 expression was markedly elevated in islets from BTBR mice after the onset of diabetes. Ptger3 encodes a GPCR for prostaglandin (PG)E2, termed prostaglandin E receptor 3 (EP3), the only one of four PGE2 receptors that couples to G-proteins of the Gi subfamily, all of which negatively regulate cAMP production (8). We confirmed increased Ptger3 expression by quantitative real-time (qRT) PCR, and further, we explored the expression patterns of genes in the synthetic pathway of PGE2, the endogenous ligand for EP3. Interestingly, several PGE2 synthetic genes, including prostaglandin-endoperoxidase synthase 2 (Ptgs2, i.e., cyclooxygenase-2 or COX-2) and prostaglandin E synthase (Ptges) were also upregulated, correlating with increased PGE2 production in pancreatic islets from both diabetic mice and humans. We hypothesized that increased PGE2 production, coupled with increased EP3 receptor expression, mediates a negative autocrine/paracrine signaling pathway in diabetic islets. Our results identify a mechanism by which this pathway antagonizes therapeutic agents that act through GLP-1 and suggest that this pathway contributes to the β-cell dysfunction of T2D patients.
RESEARCH DESIGN AND METHODS
Lean and BTBR mice with genetically imposed obesity (leptinob/ob mutation) were derived from in-house breeding colonies in the University of Wisconsin Biochemistry Department (7). All animals were treated in accordance with the standards set forth by the National Institutes of Health Office of Animal Care and Use.
Mouse islet isolation and culture.
Intact pancreatic islets were isolated from 10-week-old mice using a collagenase digestion protocol (9). Islets were cultured overnight in RPMI 1640 containing 11.1 mmol/L glucose and 10% heat-inactivated FBS and penicillin/streptomycin (9).
Human islet culture.
Human islets were obtained through the Integrated Islet Distribution Program. Islets were cultured overnight in RPMI containing 8 mmol/L glucose, 10% heat-inactivated FBS, and penicillin/streptomycin to confirm viability and sterility. Islets were then handpicked and cultured for an additional day before assay. For some experiments, 0.5 mmol/L xylitol in PBS or an equivalent amount of PBS was added to the culture medium (10).
Islet and Ins-1 (832/3) insulin secretion assays.
Islet insulin secretion assays were performed in mesh-bottomed glass tubes essentially as previously described (9). Four islets were used per replicate. Ins-1 (832/3) insulin secretion assays were performed in 96-well plates essentially as outlined in the study by Bhatnagar et al. (11), as described for Ins-1 (832/13) cells. In some experiments, various concentrations of L-798106, PGE1, sulprostone, GLP-1, exendin 9-39, or an equivalent volume of DMSO was added to the assay buffer. Insulin secretion as a percentage of total insulin content was determined by ELISA (9).
Islet PGE2 production assays.
Islet culture medium was subjected to PGE2 analysis as recommended by the manufacturer (PGE2 monoclonal EIA kit; Cayman Chemical, Ann Arbor, MI). PGE2 concentration was normalized to the total number of cultured islets to obtain PGE2 production/islet/24 h.
Islet and Ins-1 (832/3) cellular cAMP production assays.
cAMP production assays were performed essentially as described previously using the cAMP Direct BioTrak EIA with novel lysis reagents (GE Healthcare Life Sciences) (12). Briefly, cAMP production assays were conducted on 13–15 islets per replicate in the presence of 200 μmol/L isobutylmethylxanthine to block cAMP degradation. In some instances, 10 μmol/L forskolin was added to stimulate cAMP production. Ins-1 (832/3) cAMP production assays were performed essentially as described above for insulin secretion assays, except that the stimulation medium was discarded and the cells frozen at −80°C until the day of the cAMP EIA. In some experiments, various concentrations of L-798106, sulprostone, or GLP-1 or an equivalent volume of DMSO were added to the assay. The cAMP production for each sample was normalized to its protein content using bicinchoninic acid assay (Thermo Scientific, Rockford, IL).
qRT PCR.
Mouse islet copy DNA (cDNA) was generated as previously described (7). Human islet cDNA was generated in the same manner from samples of cultured human islets received from the Integrated Islet Distribution Program (BMI panel) or from Beta-Pro (Charlottesville, VA) (nondiabetic vs. T2D panel). mRNA-specific primers were designed to span exon-exon junctions (primer sequences available upon request). Quantitative RT-PCR was performed as previously described (13). cDNA dilution series were performed with each primer set in order to determine the primer efficiency, allowing calculation of relative cDNA concentrations. Melting curves and agarose gel electrophoresis of PCR products were performed to ensure primer specificity (data not shown).
Statistical analysis.
Data are expressed as means ± SEM unless otherwise noted. Statistical significance was determined by paired or unpaired t test as appropriate (GraphPad Prism version 5; GraphPad Software, San Diego, CA). Statistical significance was determined as P < 0.05.
RESULTS
Mouse islet EP3 expression is elevated with the development of diabetes.
