Abstract
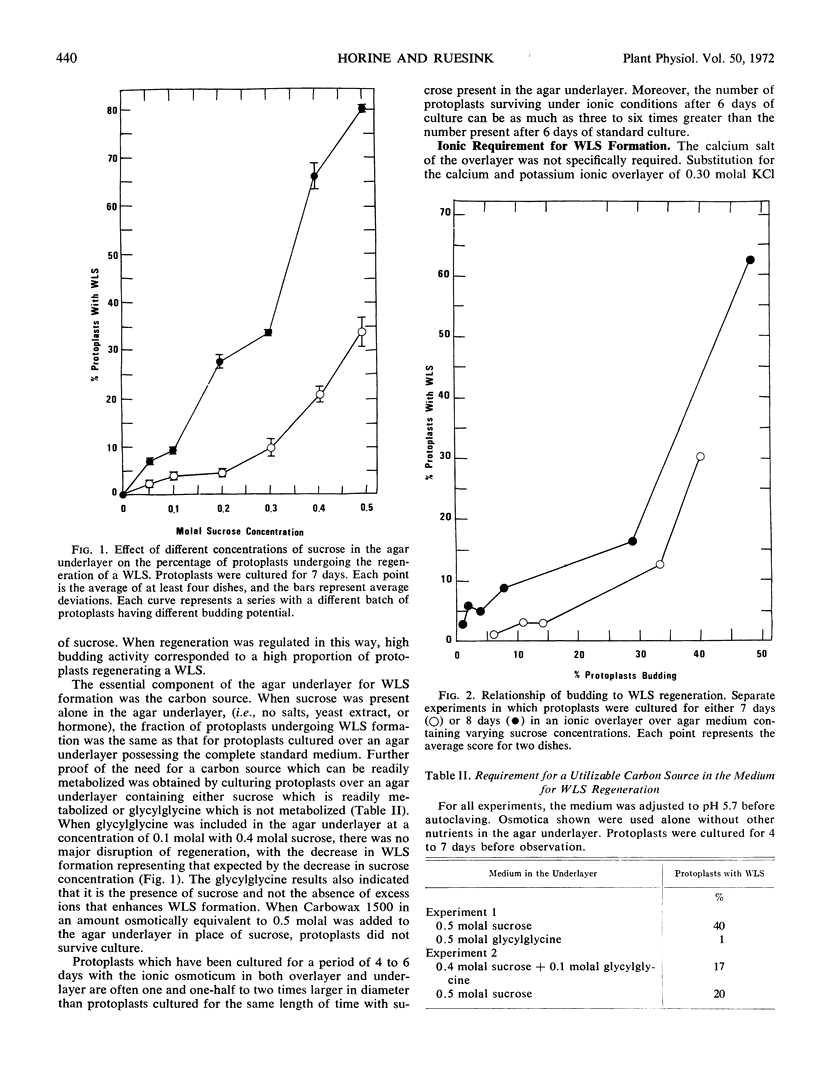
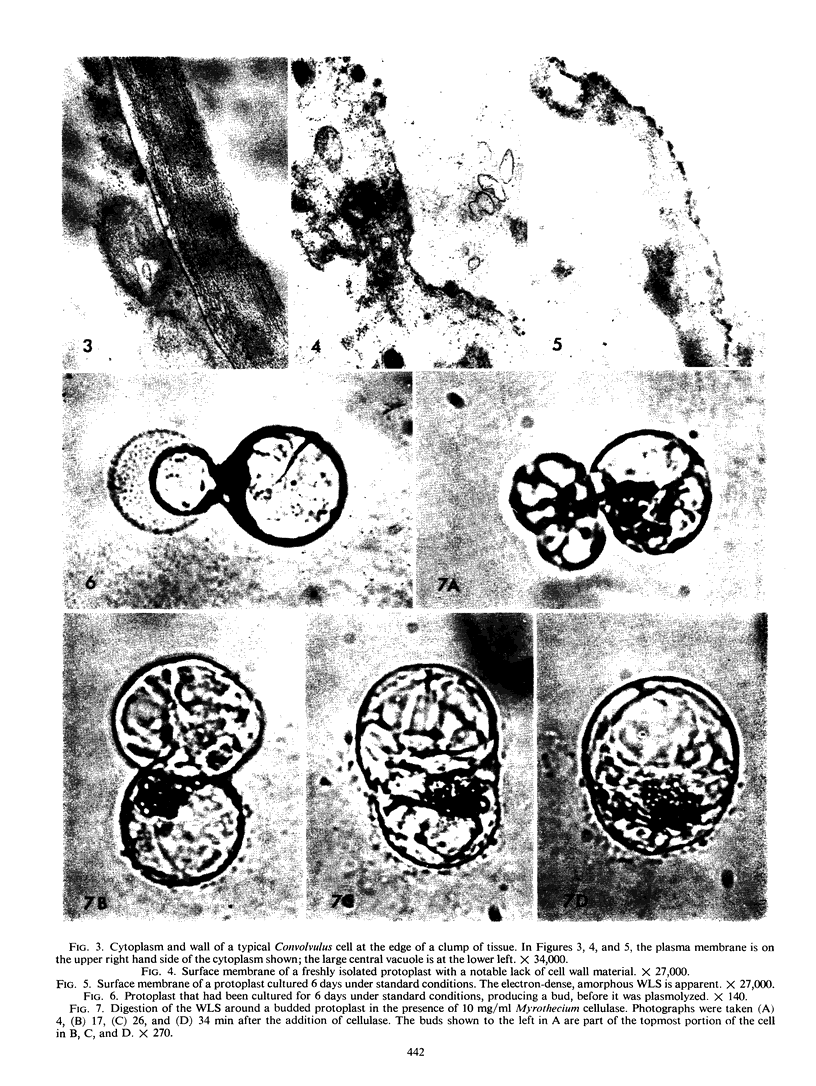
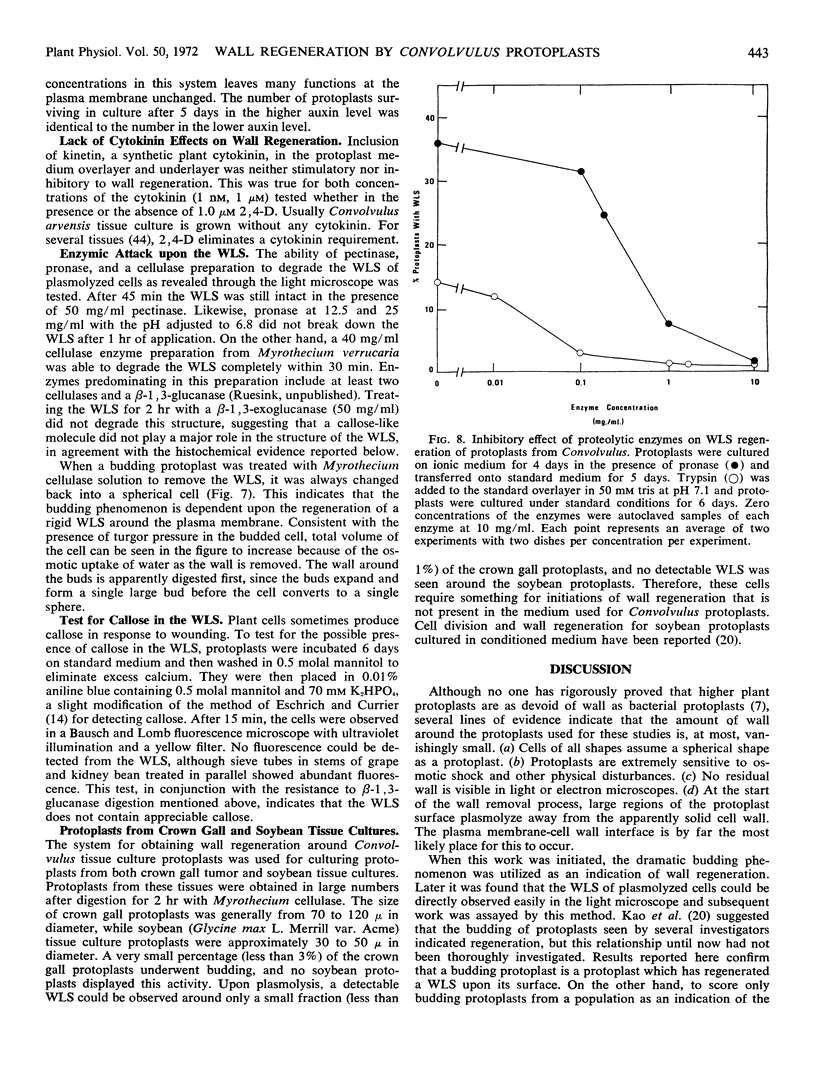
Protoplasts of Convolvulus arvensis L. tissue culture regenerated a wall-like structure within 3 days in culture. Although unusually electron dense and atypically amorphous in the electron microscope, this structure could be digested with Myrothecium cellulase but was resistant to protease, a Rohm and Haas pectinase, and a β-1, 3-exoglucanase just like the original wall. A cytochemical test for callose was negative. Wall regeneration required a readily metabolized external carbon source and was not inhibited by a high concentration of cycloheximide, puromycin, or actinomycin D. Protoplast budding was correlated with the wall regeneration, and the latter was related quantitatively to the sucrose concentration in the medium. Although a concentration of 1 μm 2,4-dichlorophenoxy acetic acid is used normally for both general culture of the tissue and for wall regeneration, concentrations of 0 and 0.1 mm, which are highly deleterious to growth, have no appreciable effect on the incidence of the wall-like structure regenerated around protoplasts. The ability of protoplasts to undergo cell wall regeneration was decreased when they were cultured in the presence of proteolytic enzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A., Ray P. M. Regulation by auxin of carbohydrate metabolism involved in cell wall synthesis by pea stem tissue. Plant Physiol. 1971 Apr;47(4):537–544. doi: 10.1104/pp.47.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHMANN B. J., BONNER D. M. Protoplasts from Neurospora crassa. J Bacteriol. 