Abstract
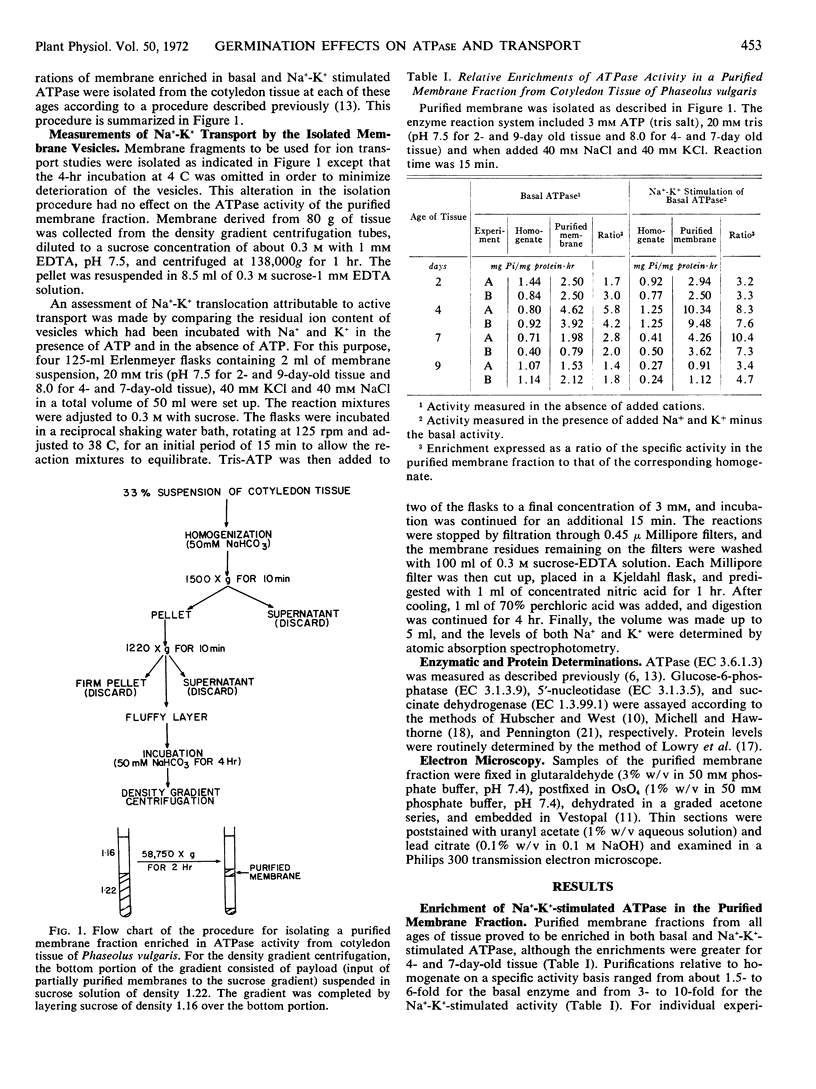
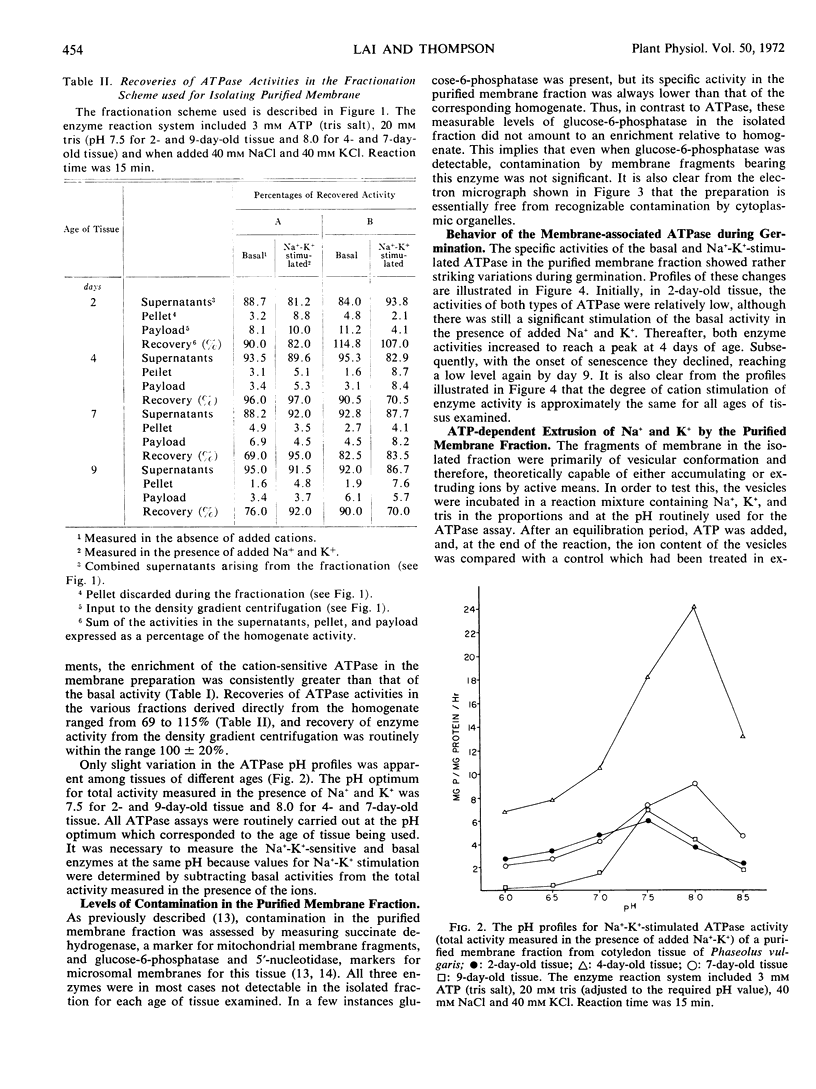
A purified membrane fraction featuring ATPase activity was isolated from cotyledon tissue of Phaseolus vulgaris at different stages of germination. The fraction is enriched in both basal and Na+-K+-stimulated ATPase and is relatively free of contamination by fragments of mitochondrial membrane and microsomes. The isolated membranes have been tentatively identified as partially purified plasma membrane.
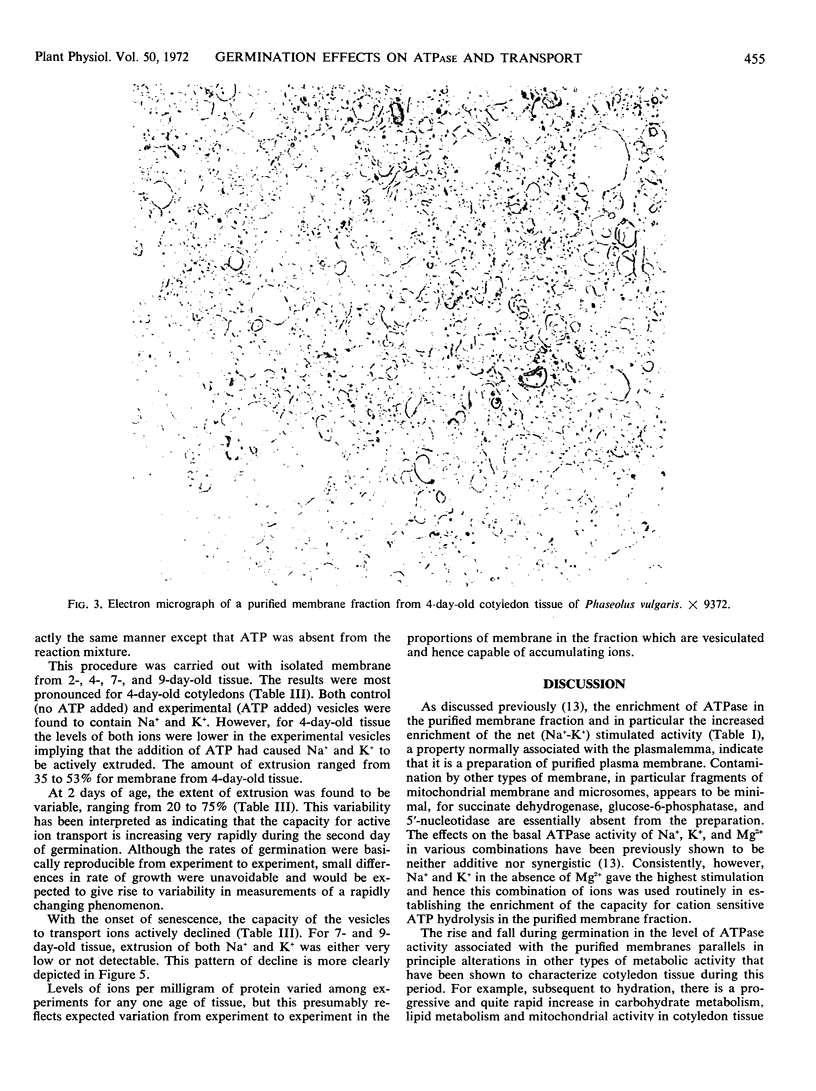
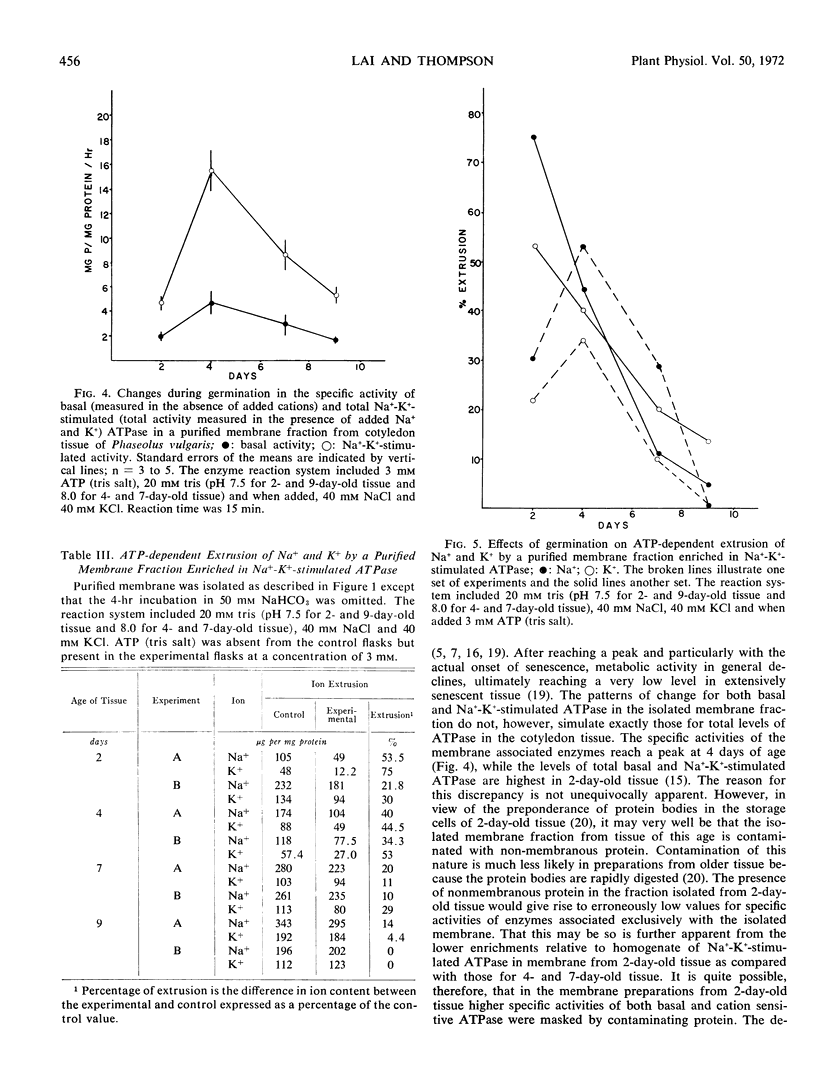
The specific activities of the basal- and cation-sensitive ATPases were high in membrane preparations from young cotyledon tissue but decreased with advancing senescence. Electron microscopy of the preparation showed that the isolated membranes were of primarily vesicular