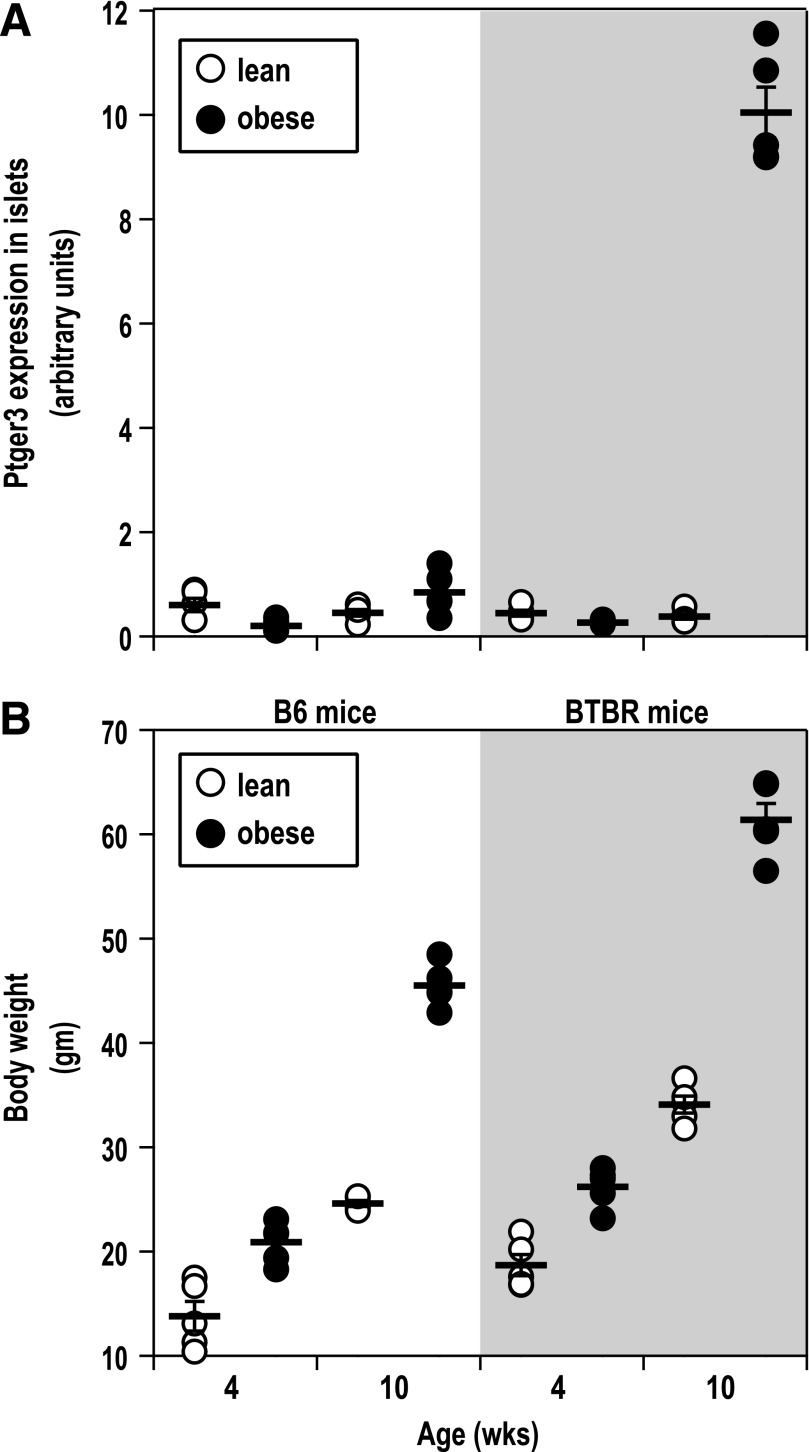
We previously published the results of a microarray analysis of six different insulin-sensitive tissues from diabetes-resistant C57Bl/6 (B6) and diabetes-susceptible BTBR mice, both lean and harboring the leptinob/ob mutation (Ob), at 4 and 10 weeks of age (7). In analyzing the islet expression of Ptger3, we found that Ptger3 expression appeared to be specifically upregulated in islets from diabetic, 10-week-old BTBR-Ob islets only (Fig. 1A). This is in comparison with islets isolated from 10-week-old B6-Ob mice, which are still morbidly obese, yet are normoglycemic (7). The weights of the mice used in isolating islets for this study are shown in Fig. 1B. Although on average, BTBR-Ob mice are heavier than B6-Ob mice, the BTBR mouse is a larger strain in general. The percent change in body weight between the 10-week-old B6-Ob and BTBR-Ob mice compared with their 4-week-old lean controls was not significantly different between the two groups (mean ± SD 276.6 ± 64.1%, B6-Ob, vs. 258.8 ± 45.6%, BTBR-Ob). The same was true for the percent weight increase in 10-week-old B6-Ob and BTBR-Ob mice compared with their 4-week-old Ob controls (193.9 ± 16.5%, B6-Ob, vs. 201.8 ± 28.49%, BTBR-Ob).
FIG. 1.
Ptger3 expression and PGE2 production are increased in a mouse model of T2D but not a mouse model of obesity. A: Islet RNA samples from 4- or 10-week-old BTBR-lean (lean) or diabetic BTBR-Ob (obese) (5 mice per group) were subjected to microarray analysis using Agilent custom ink-jet microarrays as previously described (7). Ptger3 intensity was significantly increased in islets from diabetic BTBR-Ob mice compared with all other groups. B: Body weights of the mice used to generate the islet gene expression data in A at the time of islet isolation showing equivalent percent increases in body mass between the B6 and BTBR groups. wks, week.
To confirm the change in Ptger3 (EP3) expression and to explore the expression of other components of the PGE2 synthesis and signaling pathways, we prepared islet cDNA samples from 10-week-old nondiabetic and diabetic BTBR mice suitable for qRT PCR analysis. The primers used were specific for the PGE2 receptor family (EP1, EP2, EP3, and EP4, including specific primers for the α-, β-, and γ-EP3 splice variants), the prostaglandin-endoperoxidase synthase family (Ptgs1, Ptgs2, and Ptgs3), and the prostaglandin E synthase family (Ptges, Ptges2, and Ptges3). The expression of total EP3 mRNA, as well that of all three splice variants, was elevated 40- to 200-fold (P < 10−4) in diabetic islets compared with nondiabetic islets (Fig. 2A). The α-, β-, and γ-splice variants are 90% identical (8,14), and all couple to inhibitory G-proteins of the Gi subfamily (8,15). There was little difference in the expression of any of the other PGE2 receptors, except for an approximate twofold increase in EP4 expression with diabetes (Fig. 2A). Among the genes involved in PGE2 synthesis, Ptgs2 and Ptges were significantly upregulated (Fig. 2A). Accordingly, the production of PGE2 was increased in islets from the diabetic BTBR mice (Fig. 2B).
FIG. 2.
Both EP3 expression and PGE2 production are upregulated in a mouse model of T2D. A: qRT PCR was performed on cDNA samples generated from nondiabetic and diabetic BTBR mouse islets. EP3 total, a primer set to a region common in all three splice variants (α, β, and γ). The gene expression of the other EP receptors (EP1, EP2, and EP4), prostaglandin-endoperoxidase synthases (Ptgs1, Ptgs2, and Ptgs3, i.e., COX-1, -2 and -3), and the prostaglandin E synthases (Ptges, Ptges2, and Ptges3) was also determined. Data were normalized within each sample to the Ct of a mouse β-actin primer set. ΔCt values for nondiabetic and diabetic islets were compared by unpaired t test (n = 5; *P < 0.05; **P < 1 × 10−4). NE, not expressed. B: PGE2 production was measured from islets from nondiabetic or diabetic BTBR mice. Islets were cultured for 24 h (hr), and the PGE2 that was secreted into the medium was normalized to the total number of islets. Data were compared by unpaired t test (n = 3. *P < 0.05).
PGE2/EP3 pathway causes dysfunction in diabetic mouse and human β-cells.