1959 Oct;78:550–556. doi: 10.1128/jb.78.4.550-556.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. W., Hassid W. Z. Xylan synthesis from uridine-diphosphate-d-xylose by particulate preparations from immature corncobs. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1586–1593. doi: 10.1073/pnas.56.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. B., Ray P. M. Direct and Indirect Effects of Auxin on Cell Wall Synthesis in Oat Coleoptile Tissue. Plant Physiol. 1965 Mar;40(2):345–352. doi: 10.1104/pp.40.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKING E. C. Action of growth substances, chelating agents and antibiotics on isolated root protoplasts. Nature. 1962 Mar 10;193:998–999. doi: 10.1038/193998a0. [DOI] [PubMed] [Google Scholar]
- Cocking E. C. Virus uptake, cell wall regeneration, and virus multiplication in isolated plant protoplasts. Int Rev Cytol. 1970;28:89–124. doi: 10.1016/s0074-7696(08)62541-3. [DOI] [PubMed] [Google Scholar]
- EDDY A. A., WILLIAMSON D. H. Formation of aberrant cell walls and of spores by the growing yeast protoplast. Nature. 1959 Apr 18;183(4668):1101–1104. doi: 10.1038/1831101a0. [DOI] [PubMed] [Google Scholar]
- Earle E. D., Torrey J. G. Colony Formation by Isolated Convolvulus Cells Plated on Defined Media. Plant Physiol. 1965 May;40(3):520–528. doi: 10.1104/pp.40.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M. Formation and regeneration of protoplasts in the mold Rhizopus nigricans. Folia Microbiol (Praha) 1968;13(3):231–234. doi: 10.1007/BF02871039. [DOI] [PubMed] [Google Scholar]
