Abstract
Obesity-induced chronic low-grade inflammation originates from adipose tissue and is crucial for obesity-driven metabolic deterioration, including insulin resistance and type 2 diabetes. Chronic inflammation may be a consequence of a failure to actively resolve inflammation and could result from a lack of local specialized proresolving lipid mediators (SPMs), such as resolvins and protectins, which derive from the n-3 polyunsaturated fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). We assessed obesity-induced changes of n-3–derived SPMs in adipose tissue and the effects of dietary EPA/DHA thereon. Moreover, we treated obese mice with SPM precursors and investigated the effects on inflammation and metabolic dysregulation. Obesity significantly decreased DHA-derived 17-hydroxydocosahexaenoic acid (17-HDHA, resolvin D1 precursor) and protectin D1 (PD1) levels in murine adipose tissue. Dietary EPA/DHA treatment restored endogenous biosynthesis of n-3–derived lipid mediators in obesity while attenuating adipose tissue inflammation and improving insulin sensitivity. Notably, 17-HDHA treatment reduced adipose tissue expression of inflammatory cytokines, increased adiponectin expression, and improved glucose tolerance parallel to insulin sensitivity in obese mice. These findings indicate that impaired biosynthesis of certain SPM and SPM precursors, including 17-HDHA and PD1, contributes to adipose tissue inflammation in obesity and suggest 17-HDHA as a novel treatment option for obesity-associated complications.
Obesity is associated with a chronic low-grade inflammation that plays a key role in the development of insulin resistance, leading the way to type 2 diabetes and cardiovascular disease (1,2). Obesity-driven low-grade inflammation originates from the adipose tissue and is characterized by increased accumulation of macrophages and other inflammatory cells (3,4) and a shift from an anti-inflammatory M2-like (CD206+) to an inflammatory M1-like (CD11c+) macrophage phenotype that expresses inflammatory cytokines and contributes to insulin resistance (5–9). Alteration of the macrophage phenotype significantly contributes to adipose tissue inflammation and its metabolic consequences such as insulin resistance (10–12).
Secretion of inflammatory cytokines, such as monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and osteopontin (OPN), is increased in obesity-induced adipose tissue inflammation, whereas production of anti-inflammatory and insulin-sensitizing adiponectin is reduced (13,14). In addition to peptide mediators, adipose tissue produces considerable amounts of free fatty acids and fatty acid–derived bioactive lipid mediators with potent pro- and anti-inflammatory actions. Particularly, the recently described n-3 polyunsaturated fatty acid (PUFA)–derived lipid mediators resolvins and protectins are of major interest because they have been characterized as a novel genus of potent anti-inflammatory and proresolving lipid mediators that are produced during self-limited acute inflammation in inflammatory exudates and promote resolution (i.e., active termination of inflammation) (15,16). The biosynthesis of these locally acting specialized proresolving mediators (SPMs) is regulated by the availability of n-3 PUFA eicosapentaenoic acid (EPA; C20:5n-3) and docosahexaenoic acid (DHA; C22:6n-3) and the spatial and temporal control of specific lipoxygenase pathways (17–19). Briefly, in murine tissues, EPA-derived 18-hydroxyeicosapentaenoic acid (18-HEPE) and the DHA-derived leukocyte-type 12/15-lipoxygenase (12/15-LOX) products/intermediates 17-hydroxydocosahexaenoic acid (17-HDHA) and 17-H(peroxy)DHA interact with 5-LOX to generate the SPMs resolvin E1 (RvE1), resolvin D1 (RvD1), or PD1, respectively (Fig. 1A). Uncontrolled inflammation and failed resolution could be a critical component in the pathogenesis of many chronic inflammatory diseases, including cardiovascular diseases, cancer, and metabolic disorders (17,20).
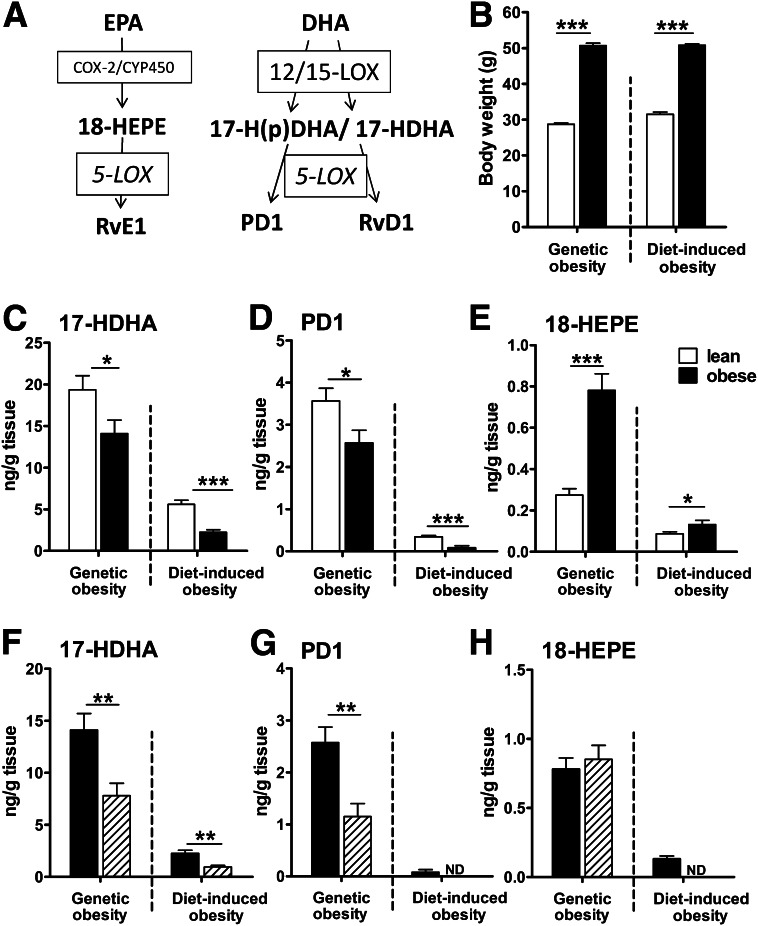
FIG. 1.
Obesity significantly reduces levels of the n-3 docosanoid lipid mediator 17-HDHA and PD1 in gonadal adipose tissue. (A) Simplified biosynthetic pathway of EPA- and DHA-derived SPMs and their precursors. (B) Mean body weight of lean (□) and obese mice (■; n = 10 animals per group). Genetically obese animals developed similar obesity compared with WT mice fed a high-fat (HF) diet for 18 weeks (diet-induced obesity). n-3 PUFA–derived 17-HDHA (C), PD1 (D), and 18-HEPE (E) were analyzed in murine gonadal adipose tissue of lean (n = 20 animals per group, pooled for n = 10 samples) and obese mice (n = 10 animals per group) in two different mouse models of obesity using solid-phase extraction and LC-MS/MS. Genetically obese db/db mice were compared with lean db/+ littermates, both fed a normal standard chow, and HF diet-induced obese WT mice were compared with lean WT mice on an LF diet. Lipid mediator concentration of 17-HDHA (F), PD1 (G), and 18-HEPE (H) in gonadal adipose tissue (■) was compared with subcutaneous adipose tissue (▨) of obese db/db and WT HF mice (n = 10 animals per group). All data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. COX-2, cyclooxygenase-2; CYP450, cytochrome P450 enzymes; ND, not detected.