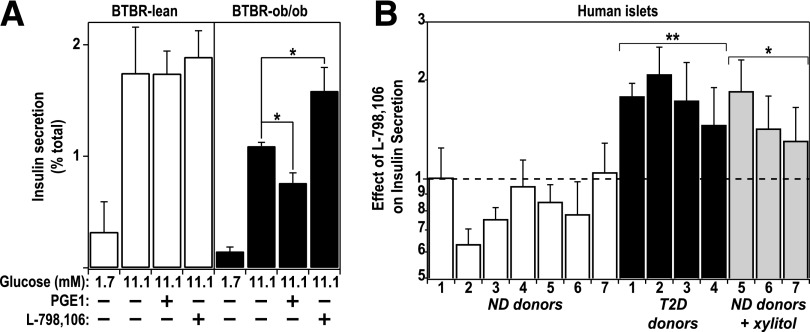
The elevated expression of EP3, coupled with the increased production of PGE2, raised the possibility of an autocrine or paracrine EP3-mediated signaling pathway present in islets from diabetic mice. We hypothesized that suppression of EP3 signaling would specifically enhance β-cell function in diabetic islets. We measured GSIS in islets isolated from nondiabetic and diabetic BTBR mice in the presence or absence of the specific EP3 antagonist L-798106 (16,17). L-798106 augmented GSIS only in diabetic BTBR islets, suggesting a competition for endogenous PGE2 at EP3 (Fig. 3A). The dose of L-798106 chosen for these studies (10 μmol/L) was determined by performing dose-response experiments in islets from BTBR-Ob mice, indicating a dose-dependent potentiation of GSIS when the L-798106 concentration was raised from 100 nmol/L to 10 μmol/L (Supplementary Fig. 1). Adding a partially selective agonist of EP3, PGE1, further reduced GSIS only in diabetic BTBR islets, suggesting that the concentration of endogenous PGE2, while high enough to elicit a biological response, was not sufficient to saturate the receptor (Fig. 3A).
FIG. 3.
An endogenous EP3 signaling cascade mediates β-cell dysfunction in diabetic mouse and human islets. A: Insulin secretion from islets isolated from 10-week-old BTBR mice that were either lean and nondiabetic or obese and diabetic. Glucose (11.1 mmol/L) was used to stimulate insulin secretion in the absence (−) or presence (+) of a semi–EP3-selective agonist, PGE1 (10 μmol/L), or an EP3-specific antagonist, L-798106 (20 μmol/L). Insulin secretion in 11.1 mmol/L glucose was significantly different than nonstimulatory glucose (1.7 mmol/L) for all conditions. Data were compared by paired t test (n = 5) *P < 0.05. B: Insulin secretion was measured from islets obtained from human donors that were either nondiabetic or confirmed T2D patients. Patient demographics can be found in Supplementary Table 1. Glucose (16.7 mmol/L) was used to stimulate insulin secretion in the absence or presence of L-798106 (20 μmol/L) in a 45-min static incubation. Data are represented as the fold change in insulin secretion relative to 16.7 mmol/L glucose alone. Islets obtained from nondiabetic donors 5–7 were incubated overnight in medium containing 0.5 mmol/L xylitol in addition to basal glucose. Data were compared by unpaired t test (n = 3–7. *P < 0.05; **P < 1 × 10−4).
Each of the mouse EP3 splice variants has a homolog in the human genome; thus, we aimed to determine whether the EP3 signaling pathway modulates insulin secretion in islets obtained from T2D humans. Cultured islets were obtained from organ donors who were either nondiabetic or confirmed T2D patients. Donor demographic information can be found in Supplementary Table 1. All islets were cultured overnight in 8 mmol/L glucose growth medium prior to assessment of their function. A subset of nondiabetic human islets was also incubated with 0.5 mmol/L xylitol, which has the same impact as 20 mmol/L glucose on carbohydrate response element–binding protein–responsive genes without inducing any detectable carbohydrate metabolism, as measured by accumulation of glucose-6-phosphate (10). This allows the separation of glucose-responsive effects from the compounding impact of insulin secretion, which itself can impact on cellular signaling. After overnight culture, GSIS was measured in the presence and absence of L-798106. For correction for the variable glucose responsiveness of cultured human islets, the data from each human islet sample were normalized to the insulin secretion elicited by glucose alone, in effect isolating the influence of L-798106 on GSIS. In human islets, as with mouse islets, L-798106 only potentiated GSIS in islets from T2D donors (Fig. 3B). Adding xylitol to the overnight culture medium to nondiabetic islets partially restored L-798106 responsiveness observed for T2D islets maintained in standard medium, suggesting that elevated glycemia may be at least in part responsible for increased EP3 signaling (Fig. 3B).
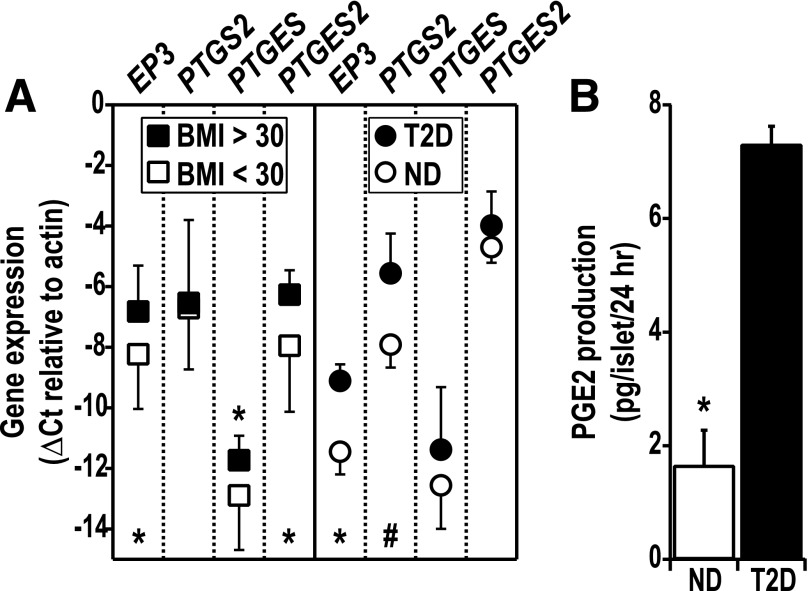
To determine the mechanisms underlying the increased L-798106 responsiveness of T2D human islets, we performed qRT PCR analyses on a series of cultured human islets that were classified based on a BMI <30 kg/m2 (nonobese) or ≥30 kg/m2 (obese). PTGER3, PTGES, and PTGES2 were significantly upregulated by obesity (Fig. 4A), while PTGS2 was unchanged. Next, we surveyed gene expression in human islets from nondiabetic and confirmed T2D cadaveric donors matched as closely as possible in donor demographics (Fig. 4A). PGTER3 mRNA expression was approximately sevenfold higher in islets from T2D donors (P < 0.05 [Fig. 4A]). There was also a trend toward increased PTGS2 mRNA expression in islets from T2D donors (approximately fivefold; P = 0.11). The expression of PTGES and PTGES2 was not significantly upregulated in diabetic human islets, although the low difference in cycles to threshhold between PTGES2 and β-actin (ΔCt) of PTGES2 is consistent with a high constitutive expression (−4.7 ± 0.3 and −4.0 ± 0.6 vs. β-actin for nondiabetic and T2D, respectively). Similar to islets from diabetic mice, islets isolated from T2D humans exhibited significantly increased PGE2 production in vitro (Fig. 4B).