- Hassid W. Z. Biosynthesis of oligosaccharides and polysaccharides in plants. Science. 1969 Jul 11;165(3889):137–144. doi: 10.1126/science.165.3889.137. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Abbott J., Lash J., Holtzer S. THE LOSS OF PHENOTYPIC TRAITS BY DIFFERENTIATED CELLS IN VITRO, I. DEDIFFERENTIATION OF CARTILAGE CELLS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1533–1542. doi: 10.1073/pnas.46.12.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao K. N., Keller W. A., Miller R. A. Cell division in newly formed cells from protoplasts of soybean. Exp Cell Res. 1970 Oct;62(2):338–340. doi: 10.1016/0014-4827(70)90563-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Motoyoshi F. Protoplasts isolated from callus cells of maize endosperm. Exp Cell Res. 1971 Oct;68(2):452–456. doi: 10.1016/0014-4827(71)90173-x. [DOI] [PubMed] [Google Scholar]
- Necas O., Svoboda A., Kopecká M. The effect of cycloheximide (actidione) on cell wall synthesis in yeast protoplasts. Exp Cell Res. 1968 Oct;53(1):291–293. doi: 10.1016/0014-4827(68)90378-9. [DOI] [PubMed] [Google Scholar]
- PHILLIPS H. J., TERRYBERRY J. E. Counting actively metabolizing tissue cultured cells. Exp Cell Res. 1957 Oct;13(2):341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- Poste G. Tissue dissociation with proteolytic enzymes. Adsorption and activity of enzymes at the cell surface. Exp Cell Res. 1971 Apr;65(2):359–367. doi: 10.1016/0014-4827(71)90014-0. [DOI] [PubMed] [Google Scholar]
- Power J. B., Cummins S. E., Cocking E. C. Fusion of isolated plant protoplasts. Nature. 1970 Mar 14;225(5237):1016–1018. doi: 10.1038/2251016a0. [DOI] [PubMed] [Google Scholar]