Treatment with n-3 PUFA attenuates obesity-associated adipose tissue inflammation and insulin resistance, but underlying mechanisms remain elusive (21,22). Recent studies demonstrated that n-3 PUFA feeding of mice elevates adipose tissue levels of 17-HDHA, PD1, and RvD1 (22,23). Transgenic restoration of n-3 PUFA in fat-1 transgenic mice increased tissue levels of n-3 PUFA–derived SPMs and SPM precursors, such as PD1 and 17-HDHA (24–26), and protected against obesity-linked insulin resistance (26). Accordingly, the effect of obesity itself on SPM biosynthesis in adipose tissue is of particular interest. Because obesity-induced alterations of SPM biosynthesis in adipose tissue could provide a clue for obesity-driven chronic inflammation and a rationale for novel potential treatment options, we characterized n-3 PUFA–derived lipid mediator profiles in adipose tissue of genetically (db/db) and high-fat (HF) diet–induced obese mice and respective lean controls. On the basis of these results, we treated obese mice with the SPM precursor 17-HDHA. Here, we report an obesity-induced decrease in endogenous biosynthesis of the DHA-derived lipid mediators 17-HDHA and PD1 as an potential mechanism involved in chronic adipose tissue inflammation further leading to metabolic complications. Treatment of obese mice with 17-HDHA, SPM precursor and marker of the local tissue PD1 and RvD1 biosynthetic pathways, attenuated adipose tissue inflammation and improved glucose tolerance. Hence, we could identify 17-HDHA as a potential treatment for obesity-induced adipose tissue inflammation and associated metabolic complications.
RESEARCH DESIGN AND METHODS
Animals and dietary interventions.
Male C57BL/6J wild-type (WT) mice, male BKS.Cg-Dock7m+/+Leprdb/J (db/db) mice, and lean nondiabetic littermates (db/+) were purchased from Charles River Laboratories (Sulzfeld, Germany). At 8 weeks of age, WT mice were fed an HF diet (60% kcal from fat, D12492; Research Diets Inc., New Brunswick, NJ), a low-fat (LF) control diet (10% kcal from fat, D12450B; Research Diets Inc.) for 18 weeks, or a short-term HF diet for 4 or 14 days and compared with WT mice fed normal standard chow. For genetic obesity, db/db and db/+ mice fed normal chow were killed at 16 weeks of age. For dietary treatment with different fatty acid compositions, db/db and db/+ mice were fed for 6 weeks an LF control diet (3.2 kcal/g) or isocaloric HF diets (4.2 kcal/g) that included 40% kcal from lard oil (rich in saturated and monounsaturated fatty acids, HF/S), safflower oil (rich in n-6 PUFA, HF/6), or an HF/S diet with 30% (v/v) of lard oil being replaced by the n-3 PUFA concentrate EPAX6000TG (containing 290 mg EPA/g oil and 190 mg DHA/g oil; HF/S3 diet containing ∼3% EPA- and 2.3% DHA-derived calories) provided by EPAX AS (Aalesund, Norway), as described (21,27). Detailed analysis of fatty acid composition of diets was performed previously (27). Diets were purchased from Altromin (Germany).
Lipid mediator treatment.
After 17 weeks of the HF diet, WT mice were treated with DHA (4 µg/g body weight), 17S-HDHA (50 ng/g body weight), both purchased from Cayman Chemicals, or vehicle control (0.9% NaCl containing 3% delipidated fatty acid-free BSA and 2% ethanol) by intraperitoneal injection every 12 h for 8 days or continuous application with osmotic (Alzet) pumps (∼120) for 15 days. All mice were housed in a pathogen-free facility on a 12-h light/dark cycle with free access to food and water. Food intake and weight gain were monitored throughout the studies. Blood was drawn after a 3-h fast immediately before mice were killed. Tissues were collected and immediately snap frozen in liquid nitrogen. The study protocols were approved by the Austrian Federal Ministry for Science and Research and followed the guidelines on accommodations and care of animals formulated by the European Convention for Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes.
Metabolic measurements.
Triglyceride, free fatty acid, and cholesterol concentration was analyzed in plasma using an automated analyzer (Falcor 350, A. Menarini Diagnostics, Florence, Italy). Enzyme-linked immunosorbent assay kits were used to determine plasma insulin (Mercodia AB, Uppsala, Sweden), adiponectin, and C-reactive protein (both Alpco Diagnostics, Salem, NH). We calculated the homeostasis model assessment of insulin resistance (HOMA-IR) as an index for insulin resistance (28). Insulin sensitivity was assessed by insulin tolerance test after a 5-h fast. Briefly, an intraperitoneal injection of recombinant human insulin (Novorapid, Novo Nordisk A/S, Bagsvaerd, Denmark), at a dose of 2 and 0.75 units/kg body weight, was given to db/db and WT HF mice, respectively. Blood glucose concentrations were determined before and 30, 60, 90 and 120 min after insulin injection. The glucose tolerance test was performed after overnight fasting, and blood glucose was measured before and 15, 45, 75, 105 and 135 min after an intraperitoneal injection of 20% glucose (0.75 g/kg body weight).
Lipid mediator analysis of adipose tissue samples.
Lipid mediators were extracted from adipose tissue using solid phase extraction. From the lean groups, tissue of two animals was pooled for one sample; from obese animals, tissue samples were analyzed in duplicates. Briefly, 300 mg tissue was homogenized in methanol after deuterated prostaglandin E2 (PGE2-d4; Cayman Chemical, Ann Arbor, MI) was added as an internal standard. Cleared supernatants were acidified to pH 3.0, loaded onto Oasis-HLB Extraction Cartridges (Waters, Milford, MA), and eluted with 1% ethyl acetate in methanol. Extracted samples were analyzed by high-performance liquid chromatography–tandem mass spectrometry (LC-MS/MS) using a triple quadrupole mass spectrometer (API5000 AB SCIEX, USA/Canada) equipped with a reversed phase column (ACE3 C18-AR, Advanced Chromatography Technologies, Aberdeen, U.K.). MS analysis was conducted in electrospray negative ionization mode, and lipid mediators were identified by multiple reaction monitoring using the following transitions: 17-HDHA (343.3:245.2m/z), PD1 (359.3:136.1m/z), 18-HEPE (317.3:215.2m/z), RvE1 (349.3:195.1m/z), RvD1 (375.3:164.1m/z), and PGE2 (351.3:315.3m/z). For enantiomeric separation of 17(R/S)-HDHA, a Chiralpak AD-RH (Daicel, Germany) was used. Calibration curves, retention times, and multiple reaction monitoring parameters were established and optimized, as previously described (29), using synthetic standards (Cayman Chemical).
RT-PCR and gene expression analysis.
Tissue samples were homogenized in TRIzol reagent (Invitrogen), and RNA was isolated according to the manufacturer’s protocol. One microgram of total RNA was treated with DNase I and transcribed to cDNA using Superscript II and random hexamer primers (all Invitrogen), as described (30). Gene expression of F4/80 (Emr1, Mm00802530_m1), MCP-1 (Ccl2, Mm00441242_m1), TNF-α (Tnf, Mm00443258_m1), IL-6 (Il6, Mm00446190_m1), OPN (Spp1, Mm00436767_m1), GLUT-4 (Slc2a4, Mm00436615_m1), adiponectin (Adipoq, Mm00456425_m1), peroxisome proliferator–activated receptor (PPAR) γ (PPARg, Mm01184322_m1), PPARα (Ppara, Mm00440939_m1), nuclear factor (NF)-κB (Nfkb1, Mm00476361_m1), 12/15-LOX (Alox15, Mm01250458_m1), and 5-LOX (Alox5, Mm01182749_m1) was analyzed by quantitative real-time RT-PCR on an ABI Prism 7000 cycler using assays-on-demand kits (TaqMan Gene Expression Assay, Applied Biosystems) and normalized to UbiquitinC mRNA (Ubc, Mm01198158_m1).
Isolation of adipose tissue macrophages and flow cytometry analysis.
Stromal vascular cells of gonadal adipose tissue were isolated by collagenase digestion and centrifugation to remove adipocytes, as described (7). For flow cytometry analysis of M1-like and M2-like macrophage phenotypes, stromal vascular cells were stained for three-color immunofluorescence analysis by dye-labeled antibodies according to standard procedures using CD16/CD32 blocking antibodies (BD Biosciences), F4/80-PE, CD206(MR)-AlexaFluor488 (both AbDSerotec), and CD11c-APC (BD Biosciences).
Immunohistochemistry.