FIG. 4.
Human islet PTGER3 and PGE2 synthetic enzyme expression is positively associated with donor obesity and T2D status, correlating with increased PGE2 production from confirmed T2D human islets. A: Cultured islets from human cadaveric donors were grouped as nonobese (BMI <30 kg/m2, n = 13) or obese (BMI ≥30 kg/m2, n = 12) (left panel) or confirmed T2D (n = 3) vs. nondiabetic (ND) (n = 3) (right panel). Confirmed T2D donors were matched as closely as possible with nondiabetic donors for sex, race, age, and BMI (all female; both groups with Caucasian and African American donors; mean ± SD age 46.3 ± 2.1 years (nondiabetic) vs. 54.3 ± 5.7 years (T2D; P = 0.32) and BMI 28.1 ± 7.3 kg/m2 (nondiabetic) vs. 25.4 ± 2.1 kg/m2 (T2D; P = 0.57). Islet cDNA was subjected to qRT PCR analysis for PTGER3, PTGS2, PTGES, and PTGES2 expression. Expression data were compared by unpaired t test (#P = 0.11; *P < 0.05). B: PGE2 production was measured from islets obtained from nondiabetic or confirmed T2D human donors. Islets were cultured for 24 h (hr), and the PGE2 secreted into the medium was normalized to the total number of islets. Data were compared by unpaired t test (n = 3). *P < 0.05.
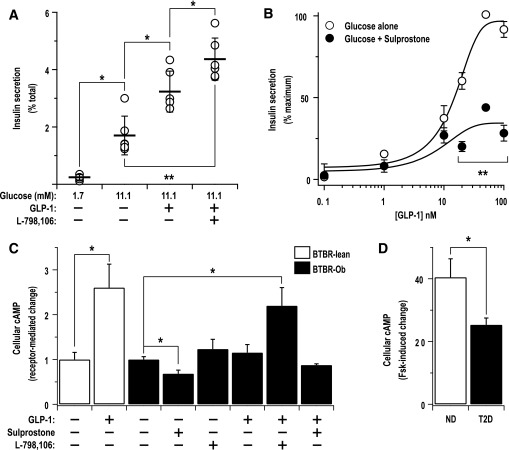
Given that the EP3- and GLP-1–mediated pathways have opposing effects on cellular cAMP production, we asked whether the modulation of EP3 signaling affects GLP-1 function in islets from diabetic BTBR mice. First, we determined whether the increased EP3 activity with diabetes suppresses the stimulatory effect of GLP-1 on GSIS by examining GLP-1 potentiation of GSIS in the presence or absence of L-798106. Interestingly, L-798106 significantly augmented the GLP-1 effect (Fig. 5A). Next, we performed a GLP-1 dose response experiment in the presence or absence of sulprostone, a specific EP3 agonist. Sulprostone blunted the maximal effect of GLP-1, while having little effect on the half-maximal effective concentration (EC50), indicating a noncompetitive antagonism of the GLP-1–dependent pathway (Fig. 5B). To confirm that the impact of EP3 receptor activation on GLP-1 receptor signaling occurs through modulation of cAMP levels, we determined the effect of GLP-1, sulprostone, or combinations thereof on intracellular cAMP levels in islets from 10-week-old lean or obese BTBR mice. Lean islets were used to confirm expected impact of various GLP-1 agonists on cAMP production; 50 nmol/L native GLP-1 increased cAMP production by 160% (Fig. 5C) and was therefore chosen for the rest of the study for physiological relevance and to maintain consistency between our GSIS studies. In comparison with BTBR lean islets, GLP-1 had a weaker, but still stimulatory, effect on cAMP production in diabetic BTBR-Ob islets (17%; P = 0.07). L-798106 also had a stimulatory effect on BTBR-Ob islet cAMP production (25%; P = 0.05). Combining L-798106 and GLP-1, though, significantly increased BTBR-Ob islet cAMP production (118% increase). Furthermore, sulprostone elicited a 30% decrease in cAMP production in BTBR-Ob islets, and combining GLP-1 and sulprostone completely blocked the stimulatory effect of GLP-1 (24.1% decrease; P = 0.24). To confirm the existence of a constitutive block on cAMP production in islets from diabetic BTBR mice, we performed cAMP production assays by incubating islets with 10 μmol/L forskolin, which strongly activates cAMP production, and recorded the change in cAMP levels from baseline. Islets from diabetic BTBR mice exhibited a 37% decrease in the ability of forskolin to promote cAMP production, suggestive of a blockade in the cAMP production pathway that is consistent with the proposed effects of increased EP3 signaling (Fig. 5D).
FIG. 5.
An EP3 antagonist augments GLP-1–potentiated GSIS and cAMP production from diabetic mouse islets, while an EP3 agonist acts as a noncompetitive inhibitor of GLP-1 signaling. A: Insulin secretion assays were performed with islets isolated from diabetic BTBR mice in the presence or absence of GLP-1 (50 nmol/L) with or without L-798106 (20 μmol/L). Data were compared by paired t test (n = 6; *P < 0.05; **P < 10−4). B: Insulin secretion assays were performed with 11.1 mmol/L glucose and various doses of GLP-1 in the presence or absence of 1 nmol/L sulprostone, an EP3-specific agonist. Each experimental dataset was normalized to the effect of glucose alone with or without sulprostone as 0% and the top plateau for the GLP-1 dose response as 100%. The combined experimental datasets were fit to a sigmoid dose response equation. The EC50s of the two curves are similar (15 nmol/L for glucose vs. 10 nmol/L for glucose plus sulprostone) (n = 3; **P < 10−4). C: cAMP production was measured in the presence of 11.1 mmol/L glucose with and without sulprostone (10 nmol/L), GLP-1 (50 nmol/L), L-798106 (10 μmol/L), or a combination of GLP-1 and sulprostone or L-798106. Only the combination of GLP-1 and L-798106 significantly increased cAMP levels in diabetic BTBR islets. Data were compared by paired t test (n = 3; *P < 0.05). D: Forskolin (Fsk)-stimulated cAMP production assays were performed in islets isolated from lean or diabetic BTBR mice, indicating a reduced intrinsic ability of BTBR-Ob islets to stimulate cAMP production. Data were compared by paired t test (n = 3; *P < 0.05).