- Ruesink A. W., Thimann K. V. Protoplasts from the Avena coleoptile. Proc Natl Acad Sci U S A. 1965 Jul;54(1):56–64. doi: 10.1073/pnas.54.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife J. F., Brohée H. A quantitative measurement of cell damage by means of 51Cr-binding. Exp Cell Res. 1967 Jun;46(3):612–615. doi: 10.1016/0014-4827(67)90391-6. [DOI] [PubMed] [Google Scholar]
- Svoboda A., Necas O. Mechanism of regeneration of yeast protoplasts. VI. An experimental blocking of regeneration of protoplasts. Folia Biol (Praha) 1968;14(5):390–397. [PubMed] [Google Scholar]
- Villemez C. L., Lin T. Y., Hassid W. Z. Biosynthesis of the polygalacturonic acid chain of pectin by a particulate enzyme preparation from Phaseolus aureus seedlings. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1626–1632. doi: 10.1073/pnas.54.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemez C. L., Swanson A. L., Hassid W. Z. Properties of a polygalacturonic acid-synthesizing enzyme system from Phaseolus aureus seedlings. Arch Biochem Biophys. 1966 Sep 26;116(1):446–452. doi: 10.1016/0003-9861(66)90051-8. [DOI] [PubMed] [Google Scholar]
- Werz G. Differenzierung und Zellwandibildung in isoliertem Cytoplasma aus Acetabularia. Protoplasma. 1968;65(3):349–357. doi: 10.1007/BF01682538. [DOI] [PubMed] [Google Scholar]
- Witham F. H. Effect of 2,4-dichlorophenoxyacetic Acid on the cytokinin requirement of soybean cotyledon and tobacco stem pith callus tissues. Plant Physiol. 1968 Sep;43(9):1455–1457. doi: 10.1104/pp.43.9.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]