Adipose tissue was fixed with 4% paraformaldehyde and embedded in paraffin. After sections were dewaxed and rehydrated, immunohistochemical staining for MAC-2 (anti-MAC-2/galectin-3 antibody, Cedarlane Laboratories, Burlington, ON, Canada) was performed using the Vectastain ABC Kit (Vector Laboratories) and Sigma Fast 3,3′-diaminobenzidine (Sigma) as the substrate, according to the manufacturers’ recommendations. Sections were counterstained with hematoxylin. Samples were analyzed with Nikon Eclipse E800 light microscope, and digital images were captured with a DXM 1200 camera. Crown-like structure (CLS) density was obtained by counting the total number of CLS in each section compared with the total number of adipocytes (31).
Western blotting.
Lysates of gonadal adipose tissue were prepared as previously described (21). Briefly, gonadal adipose tissue was homogenized and lysed on ice for 30 min in Tris-buffered saline (pH 7.4) containing 1% Triton X-100 (Pierce) and phosphatase and protease inhibitors. The tissue extract was cleared from fat, nuclei, and debris by centrifugation. Identical amounts of protein were separated by SDS-PAGE and blotted onto nitrocellulose membranes and probed for IκBα (anti-IκBα rabbit antibody, 400001, Calbiochem) and β-tubulin (anti–β-tubulin mouse antibody, T4026, Sigma). Chemiluminescence was generated by a BM chemiluminescence substrate (Roche), and quantification of band intensities was performed using AlphaEase FC Software version 3.2.1.
Statistics.
Data are given as means ± SEM. Comparisons between lean and obese mice were assessed by unpaired two-tailed Student t test. Treatment effects within a genotype were analyzed with univariate ANOVA using the Dunnett t test for post hoc analysis. For correlation analysis, Spearman rank correlations were calculated. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
Genetic and diet-induced obesity decreases adipose tissue levels of the n-3 PUFA–derived lipid mediators 17-HDHA and PD1.
We first aimed to investigate the effect of obesity on n-3 PUFA–derived lipid mediator levels in murine adipose tissue. Therefore, gonadal adipose tissue from two different mouse models of obesity, genetic (db/db), and diet-induced obesity, was analyzed using LC-MS/MS. Mean body weight is presented in Fig. 1B. We identified significant levels of the n-3 PUFA lipid mediators 17-HDHA and PD1, which derive from DHA by 12/15-LOX activity, as well as the EPA-derived RvE1 precursor 18-HEPE in murine adipose tissue of lean and obese animals, whereas RvE1 and RvD1 levels were below the detection limit. Of note, obesity significantly reduced 17-HDHA and PD1 in gonadal adipose tissue of db/db mice compared with lean littermates (db/+) fed a normal standard chow, whereas 18-HEPE was significantly increased (Fig. 1C–E). Obesity-induced alterations of n-3 PUFA–derived lipid mediators were confirmed in WT mice fed an HF diet for 18 weeks compared with counterparts fed the LF diet. These findings indicate that obesity per se and HF-feeding leads to a deficiency in DHA-derived mediators of resolution in adipose tissue.
Levels of 17-HDHA, PD1, and partly 18-HEPE (in WT HF mice) were either significantly lower or even below the detection limit in subcutaneous adipose tissue compared to gonadal adipose tissue, indicating predominant production in the metabolically more relevant gonadal adipose tissue (Fig. 1F–H).
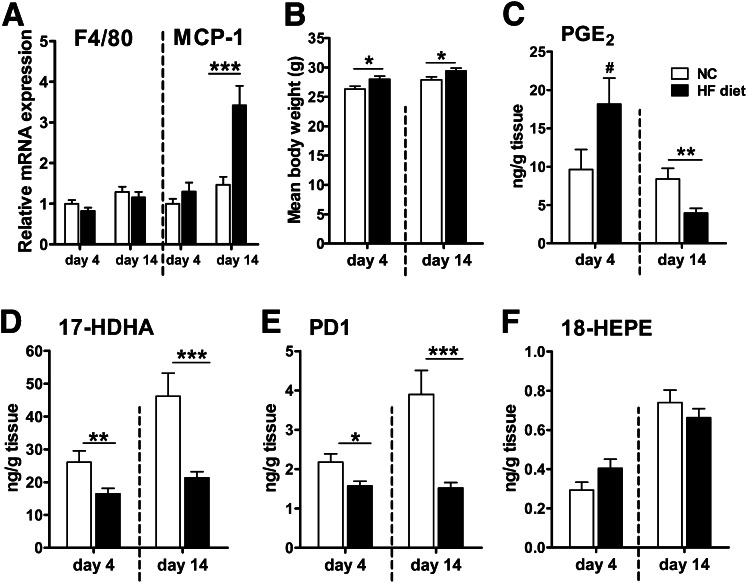
Adipose tissue levels of 17-HDHA and PD1 are already affected after 4 days of HF diet.
Considerable acute inflammatory alterations in adipose tissue may occur within a few days of HF diet treatment (32,33). Because induction of SPM biosynthesis during acute inflammation is critical for subsequent resolution, we examined if altered lipid mediator levels could be found after a short-term HF diet compared with normal chow for 4 and 14 days in WT mice when weight change was minimal and accumulation of macrophages had not yet occurred (Fig. 2A and B). Significantly lower concentrations of 17-HDHA and PD1 were detected in gonadal adipose tissue in animals fed the HF diet for 4 days than in animals fed normal chow, whereas 18-HEPE was not altered. In addition, PGE2 tended to be elevated in adipose tissue, indicating an acute inflammation after 4 days of the HF diet (Fig. 2C–F). Differences between HF- and normal chow–fed animals in adipose tissue 17-HDHA and PD1 levels were more pronounced after 14 days, when PGE2 levels were also decreased and the first inflammatory changes (increased MCP-1 expression, Fig. 2A and C) were identified. These findings show that changes in SPMs or their precursors belong to the earliest alterations in diet-induced inflammation, and hence, inefficient biosynthesis of PD1 and 17-HDHA as well as other SPMs might contribute to chronic low-grade inflammation in adipose tissue.
FIG. 2.
Adipose tissue reduction of 17-HDHA and PD1 represents one of the earliest alterations in diet-induced inflammation. WT mice were fed a normal standard chow (NC; □) or an HF diet (■) for 4 or 14 days. (A) mRNA expression of the genes for macrophage marker F4/80 (encoded by Emr1 gene) and MCP-1 (encoded by Ccl2) was determined in gonadal adipose tissue using quantitative real-time RT-PCR (n = 12 animals per group). (B) Mean body weight after the NC or HF diet for 4 and 14 days (n = 12–15 animals per group). (C–F) Lipid mediator levels were analyzed in gonadal adipose tissue. PGE2 was increased after 4 days but was significantly lower after 14 days of the HF diet. The HF diet decreased adipose tissue levels of 12/15-LOX–derived 17-HDHA and PD1 after 4 days and demonstrated an even stronger impact after 14 days (n = 12–15 animals per group). All data are mean ± SEM. #P = 0.088; *P < 0.05; **P < 0.01; ***P < 0.001.
To assess whether the obesity-induced decrease of 17-HDHA and PD1 could be due to altered relevant enzyme expression, we examined expression of the genes for 12/15-LOX (Alox15) and 5-LOX (Alox5) in adipose tissue. We detected considerable mRNA levels of 12/15-LOX and 5-LOX in gonadal adipose tissue of db/db and WT HF animals (Supplementary Fig.1A and B) and significantly lower levels in subcutaneous adipose tissue (P < 0.05; data not shown) in concordance with the different 17-HDHA and PD1 concentrations within the different fat pads (Fig. 1F and G). However, 12/15-LOX was moderately decreased in diet-induced obesity, whereas genetic obesity had no effect (Supplementary Fig. 1A).