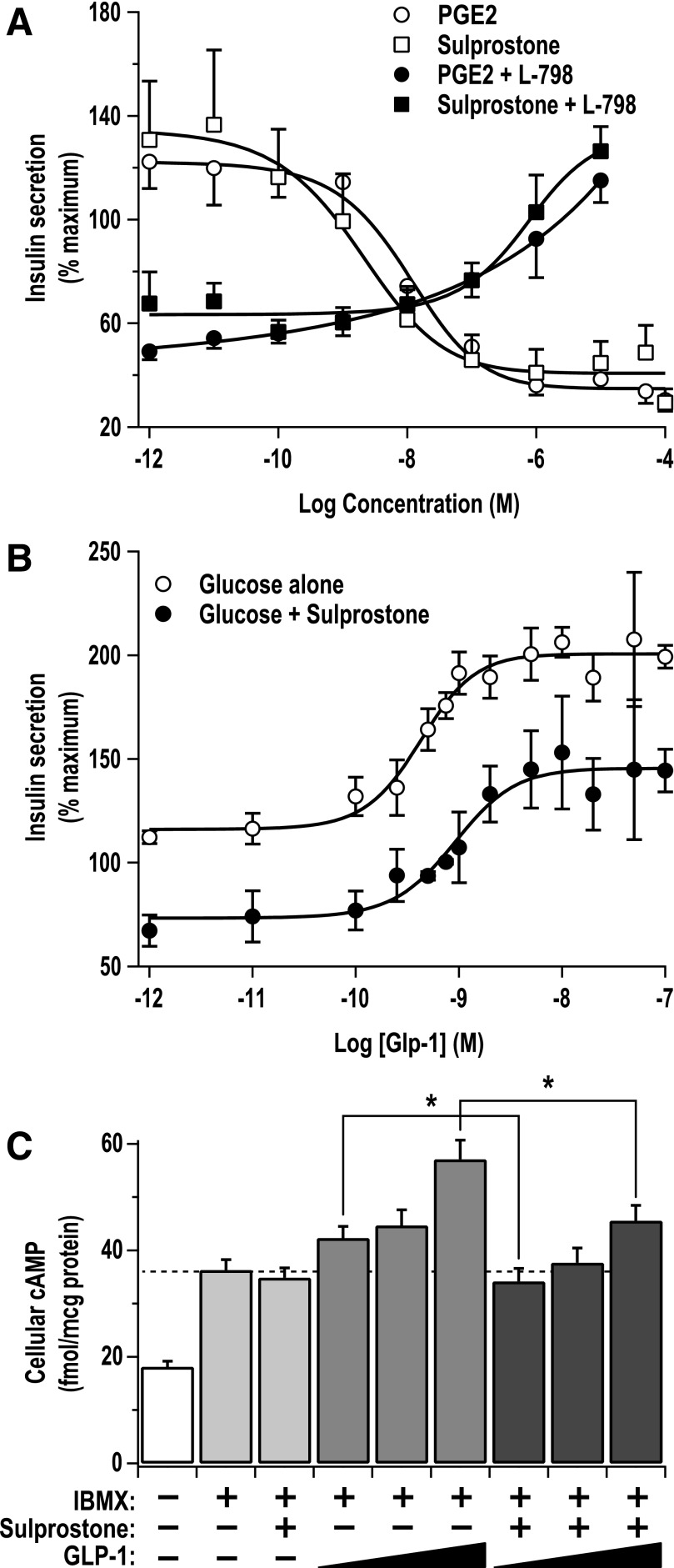
To further characterize the pharmacology of the EP3 and GLP-1 receptors on cAMP production and downstream GSIS, we used the Ins-1 (832/3) cell model, a highly glucose-responsive insulinoma cell line that is also quite responsive to cAMP modulation (18,19). Furthermore, EP3 is highly expressed in the Ins-1 (832/3) cell line, as determined by qRT PCR (Supplementary Fig. 2A and B). EP3 receptor expression was confirmed by functional assays with sulprostone and PGE2, the endogenous, nonselective agonist of the E prostanoid receptor family (20). Both sulprostone and PGE2 elicited a dose-dependent decrease in Ins-1 (832/3) cell GSIS, and these curves nearly overlaid one another, indicating a primacy of the EP3 receptor in the Ins-1 (832/3) line (Fig. 6A). Furthermore, the impact of a subsaturating dose of PGE2 (50 nmol/L) or sulprostone (10 nmol/L), chosen to be close to the EC50 for these agonists, could be completely competed away by increasing concentrations of L-798106 (Fig. 6A), consistent with the effect of these agonists being mediated specifically by the EP3 receptor.
FIG. 6.
EP3 signaling in Ins-1 (832/3) cells noncompetitively inhibits GLP-1 receptor signaling. A: Insulin secretion assays were performed in Ins-1 (832/3) cells in the presence or absence of various concentrations of the nonselective EP receptor agonist, PGE2, or the specific EP3 agonist, sulprostone. Concentrations near the EC50 for both agonists (PGE2 = 50 nmol/L and sulprostone = 10 nmol/L) were chosen to test the ability of L-798106 (L-798) to compete their effects. Data were compared by unpaired t test (N = 4–7; *P < 0.05). B: Increasing doses of GLP-1 were incubated with Ins-1 (832/3) cells with and without the addition of 10 nmol/L sulprostone, reducing the maximal effect of GLP-1 (N = 4; *P < 0.05). C: cAMP production was measured in the presence of 11.1 mmol/L glucose with and without the addition of isobutylmethylxanthine (IBMX) (200 μmol/L), sulprostone (100 pmol/L to 10 nmol/L), GLP-1 (100 pmol/L to 10 nmol/L), or a combination of GLP-1 and 1 nmol/L sulprostone. The addition of sulprostone blunted the effect of GLP-1 at every concentration tested, consistent with its impact on GSIS in B. Data were compared by paired t test (N = 4; *P < 0.05).