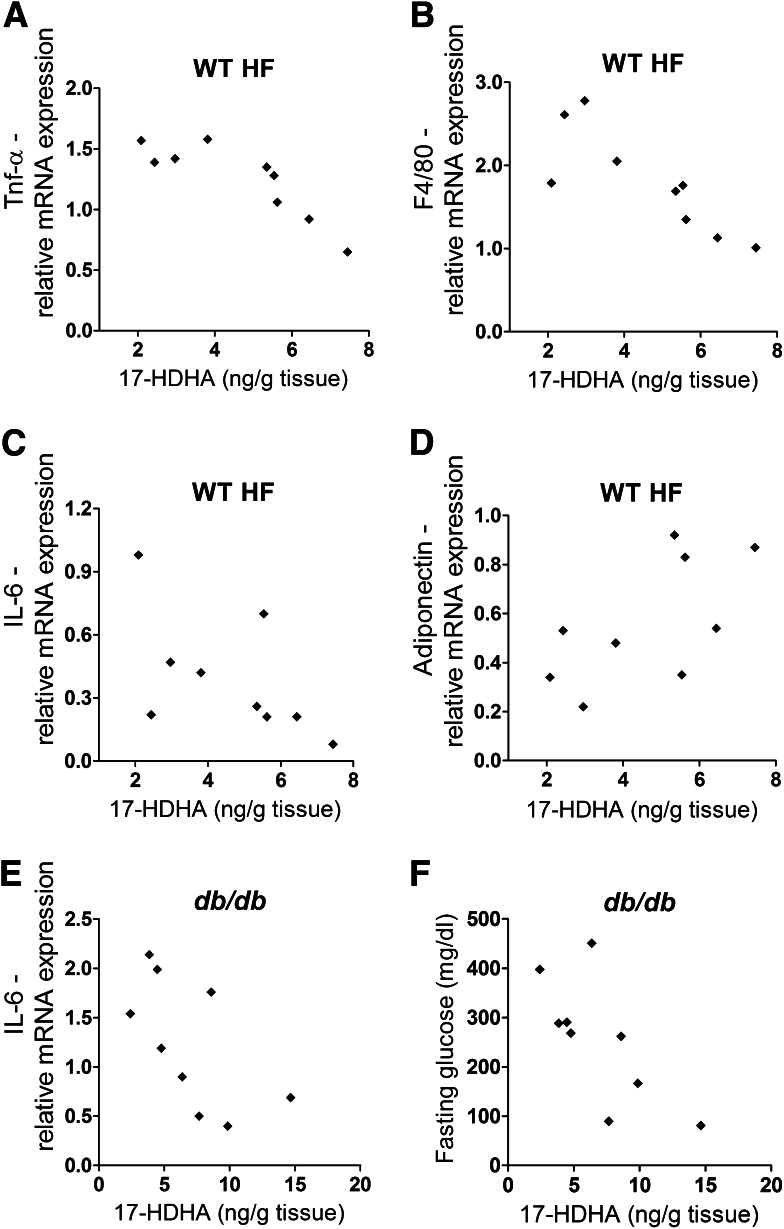
Adipose tissue 17-HDHA is associated with reduced adipose tissue inflammation.
In a different set of experiments, we evaluated possible correlations of n-3 PUFA–derived lipid mediators with markers of adipose tissue inflammation in obese animals. Gonadal adipose tissue level of 17-HDHA negatively correlated with expression of the genes for TNF-α (Fig. 3A), F4/80 (Fig. 3B), and IL-6 (Fig. 3C) in diet-induced obesity. In contrast, mRNA expression of adiponectin tended to correlate with 17-HDHA adipose tissue concentration (Fig. 3D), without reaching statistical significance. In genetic obesity, 17-HDHA level negatively correlated with gene expression of IL-6 and fasting glucose (Fig. 3E and F). These results indicate a possible link between obesity, adipose tissue inflammation, and 17-HDHA level.
FIG. 3.
Adipose tissue 17-HDHA concentration negatively correlates with markers of adipose tissue inflammation in murine obesity. Gonadal adipose tissue level of 17-HDHA in obese animals (WT HF-fed mice) negatively correlated with expression of genes for TNF-α (Tnf) (ρ = −0.88; P = 0.002) (A), F4/80 (Emr1) (ρ = −0.87; P = 0.002) (B), and IL-6 (Il6) (ρ = −0.73; P = 0.026) (C), whereas mRNA expression of the gene encoding adiponectin (AdipoQ) (D) tended to correlate with 17-HDHA adipose tissue level (ρ = 0.63; P = 0.067). In db/db animals, Il6 gene expression (ρ = −0.68; P = 0.04) (E) and fasting glucose (ρ = −0.77; P = 0.016) (F) negatively correlated with the 17-HDHA level in adipose tissue (n = 9 animals per group).
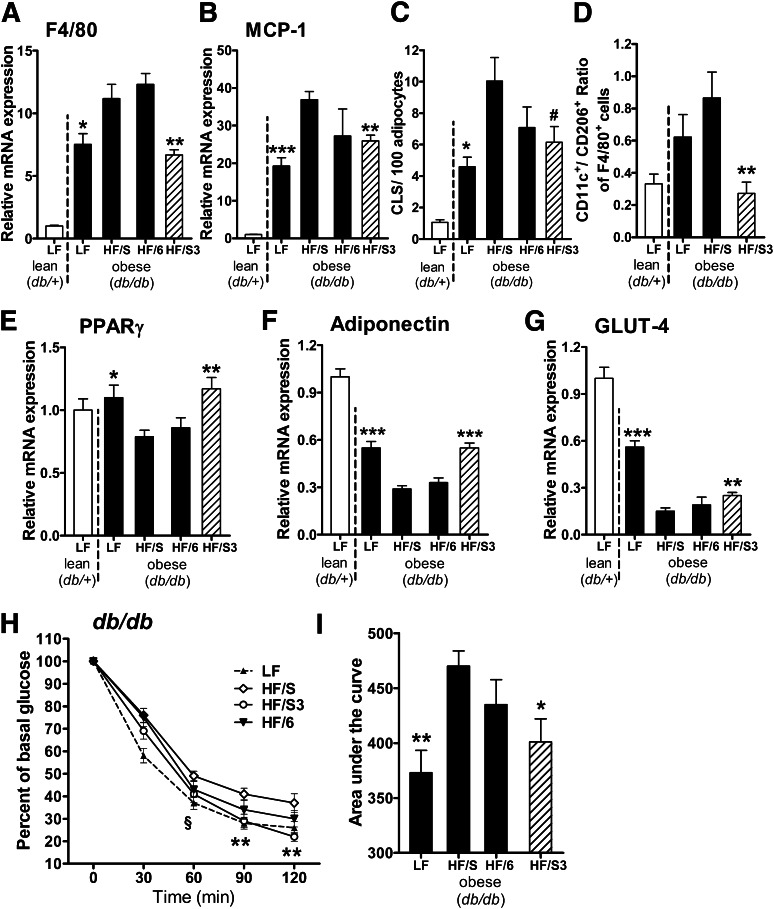
Attenuated inflammation and improved insulin sensitivity induced by dietary n-3 PUFA treatment are linked to increased adipose tissue levels of SPMs and their precursors.
We showed in a previous study that PUFA-supplemented diets led to an enrichment of respective fatty acids in the adipose tissue (27). Here, we further investigated whether the known beneficial effects of n-3 PUFA on adipose tissue inflammation and metabolic control in obese mice (21,34–36) are linked to an alleviation of the obesity-induced perturbations in endogenous n-3 PUFA–derived lipid mediator synthesis. Obese diabetic mice (db/db) and lean (db/+) littermates were fed for 6 weeks a LF control diet or three different HF diets rich in saturated/monounsaturated fatty acids (HF/S), n-6 PUFA (HF/6), and an HF/S diet including long-chain n-3 PUFA (HF/S3), similar to our previous study (21). The HF/S3 diet increased plasma adiponectin, reduced overnight fasting glucose concentration compared with db/db animals fed a HF/S diet (Supplementary Table 1), and significantly decreased gene expression of F4/80 and MCP-1 in gonadal adipose tissue of db/db mice, whereas the HF/6 diet had no significant impact (Fig. 4A and B). In addition, the HF/S diet-induced increase of CLSs, which are formed by macrophages around adipocytes (31), was reduced after the HF/S3 diet (Fig. 4C).
FIG. 4.
Dietary n-3 PUFA treatment attenuates adipose tissue inflammation and improves insulin sensitivity. Lean (db/+) mice were fed an LF diet and obese (db/db) animals were fed an LF diet or three different isocaloric HF diets: 1) HF/S diet rich in saturated/monounsaturated fatty acids; 2) HF/6 diet rich in n-6 PUFA; and 3) HF/S3 (HF/S diet supplemented with n-3 PUFA) for 6 weeks. Gonadal adipose tissue expression of the genes for macrophage marker F4/80 (Emr1) (A) and MCP-1 (Ccl2) (B) after dietary treatment was analyzed (n = 10 animals per group). (C) CLS formation, a hallmark of obesity-associated inflammation, was assessed by MAC-2 staining, and the number of CLS in gonadal adipose tissue was calculated per 100 adipocytes (n = 5 animals per group). (D) Flow cytometry analysis of the CD11c+CD206−-to-CD11c−CD206+ ratio of adipose tissue macrophages (F4/80+ cells) obtained from stromal vascular fractions of the db/+ LF control and db/db animals after dietary treatment with indicated diets (n = 8 animals per group). Expression of the genes for PPARγ (Pparg) (E), adiponectin (Adipoq) (F), and GLUT-4 (Slc2a4) (G) after dietary treatment in gonadal adipose tissue (n = 10 animals per group). (H and I) Insulin sensitivity was determined in db/db mice after LF control, HF/S, HF/6, or HF/S3 diet. Blood glucose was measured before and 30, 60, 90, and 120 min after intraperitoneal injection of insulin (2.0 units/kg body weight; n = 7–9 animals per group), and the area under the curve was calculated (n = 7–9 animals per group). For statistical analysis, db/db mice were compared with those fed the HF/S diet. All data are mean ± SEM. #P = 0.067; *P < 0.05; **P < 0.01; ***P < 0.001.
Of note, flow cytometric analysis revealed a reduction in the proportion of inflammatory CD11c+ adipose tissue macrophages (F4/80+CD11c+CD206-; P = 0.004) and an increase of anti-inflammatory M2-like (F4/80+CD11c-CD206+) macrophages (P = 0.07) leading to a significantly decreased CD11c+-to-CD206+ ratio after the HF/S3 diet compared with the HF/S diet (Fig. 4D). Moreover, the HF/S3 diet was associated with significant upregulation in adipose tissue of the genes involved in insulin sensitivity (Adipoq and Pparg) and glucose transport (Slc2a4 coding for GLUT-4) compared with animals fed the HF/S and HF/6 diets (Fig. 4E–G). As shown by the insulin tolerance test, the HF/S3 diet significantly improved insulin sensitivity compared with HF/S and HF/6 diets to a similar extent as seen in LF-fed db/db animals (Fig. 4H and I).
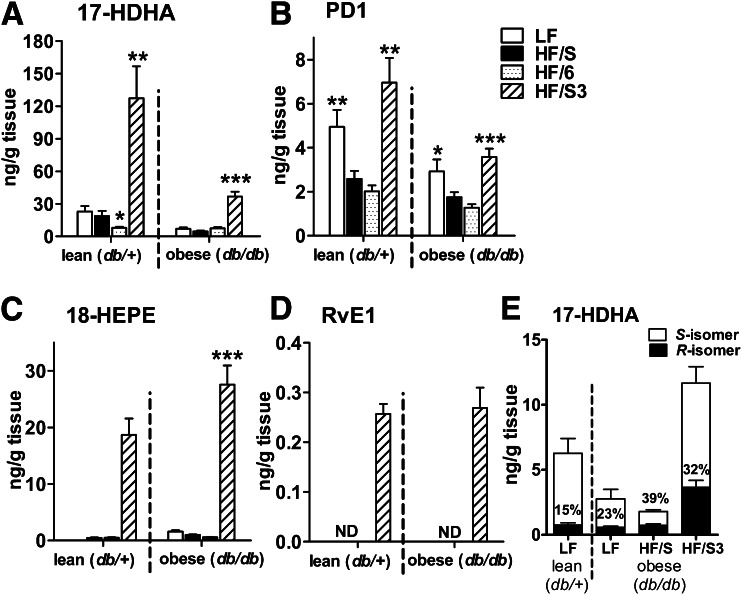
In parallel with decreased adipose tissue inflammation and improved insulin sensitivity, the HF/S3 diet dramatically increased the concentration of 17-HDHA, PD1, and 18-HEPE in gonadal adipose tissue of db/db mice (Fig. 5A–C), and RvE1 became detectable in adipose tissue after the HF/S3 diet (Fig. 5D). Of note, db/db mice fed the HF/S3 diet showed significantly lower levels of 17-HDHA and PD1 than their lean (db/+) counterparts fed the same diet (P < 0.01; Fig. 5A and B), indicating an inhibitory effect of obesity on SPM synthesis despite the HF/S3 diet that favors SPM synthesis. In addition, the HF/S diet increased the percentage of the R-isoform of 17-HDHA, whereas the HF/S3 diet enhanced adipose levels of both 17-HDHA enantiomers, with 17S-HDHA remaining the predominant isoform (Fig. 5E).
FIG. 5.
Dietary n-3 PUFA treatment increases synthesis of n-3 PUFA–derived SPMs and their precursors in the adipose tissue of genetically obese mice. Lipid mediator concentration was determined in gonadal fat pads of db/+ and db/db mice fed the LF, HF/S, HF/6, or HF/S3 diet using LC-MS/MS. HF/S3 treatment of db/db animals significantly increased the adipose tissue concentration of DHA-derived 17-HDHA (A) and PD1 (B) as well as EPA-derived 18-HEPE (C) and RvE1 (D) (n = 10 animals per group). Dietary effects within db/db and db/+ animals were compared with the respective HF/S groups. (E) Stereoselective analysis using chiral-based LC-MS/MS revealed 17S-HDHA as the main naturally occurring stereoisomer in gonadal adipose tissue of db/+ and db/db animals. The percentage of 17R-HDHA calculated from the total 17-HDHA in adipose tissue was increased after HF feeding (n = 10 animals per group). All data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. ND, not detected.
Altogether, these findings strongly suggest that dietary n-3 PUFA treatment reduces obesity-associated adipose tissue inflammation and insulin resistance by increasing endogenous SPM biosynthesis.
Treatment with the DHA product 17-HDHA reduces obesity-induced adipose tissue inflammation.