Next, we explored the impact of EP3 signaling on the GLP-1 receptor in the Ins-1 (832/3) cell line. Regardless of the presence of sulprostone, GLP-1 elicited a dose-dependent increase in GSIS, but its maximal effect was significantly blunted in the presence of 10 nmol/L sulprostone, while the EC50 was essentially unchanged, indicating a noncompetitive antagonism (Fig. 6B). In comparison, the effect of exendin 9-39, an inverse agonist of the GLP-1 receptor (21), on GLP-1 potentiation of GSIS followed a traditional competitive inhibition, where the EC50 was right shifted and the maximal effect plateaued at nearly the same value (Supplementary Fig. 2D). cAMP production assays were performed to confirm the mechanism of EP3 and GLP-1 receptor signaling in the Ins-1 (832/3) cell line. GLP-1 elicited a dose-dependent increase in cAMP production, while combining GLP-1 with a subsaturating dose of sulprostone blunted the ability of GLP-1 to elicit increases in cAMP production at every dose tested (19, 15, and 20% decrement at 10 pmol/L, 1 nmol/L, and 10 nmol/L GLP-1, respectively [Fig. 6C]).
DISCUSSION
Endogenous prostanoids such as PGE1 and PGE2 have long been known as inhibitors of insulin secretion (22). The prostaglandin-endoperoxidase synthase enzymes (PTGS1 and PTGS2, i.e., COX-1 and COX-2) catalyze the committed step in the PGE2 synthetic pathway, generating PGH2 from arachidonic acid. PTGS1 is constitutively expressed, whereas the expression of PTGS2 in many cells, including pancreatic islets, is induced by numerous stimuli (23,24). It may be the relatively high level of unstimulated PTGS2 expression that distinguishes pancreatic islet cells from most other cell types (25). PTGS2 has previously been suggested as a potential therapeutic target for T2D (24,26,27).
Human islets treated with high glucose or ligands of the advanced glycation end product–specific receptor show a 2.5-fold or 6.0-fold increase in PTGS2 expression, respectively (28). The inflammatory cytokine interleukin (IL)-1β also stimulates PTGS2 expression (29). Interestingly, Tran, Gleason, and Robertson demonstrated that IL-1β simultaneously increased Ptgs2 and EP3 mRNA expression in rat islets by an NF-kB–dependent mechanism—an effect that was blocked by sodium salicylate (29). Recently, Parazzoli and colleagues confirmed that PTGES2, and not PTGES, mediated the effects of IL-1β on promoting PGE2 synthesis in rat, mouse, and human islets, even though PTGES expression was induced by IL-1β (30). This fits well with our qRT PCR data from mouse and human islets (Figs. 2 and 3), in which we saw significant increases in PTGS2 mRNA with diabetes without a corresponding change in PTGES2 expression. Thus, even though PTGES expression is upregulated in our diabetic islets, it may not be playing a role in their increased PGE2 production.
While the mechanisms that regulate cellular PGE2 production are becoming understood, it is unclear whether PGE2 release from cells occurs simply as a result of increased PGE2 production or whether it is a regulated secretion. In human umbilical vein endothelial cells, PGE2 synthesis and PGE2 release were separated by disruption of the actin cytoskeleton; PTGS2 was required for increased PGE2 synthesis, but PGE2 release was significantly augmented by treatment with a microfilament disruptor (31). In the kidney tubule, flow shear stress can induce an MAPK-dependent signaling cascade that induces expression of cytosolic phospholipase A2 (cPLA2), which cleaves arachidonic acid from membrane phospholipids. Arachidonic acid is the substrate for PTGS2. Interestingly, flow shear stress itself appears to promote PGE2 release, independent of MAPK-signaling, where it acts in an autocrine/paracrine manner to regulate cation transport (32).
We propose that the elevated production and secretion of PGE2, coupled with increased islet EP3 expression, raises the possibility of an autocrine or a paracrine EP3-mediated signaling pathway in diabetic islets that negatively regulates insulin secretion. Indeed, EP3 appears to be the primary E prostanoid receptor isoform expressed in a β-cell–derived line (Supplementary Fig. 2) and mediates the effects of PGE2 on insulin secretion (Fig. 6). Even so, we cannot exclude the possibility that either PGE2 is secreted from non-β-cells or EP3 is expressed in other cell types of the islet. We profiled the expression of the four E prostanoid receptor genes and the synthetic enzymes, Ptgs2, Ptges, and Ptges2, in a mouse β-cell–derived line, Min6, and α-cell–derived line, αTC1, and compared gene expression with that observed in primary mouse islets. The pattern of EP receptor and PGE2 synthetic enzyme expression is similar in all three cell types (Supplementary Fig. 3). These results suggest that our phenotype may not necessarily be a β-cell–specific phenomenon and that it is possible that PGE2 generated from or acting on the α-cell indirectly regulates β-cell function. Glucagon can stimulate insulin secretion through a Gs-coupled receptor (33), and it is possible that if EP3 negatively regulates glucagon secretion from the α-cell, this could at least partially explain the decrement in cAMP production and insulin secretion in diabetic β-cells.
PGE2-mediated receptor internalization and desensitization have long been known as an important mode of regulation of PGE2 signaling (34–37). We believe that receptor desensitization is likely not playing a major role in the phenotype of L-798106 responsiveness of the BTBR-Ob islet. First, of EP3-α and EP3-β, only EP3-α is susceptible to receptor desensitization (38). Both EP3-α and EP3-β mRNA expression are increased >100-fold in BTBR-Ob islets; thus, even if the increased PGE2 production from diabetic islets is enough to desensitize EP3-α, EP3-β will still be available at the plasma membrane to signal to downstream effectors. EP3-γ appears to be almost completely agonist independent in inhibiting cAMP accumulation (39) and is also the least highly upregulated in the diabetic BTBR-Ob islets. Therefore, we propose that the effects of L-798106 in the diabetic mouse islet are mediated primarily through the desensitization-insensitive EP3-β. The human EP3 receptor subfamily is more diverse, and at least three of the human EP3 receptor isoforms have been shown to be capable of being desensitized, albeit two only transiently (37). As L-798106 still promotes insulin secretion in diabetic human islets, we anticipate that the receptor isoforms responsible for mediating PGE2 effects in diabetic human islets are not sensitive to desensitization either. Direct confirmation of these hypotheses awaits the development of splice-variant specific reagents.
Our studies suggest that EP3 receptor activation antagonizes GLP-1 receptor signaling via a negative effect on cellular cAMP production. In diabetic BTBR-Ob mouse islets as well as Ins-1 cells, we see a good correlation between the impact of sulprostone on GLP-1–mediated cAMP accumulation and its effect on the ability of GLP-1 to potentiate GSIS (Figs. 5 and 6). EP3 regulation of Gs-coupled receptor-mediated cAMP accumulation has previously been shown to impact glucagon-stimulated cAMP production in rat outer medullary collecting duct cells (40) and hepatocytes (41). This does not exclude other signaling pathways from being involved in an interaction between EP3 and GLP-1 signaling. In rat vascular smooth muscle cells, PGE2 signals through EP3 to induce a calcium-independent contractile pathway including novel protein kinase C isozymes and Rho kinase (ROCK), leading to increased vascular contraction (42). In addition, mouse EP3-α, -β, and -γ can all act through a RhoA-dependent mechanism to block metastatic potential in colon cancer cells by increasing cell-cell contact and reducing proliferation (43). These cAMP-independent functions of EP3 are mediated by coupling to G12 and not Gi (43). In addition to Rho signaling, EP3 has also been linked with increases in intracellular Ca2+ and generation of inositol triphosphate (37,44). An and coworkers (37) demonstrated that EP3-dependent elevation in Ca2+ and inositol triphosphate was pertussis-toxin independent, consistent with signaling through Gq. However, others have raised questions about the G-protein involved in this pathway (44). Members of the Gq and G12 families have both been implicated in mediating signaling pathways important in regulating insulin secretion (45).