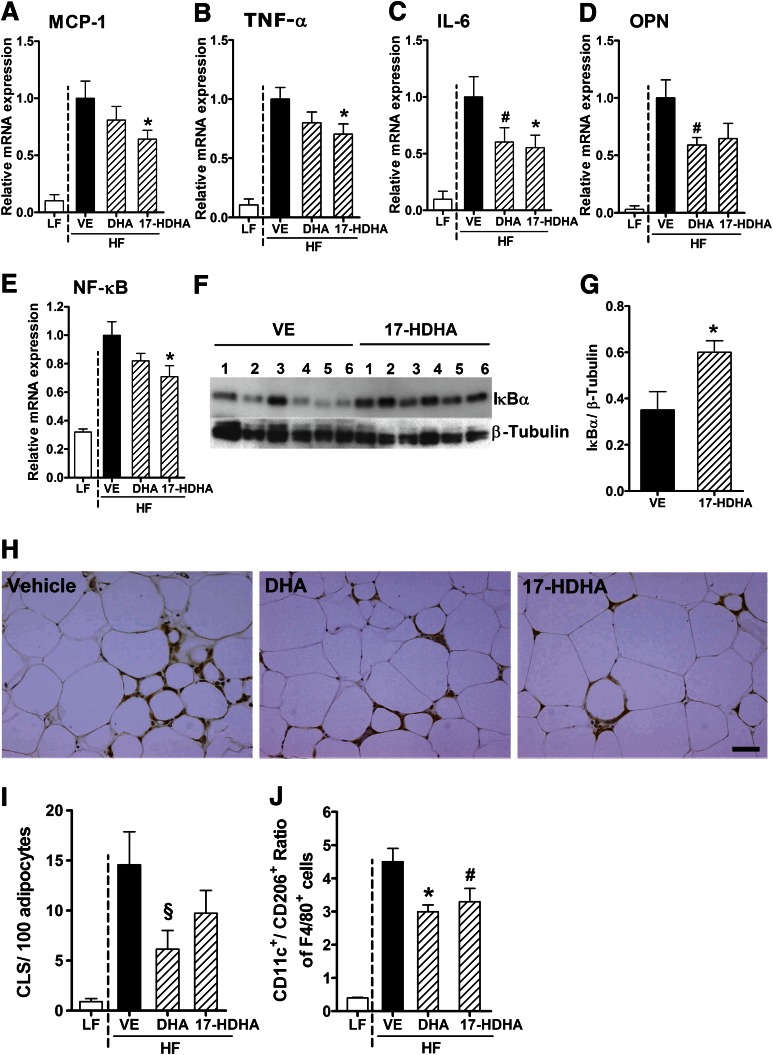
Because obesity strongly affected DHA-derived lipid mediators in adipose tissue, we next investigated whether direct treatment with DHA and its metabolite, 17-HDHA, attenuates obesity-induced adipose tissue inflammation and its associated metabolic complications. To this end, WT mice were fed an HF or LF control diet for 17 weeks, and the HF-fed animals were treated with DHA (4 µg/g body weight), 17-HDHA (50 ng/g body weight), or vehicle by intraperitoneal injection every 12 h for 8 days. As reported in Supplementary Table 2, no differences in body weight, gonadal adipose tissue weight, and fasting glucose were observed between treatments. Treatment with 17-HDHA and, less pronounced, DHA reduced adipose tissue inflammation as determined by gene expression of the inflammatory cytokines MCP-1, TNF-α, IL-6, and OPN, concomitant with decreased gene expression of NF-κB in diet-induced obese animals compared with vehicle control (Fig. 6A–E). To investigate potential effects of 17-HDHA on inflammatory signaling, we analyzed the effect on the NF-κB pathway in gonadal adipose tissue. Notably, 17-HDHA treatment increased the protein level of IκBα, the major inhibitor of NF-κB signaling in adipose tissue (Fig. 6F and G). Immunohistochemical staining for MAC-2 revealed a trend toward decreased CLS formation after 17-HDHA and DHA treatment in gonadal adipose tissue (Fig. 6H and I), whereas mean adipocyte size was not altered (not shown). Moreover, DHA and 17-HDHA treatment reduced the ratio of CD11c+ to CD206+ adipose tissue macrophages (Fig. 6J). Thus, 17-HDHA markedly attenuated adipose tissue inflammation in obese mice.
FIG. 6.
17-HDHA significantly decreases obesity-associated adipose tissue inflammation. HF-fed WT mice were treated with DHA, 17-HDHA, or vehicle (VE) control via intraperitoneal injection every 12 h for 8 days. 17-HDHA treatment reduced mRNA expression of the inflammatory genes for MCP-1 (Ccl2) (A), TNF-a (Tnf) (B), IL-6 (Il6) (C), OPN (Spp1) (D), and NF-κB (Nfkb1) (E) in gonadal adipose tissue of WT HF animals compared with VE-treated control group (n = 12–14 animals per group). (F) and (G) Immunoblot analysis and quantification of IκBα in gonadal adipose tissue of WT HF animals after VE and 17-HDHA treatment. The diagram shows means of the chemiluminescence intensity ratios from IκBα vs. β-tubulin, which was used as the loading control (G) (n = 6 animals per group). (H) Representative images of CLS formation in gonadal adipose tissue (scale bar = 50 µm). (I) Number of CLS counts per 100 adipocytes in gonadal adipose tissue after VE, DHA, or 17-HDHA treatment (n = 5–6 animals per group). (J) Flow cytometry analysis of the CD11c+CD206−-to-CD11c−CD206+ ratio of adipose tissue macrophages (F4/80+ cells) obtained from stromal vascular fractions after DHA and 17-HDHA treatment compared with VE control (n = 5–6 animals per group). For statistical analysis, DHA- and 17-HDHA–treated groups were compared with the VE-treated control group. All data are mean ± SEM. #P < 0.08; §P = 0.088; *P < 0.05. (A high-quality color representation of this figure is available in the online issue.)
Treatment with 17-HDHA improves metabolic regulation in obesity.
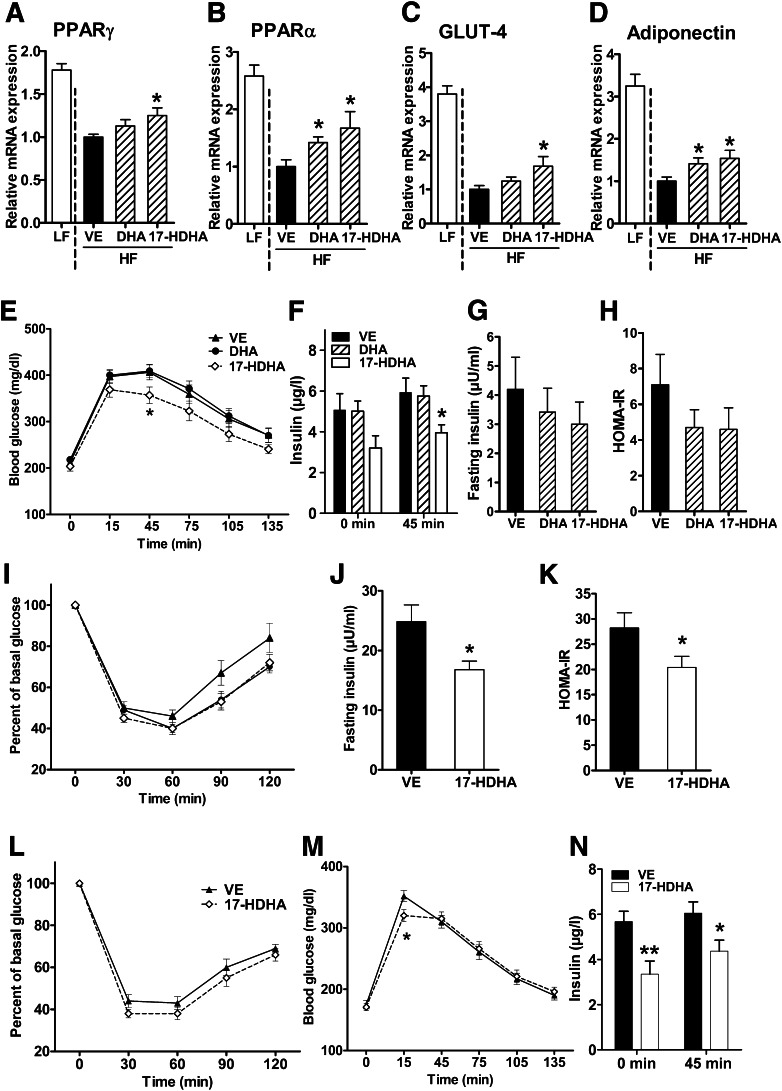
We further analyzed whether 17-HDHA treatment also affected markers of metabolic control. 17-HDHA enhanced obesity-impaired expression of the genes for PPARγ, PPARα, GLUT-4, and adiponectin (Fig. 7A–D), whereas leptin expression was not significantly altered (data not shown). In addition, 17-HDHA treatment moderately improved glucose tolerance in obese mice (Fig. 7E), concomitant with decreased plasma insulin levels in response to the glucose tolerance test at the 45-min time point (Fig. 7F).
FIG. 7.
17-HDHA treatment increases anti-inflammatory and insulin-sensitizing markers and improves glucose tolerance. Expression of genes encoding PPARγ (Pparg) (A), PPARα (Ppara) (B), GLUT-4 (Slc2a4) (C), and adiponectin (Adipoq) (D) in gonadal adipose tissue after vehicle (VE), DHA, and 17-HDHA treatment of HF-fed WT mice by intraperitoneal injection every 12 h for 8 days (n = 12–14 animals per group). Glucose tolerance test was performed by intraperitoneal injection of 0.75 g 20% glucose/kg body weight (n = 12 animals per group) (E), and plasma insulin concentration was measured at the indicated time points (n = 8 animals per group) (F). Determination of 3-h fasting plasma insulin (n = 10 animals per group) (G), HOMA-IR (n = 10 animals per group) (H), and insulin sensitivity by performing an insulin tolerance test (0.75 units/kg body weight; n = 12 animals per group) (I) upon VE, 17-HDHA, and DHA intraperitoneal injection for 8 days. Three-hour fasting plasma insulin (n = 10 animals per group) (J), HOMA-IR (n = 10 animals per group) (K), insulin sensitivity (n = 14 animals per group) (L), glucose tolerance (n = 12 animals per group) (M), and plasma insulin levels before and in response to glucose tolerance test (n = 6 animals per group) (N) were determined after prolonged 17-HDHA administration for 15 days in WT HF mice by osmotic pumps (body weight: 50.9 ± 0.8 g after VE treatment and 49.5 ± 0.8 g after 17-HDHA treatment). For statistical analysis, DHA- and 17-HDHA–treated animals were compared with the VE-treated control group. All data are mean ± SEM. *P < 0.05; **P < 0.01.
Although not statistically significant, fasting insulin concentration was reduced by ∼30% and insulin resistance, as estimated by HOMA-IR, was ∼40% lower after 17-HDHA and DHA treatment (Fig. 7G and H). A trend in improvement in the insulin tolerance test was seen after 60 (P = 0.1), 90 (P = 0.08), and 120 min (P = 0.1) upon 17-HDHA and DHA application for 8 days (Fig. 7I). In addition, 17-HDHA treatment for 15 days by osmotic pump resulted in increased insulin sensitivity, as illustrated by a significantly decreased fasting insulin concentration and HOMA-IR and a trend of reduced blood glucose concentration after 30 min (P = 0.09) during the insulin tolerance test (Fig. 7J–L). The glucose concentration in the glucose tolerance test was significantly lower after 15 min by 17-HDHA treatment compared with vehicle control (Fig. 7M). In addition, the group treated with 17-HDHA revealed lower plasma insulin levels before and during the glucose tolerance test (after 45 min, Fig. 7N).
Taken together, these results indicate that 17-HDHA not only reduced adipose tissue inflammation but also improved glucose tolerance and insulin sensitivity in obese mice.
DISCUSSION
In this study we demonstrate an obesity-induced deficiency of PD1 and 17-HDHA, which is a PD1 pathway biomarker and RvD1 precursor, in adipose tissue. Our data indicate that the deficiency of these n-3 PUFA–derived SPMs is linked to the development and perpetuation of obesity-driven adipose tissue inflammation that promotes type 2 diabetes. Moreover, we show that treatment with 17-HDHA mitigates obesity-driven inflammation and its metabolic consequences.
The obesity-associated disturbance of 17-HDHA and PD1 biosynthesis may result from decreased substrate availability and/or altered enzyme activities. HF feeding has been shown to reduce n-3 PUFA availability by elevating the long-chain n-6–to–n-3 PUFA ratio in adipose tissue membranes compared with counterparts fed normal chow (26). On one hand, we demonstrate an effect of HF diet on lipid mediator synthesis in adipose tissue, which is independent of obesity, because 17-HDHA and PD1 levels were affected also by short-term dietary treatment before the onset of obesity-induced adipose tissue inflammation (Fig. 2). On the other hand, our results on decreased SPMs and SPM precursor levels in db/db mice fed normal chow compared with lean littermates show that also obesity itself decreases adipose tissue levels of DHA-derived 17-HDHA available for SPM biosynthesis while EPA-derived 18-HEPE was even increased (Fig. 1).
We show here that the adipose tissue level of 12/15-LOX mRNA, a pivotal enzyme for 17-HDHA and PD1 biosynthesis in mice (37,38), is significantly decreased in diet-induced obesity, corroborating previous data that impaired 12/15-LOX activity results in a resolution-deficient phenotype (39–41). In addition to 17-HDHA, obesity also decreased 12/15-LOX–derived 12-hydroxyeicosatetraenoic acid (12-HETE) and 15-HETE levels in adipose tissue (Supplementary Fig. 1C and D), further indicating diminished 12/15-LOX activity. That 18-HEPE synthesis, despite decreased 17-HDHA and PD1 formation, is not impaired by obesity (Fig. 1E) or HF-feeding per se (Fig. 2F) might indicate that not only obesity but also the HF diet especially affects the synthesis of 12/15-LOX–derived lipid mediators. Alternatively, the significant increase of 18-HEPE could represent a lack of 5-LOX activity for conversion to RvE1 and RvE2 (42) and an impaired 12/15-LOX pathway for conversion to the recently characterized RvE3 (43). However, the decrease in 17-HDHA and PD1 levels in obesity cannot clearly be related to altered enzyme expression on the mRNA level, particularly in db/db animals (Supplementary Fig. 1A). A precise determination of relevant enzyme activity might shed light on this issue but was beyond the present scope of this study.
Our study suggests that n-3 PUFA–derived SPMs present in adipose tissue mediate crucial effects of n-3 PUFA treatment, such as decreasing obesity-induced adipose tissue inflammation, including a shift of macrophage polarization from the M1-like to the M2-like phenotype, and improving metabolic parameters such as insulin sensitivity (Fig. 4 and Fig. 5) and glucose tolerance (22,23,26,44). Previous studies demonstrated that the number of M1-like adipose tissue macrophages expressing CD11c and the M1-to-M2 macrophage ratio are both associated with insulin resistance (11,12), whereas the ablation of CD11c+ cells resulted in the normalization of insulin sensitivity and glucose tolerance in obese mice (10,45). Of note, the doses used in the animals are high, similar to other feeding experiments with n-3 PUFA (35,36,46), and cannot be reached in humans. Moreover, treatment with long-chain n-3 PUFA in high doses that are known to have anti-inflammatory effects (≥4 g/day) is rarely successful because of low adherence of patients that might be partly due to the unpleasant capsule size. Thus, similar beneficial effects with substances to be applied at much lower doses are highly desirable. The obesity-induced reduction of the SPM precursor 17-HDHA (Fig. 1C) and its negative correlation with markers of adipose tissue inflammation (Fig. 3) prompted us to focus on this compound. Treatment with 17-HDHA elicited an anti-inflammatory response in diet-induced obesity by significantly reducing inflammatory gene expression, probably by attenuating NF-κB activation (Fig. 6) and increasing expression of anti-inflammatory and insulin-sensitizing adiponectin as well as PPARγ in adipose tissue of obese mice. Notably, the anti-inflammatory effects of 17-HDHA were comparable to those of DHA at a dose that was nearly 100-fold lower. In addition to the anti-inflammatory and insulin sensitizing effects, 17-HDHA but not DHA treatment increased glucose tolerance, albeit moderately, concomitant with lower plasma insulin concentration (Fig. 7E and F), suggesting that 17-HDHA improves insulin action.
Our results with 17-HDHA and those achieved with RvE1 and RvD1 treatment of hepatic steatosis and insulin resistance (22,47) encourage follow-up of SPM precursor-based therapies in obesity.
DHA treatment affects M1-like/M2-like macrophage polarization, as evaluated here by the CD11c+-to-CD206+ macrophage ratio (Fig. 6J) (44). In in vitro studies, 17-HDHA inhibits cytokine release of human microglial cells (48) and decreases TNF-α secretion of murine RAW macrophages (49,50) indicating that 17-HDHA or SPM derived from it exert potent anti-inflammatory action on macrophage-like cells. In our study, 17-HDHA treatment decreased the M1-to-M2 ratio of adipose tissue macrophages (Fig. 6J) in a similar manner as DHA. Hence, 17-HDHA could substitute for n-3 PUFA with respect to its potent anti-inflammatory effects in obesity-associated inflammation, which is mainly driven by macrophages (51).