Interestingly, loss of the EP3 receptor itself results in a phenotype of obesity, insulin resistance, and glucose intolerance (46), appearing in direct contradiction to our ascertainment of EP3 as a potential therapeutic target for T2D. In the case of EP3-null mice, the glucose intolerance appears secondarily to a significantly increased food intake and the development of obesity and insulin resistance, whereas Gαz-null mice (the specific Gi-protein that EP3 couples to in the islet) display no changes in food intake, adiposity, or insulin sensitivity compared with wild-type controls (12,47). Thus, the EP3-null mouse phenotype could be completely explained by EP3 signaling in the hypothalamus regulating feeding behavior, the targeting of which could be avoided by a drug that did not pass the blood-brain barrier.
GPCRs are the largest protein family in the human genome and are the target of 30–40% of current pharmaceutical molecules. Six different T2D drugs target the Gs-coupled GLP-1 receptor (2). Here, we show that an agonist of the EP3 receptor could be considered a noncompetitive antagonist of the GLP-1 receptor. These results offer a potential explanation for why GLP-1–based treatments are not effective in all T2D patients. Taken together, our results demonstrate that EP3 may be a useful target for T2D therapeutics that would avoid altering systemic PGE2 synthesis and promote better GLP-1 function.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by JDRF Grant 17-2011-608, National Institutes of Health Grant DK080845, and a UW Institute for Clinical and Translational Research Pilot Award to M.E.K. and by National Institutes of Health grants DK066369 and DK58037 to A.D.A.
A.D.A is a member of Pfizer’s Cardiovascular and Metabolic Disease Therapeutic Area Scientific Advisory Board. No other potential conflicts of interest relevant to this article were reported.
M.E.K. contributed to the conception and design of the study, collected data and contributed to its analysis and interpretation, wrote the original draft of the manuscript, reviewed the penultimate draft of the manuscript, and read and approved the final version of the manuscript as submitted. M.P.K. contributed to the conception and design of the study, contributed to the interpretation of data, reviewed and revised the original version of the manuscript, reviewed the penultimate draft of the manuscript, and read and approved the final version of the manuscript as submitted. M.R.R. and J.C.N. collected data and contributed to its analysis and interpretation, reviewed the penultimate draft of the manuscript, and read and approved the final version of the manuscript as submitted. R.L.P., N.A.T., and H.K.B. collected data and contributed to its analysis, reviewed the penultimate draft of the manuscript, and read and approved the final version of the manuscript as submitted. A.D.A. contributed to the conception and design of the study, reviewed and revised the original draft of the manuscript, reviewed the penultimate draft of the manuscript, and read and approved the final version of the manuscript as submitted. M.E.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the Keystone Advances in Islet Biology Meeting, Monterrey, California, 25–30 March 2012, and at the Midwest Islet Club Meeting, Pittsburgh, Pennsylvania, 23–25 May 2012.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0769/-/DC1.
REFERENCES
- 1.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 2007;113:546–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blonde L, Montanya E. Comparison of liraglutide versus other incretin-related anti-hyperglycaemic agents. Diabetes Obes Metab 2012;14(Suppl. 2):20–32 [DOI] [PubMed] [Google Scholar]
- 3.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, Sitagliptin Study 021 Group Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006;29:2632–2637 [DOI] [PubMed] [Google Scholar]
- 4.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H, Sitagliptin Study 023 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006;49:2564–2571 [DOI] [PubMed] [Google Scholar]
- 5.Collet-Gaudillat C, Petit-Aubert G, Desforges-Bullet V, Beressi JP. Exenatide efficacy in unselected patients: comparison with clinical trials. J Diabetes Mellitus 2012;2:118–121 [Google Scholar]
- 6.Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocr Rev 2007;28:48–83 [DOI] [PubMed] [Google Scholar]
- 7.Keller MP, Choi Y, Wang P, et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 2008;18:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negishi M, Sugimoto Y, Namba T, Irie A, Narumiya S, Ichikawa A. Signal transductions of three isoforms of mouse prostaglandin E receptor EP3 subtype. Adv Prostaglandin Thromboxane Leukot Res 1995;23:255–257 [PubMed] [Google Scholar]
- 9.Rabaglia ME, Gray-Keller MP, Frey BL, Shortreed MR, Smith LM, Attie AD. Alpha-Ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice. Am J Physiol Endocrinol Metab 2005;289:E218–E224 [DOI] [PubMed] [Google Scholar]
- 10.Doiron B, Cuif MH, Chen R, Kahn A. Transcriptional glucose signaling through the glucose response element is mediated by the pentose phosphate pathway. J Biol Chem 1996;271:5321–5324 [DOI] [PubMed] [Google Scholar]
- 11.Bhatnagar S, Oler AT, Rabaglia ME, et al. Positional cloning of a type 2 diabetes quantitative trait locus; tomosyn-2, a negative regulator of insulin secretion. PLoS Genet 2011;7:e1002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimple ME, Joseph JW, Bailey CL, et al. Galphaz negatively regulates insulin secretion and glucose clearance. J Biol Chem 2008;283:4560–4567 [DOI] [PubMed] [Google Scholar]
- 13.Davis DB, Lavine JA, Suhonen JI, et al. FoxM1 is up-regulated by obesity and stimulates beta-cell proliferation. Mol Endocrinol 2010;24:1822–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugimoto Y, Namba T, Honda A, et al. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem 1992;267:6463–6466 [PubMed] [Google Scholar]
- 15.Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta 1995;1259:109–119 [DOI] [PubMed] [Google Scholar]
- 16.Belley M, Chan CC, Gareau Y, et al. Comparison between two classes of selective EP(3) antagonists and their biological activities. Bioorg Med Chem Lett 2006;16:5639–5642 [DOI] [PubMed] [Google Scholar]