17-HDHA might exert its anti-inflammatory activity and beneficial effects on metabolic regulation through different mechanisms. On one hand, 17-HDHA and its precursor 17-H(peroxy)DHA serve as SPM precursors for local conversion to RvD1 or PD1 that depends on cell type (17). On the other hand, 17S-HDHA but not 17R-HDHA directly activates PPARγ (49), which could contribute to increased adiponectin and GLUT-4 expression in adipocytes as well decreased inflammatory cytokine expression in macrophages (52); however, the possible action of 17-HDHA through PPARα remains to be elucidated. In addition, the anti-inflammatory effects of 17-HDHA treatment in obesity-induced inflammation might be due to decreased NF-κB activity, which could be partly mediated through PPAR activation (53,54). However, because PPARα target genes, such as acyl-CoA oxidase, were not altered by the treatment (data not shown), these data might argue against a dominant role of this nuclear receptor in 17-HDHA action to attenuate obesity-induced inflammation.
In conclusion, we show for the first time that obesity significantly reduced the endogenous production of 17-HDHA and PD1 in adipose tissue, which probably impairs resolution of adipose tissue inflammation. This lack of anti-inflammatory and proresolving lipid mediators could contribute to chronic adipose tissue inflammation and metabolic complications in obesity. Application of proresolving lipid mediators or 17-HDHA as a critical precursor reconstitutes endogenous resolution capacity and might thus provide a novel option for treatment and prevention of obesity-related diseases such as type 2 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the European Community’s 7th Framework Programme (FP7/2007-2013) under grant agreement No. 201608, the Austrian Science Fund (as part of the Cell Communication in Health and Disease doctoral program, W1205-B09), the Federal Ministry of Economy, Family and Youth, and the National Foundation for Research, Technology, and Development, all to T.M.S. C.N.S. acknowledges support from the National Institutes of Health (National Institute of Environmental Health Sciences) PO1-GM095467. C.N.S. is an inventor on patents [resolvins] assigned to Brigham and Women’s Hospital and licensed to Resolvyx Pharmaceuticals. C.N.S. is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. C.N.S.’s interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. No other potential conflicts of interest relevant to this article were reported.
A.N. planned and conducted experiments, researched and analyzed data, wrote the manuscript, and read and approved the final manuscript. M.Z. supervised experiments, researched data, reviewed and edited the manuscript, and read and approved the final manuscript. D.M. established and performed LC-MS/MS of lipid mediators and read and approved the final manuscript. B.K.I., L.L., E.E.H., and P.F. conducted research, reviewed the manuscript, and read and approved the final manuscript. I.M. and S.C. performed immunohistochemistry, reviewed the manuscript, and read and approved the final manuscript. C.N.S. provided some synthetic lipid mediators, contributed to discussion, critically reviewed and edited the manuscript, and read and approved the final manuscript. T.M.S. designed research, raised grants, supervised experiments, reviewed and edited the manuscript, and read and approved the final manuscript. T.M.S. and A.N. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as abstracts at the 70th Scientific Sessions of the American Diabetes Association, 25–29 June 2010, Orlando, Florida, and at the 11th International Congress on Obesity (ICO), 11–15 July 2010, Stockholm, Sweden.
The authors thank Elisabeth Matzner, Barbara Legerer, Bernhard Wernly, Eva Ratzinger, Erika Nowotny, Liliana Ionasz, Sandra Haiderer, Alexander Juerets, and Sandra Pferschy (all of the Medical University of Vienna) for excellent technical assistance and support and EPAX AS (Aalesund, Norway) for kindly providing EPAX 6000 TG.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0828/-/DC1.
REFERENCES
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 2.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaminski DA, Randall TD. Adaptive immunity and adipose tissue biology. Trends Immunol 2010;31:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008;57:3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeyda M, Gollinger K, Kriehuber E, Kiefer FW, Neuhofer A, Stulnig TM. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. Int J Obes (Lond) 2010;34:1684–1694 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 2007;282:35279–35292 [DOI] [PubMed] [Google Scholar]
- 9.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012;61:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 2008;8:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujisaka S, Usui I, Bukhari A, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009;58:2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wentworth JM, Naselli G, Brown WA, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 2010;59:1648–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance—a mini-review. Gerontology 2009;55:379–386 [DOI] [PubMed] [Google Scholar]
- 14.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol 2010;177:1576–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005;6:1191–1197 [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 2007;25:101–137 [DOI] [PubMed] [Google Scholar]
- 18.Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr 2012;32:203–227 [DOI] [PubMed] [Google Scholar]
- 19.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest 2011;121:569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan C, Ding A. Nonresolving inflammation. Cell 2010;140:871–882 [DOI] [PubMed] [Google Scholar]
- 21.Todoric J, Löffler M, Huber J, et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia 2006;49:2109–2119 [DOI] [PubMed] [Google Scholar]
- 22.González-Périz A, Horrillo R, Ferré N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J 2009;23:1946–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flachs P, Rühl R, Hensler M, et al. Synergistic induction of lipid catabolism and anti-inflammatory lipids in white fat of dietary obese mice in response to calorie restriction and n-3 fatty acids. Diabetologia 2011;54:2626–2638 [DOI] [PubMed] [Google Scholar]
- 24.Hudert CA, Weylandt KH, Lu Y, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A 2006;103:11276–11281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med 2007;13:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White PJ, Arita M, Taguchi R, Kang JX, Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes 2010;59:3066–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber J, Löffler M, Bilban M, et al. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond) 2007;31:1004–1013 [DOI] [PubMed] [Google Scholar]
- 28.Ferrannini E, Mari A. How to measure insulin sensitivity. J Hypertens 1998;16:895–906 [DOI] [PubMed] [Google Scholar]
- 29.Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol 2011;Chapter 14:Unit 14.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiefer FW, Zeyda M, Gollinger K, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 2010;59:935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 32.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 2008;49:1894–1903 [DOI] [PubMed] [Google Scholar]
- 33.Lee YS, Li P, Huh JY, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011;60:2474–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neschen S, Morino K, Dong J, et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes 2007;56:1034–1041 [DOI] [PubMed] [Google Scholar]
- 35.Kuda O, Jelenik T, Jilkova Z, et al. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia 2009;52:941–951 [DOI] [PubMed] [Google Scholar]
- 36.Neschen S, Morino K, Rossbacher JC, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes 2006;55:924–928 [DOI] [PubMed] [Google Scholar]
- 37.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 2003;278:14677–14687 [DOI] [PubMed] [Google Scholar]
- 38.Ariel A, Li PL, Wang W, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem 2005;280:43079–43086 [DOI] [PubMed] [Google Scholar]
- 39.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 2008;22:3595–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem 2005;280:15267–15278 [DOI] [PubMed] [Google Scholar]
- 41.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007;447:869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tjonahen E, Oh SF, Siegelman J, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol 2006;13:1193–1202 [DOI] [PubMed] [Google Scholar]
- 43.Isobe Y, Arita M, Matsueda S, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem 2012;287:10525–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Titos E, Rius B, González-Périz A, et al. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol 2011;187:5408–5418 [DOI] [PubMed] [Google Scholar]
- 45.Wu H, Perrard XD, Wang Q, et al. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol 2010;30:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J 2011;25:2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.González-Périz A, Planagumà A, Gronert K, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J 2006;20:2537–2539 [DOI] [PubMed] [Google Scholar]
- 50.Weylandt KH, Krause LF, Gomolka B, et al. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-α. Carcinogenesis 2011;32:897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett 2007;112:61–67 [DOI] [PubMed] [Google Scholar]
- 52.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 2008;77:289–312 [DOI] [PubMed] [Google Scholar]
- 53.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008;454:470–477 [DOI] [PubMed] [Google Scholar]
- 54.Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 2001;169:453–459 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.