- 17.Belley M, Gallant M, Roy B, et al. Structure-activity relationship studies on ortho-substituted cinnamic acids, a new class of selective EP(3) antagonists. Bioorg Med Chem Lett 2005;15:527–530 [DOI] [PubMed] [Google Scholar]
- 18.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000;49:424–430 [DOI] [PubMed] [Google Scholar]
- 19.Maida A, Lovshin JA, Baggio LL, Drucker DJ. The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances beta-cell function but does not inhibit gastric emptying in mice. Endocrinology 2008;149:5670–5678 [DOI] [PubMed] [Google Scholar]
- 20.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol 1997;122:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serre V, Dolci W, Schaerer E, et al. Exendin-(9-39) is an inverse agonist of the murine glucagon-like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3′,5′-monophosphate levels and beta-cell glucose competence. Endocrinology 1998;139:4448–4454 [DOI] [PubMed] [Google Scholar]
- 22.Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol 1996;271:C1781–C1799 [DOI] [PubMed] [Google Scholar]
- 23.Persaud SJ, Burns CJ, Belin VD, Jones PM. Glucose-induced regulation of COX-2 expression in human islets of Langerhans. Diabetes 2004;53(Suppl. 1):S190–S192 [DOI] [PubMed] [Google Scholar]
- 24.Persaud SJ, Muller D, Belin VD, et al. The role of arachidonic acid and its metabolites in insulin secretion from human islets of langerhans. Diabetes 2007;56:197–203 [DOI] [PubMed] [Google Scholar]
- 25.Robertson RP. Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes 1998;47:1379–1383 [DOI] [PubMed] [Google Scholar]
- 26.Robertson RP, Chen M, McRae JR, Metz SA. Improvement of insulin secretion in diabetics by a prostaglandin synthesis inhibitor. Adv Exp Med Biol 1979;119:227–231 [DOI] [PubMed] [Google Scholar]
- 27.Robertson RP. Prostaglandins, glucose homeostasis, and diabetes mellitus. Annu Rev Med 1983;34:1–12 [DOI] [PubMed] [Google Scholar]
- 28.Shanmugam N, Todorov IT, Nair I, Omori K, Reddy MA, Natarajan R. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (AGER), and in islets from diabetic mice. Diabetologia 2006;49:100–107 [DOI] [PubMed] [Google Scholar]
- 29.Tran PO, Gleason CE, Robertson RP. Inhibition of interleukin-1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes 2002;51:1772–1778 [DOI] [PubMed] [Google Scholar]
- 30.Parazzoli S, Harmon JS, Vallerie SN, Zhang T, Zhou H, Robertson RP. Cyclooxygenase-2, not microsomal prostaglandin E synthase-1, is the mechanism for interleukin-1β-induced prostaglandin E2 production and inhibition of insulin secretion in pancreatic islets. J Biol Chem 2012;287:32246–32253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawyer SJ, Norvell SM, Ponik SM, Pavalko FM. Regulation of PGE(2) and PGI(2) release from human umbilical vein endothelial cells by actin cytoskeleton. Am J Physiol Cell Physiol 2001;281:C1038–C1045 [DOI] [PubMed] [Google Scholar]
- 32.Flores D, Liu Y, Liu W, Satlin LM, Rohatgi R. Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct. Am J Physiol Renal Physiol 2012;303:F632–F638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai K, Yokota C, Ohashi S, Watanabe Y, Yamashita K. Evidence that glucagon stimulates insulin secretion through its own receptor in rats. Diabetologia 1995;38:274–276 [DOI] [PubMed] [Google Scholar]
- 34.Robertson RP, Little SA. Down-regulation of prostaglandin E receptors and homologous desensitization of isolated adipocytes. Endocrinology 1983;113:1732–1738 [DOI] [PubMed] [Google Scholar]
- 35.Shamma MS, Fernandez-Botran R, Suzuki T. PGE2-induced desensitization of adenylate cyclase of a murine macrophage-like cell line (P388D1). Prostaglandins 1988;36:329–341 [DOI] [PubMed] [Google Scholar]
- 36.Coffey RG, Alberts VA, Weakland LL. Prostaglandin-dependent desensitization of human monocyte cAMP responses. J Leukoc Biol 1990;48:557–564 [DOI] [PubMed] [Google Scholar]
- 37.An S, Yang J, So SW, Zeng L, Goetzl EJ. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular calcium and cAMP signals. Biochemistry 1994;33:14496–14502 [DOI] [PubMed] [Google Scholar]
- 38.Negishi M, Sugimoto Y, Irie A, Narumiya S, Ichikawa A. Two isoforms of prostaglandin E receptor EP3 subtype. Different COOH-terminal domains determine sensitivity to agonist-induced desensitization. J Biol Chem 1993;268:9517–9521 [PubMed] [Google Scholar]
- 39.Negishi M, Hasegawa H, Ichikawa A. Prostaglandin E receptor EP3gamma isoform, with mostly full constitutive Gi activity and agonist-dependent Gs activity. FEBS Lett 1996;386:165–168 [DOI] [PubMed] [Google Scholar]
- 40.Aarab L, Siaume-Perez S, Chabardès D. Cell-specific coupling of PGE2 to different transduction pathways in arginine vasopressin- and glucagon-sensitive segments of the rat renal tubule. Br J Pharmacol 1999;126:1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrity MJ, Reed MM, Brass EP. Coupling of hepatic prostaglandin receptors to adenylate cyclase through a pertussis toxin sensitive guanine nucleotide regulatory protein. J Pharmacol Exp Ther 1989;248:979–983 [PubMed] [Google Scholar]
- 42.Kobayashi K, Murata T, Hori M, Ozaki H. Prostaglandin E2-prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur J Pharmacol 2011;660:375–380 [DOI] [PubMed] [Google Scholar]
- 43.Macias-Perez IM, Zent R, Carmosino M, Breyer MD, Breyer RM, Pozzi A. Mouse EP3 alpha, beta, and gamma receptor variants reduce tumor cell proliferation and tumorigenesis in vivo. J Biol Chem 2008;283:12538–12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irie A, Segi E, Sugimoto Y, Ichikawa A, Negishi M. Mouse prostaglandin E receptor EP3 subtype mediates calcium signals via Gi in cDNA-transfected Chinese hamster ovary cells. Biochem Biophys Res Commun 1994;204:303–309 [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 2009;122:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Alavez M, Klein I, Brownell SE, et al. Night eating and obesity in the EP3R-deficient mouse. Proc Natl Acad Sci USA 2007;104:3009–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimple ME, Moss JB, Brar HK, et al. Deletion of GαZ protein protects against diet-induced glucose intolerance via expansion of β-cell mass. J Biol Chem 2012;287:20344–20355 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.