conformation. These vesicles proved to be capable of extruding Na+ and K+ in the presence of ATP. Moreover, the degree of ATP-dependent extrusion varied during germination in a pattern that resembled variations during the same period in the Na+-K+-stimulated ATPase of the isolated fraction. This indicates that as the level of cation-sensitive ATPase on the membrane rises or falls, there is a corresponding change in the ability of the membrane to actively translocate Na+ and K+.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman H. M., Gram W., Spirtes M. A. An improved, reproducible method opreparing rat liver plasma cell membranes in buffered isotonic sucrose. Biochim Biophys Acta. 1969 Jun 3;183(1):10–18. doi: 10.1016/0005-2736(69)90124-2. [DOI] [PubMed] [Google Scholar]
- Brown A. P., Wray J. L. Correlated changes of some enzyme activities and cofactor and substrate contents of pea cotyledon tissue during germination. Biochem J. 1968 Jul;108(3):437–444. doi: 10.1042/bj1080437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. D., Altschul A. M. Glycoside-sensitive ATPase from Arachis hypogaea. Biochem Biophys Res Commun. 1964 Apr 22;15(5):479–483. doi: 10.1016/0006-291x(64)90490-5. [DOI] [PubMed] [Google Scholar]
- Brown H. D., Neucere N. J., Altschul A. M., Evans W. J. Activity patterns of purified ATPase from Arachis hypogaea. Life Sci. 1965 Jul;4(14):1439–1447. doi: 10.1016/0024-3205(65)90023-8. [DOI] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylin A., Gee R. Adenosine Triphosphatase Activities in Leaves of the Mangrove Avicennia nitida Jacq: Influence of Sodium to Potassium Ratios and Salt Concentrations. Plant Physiol. 1970 Feb;45(2):169–172. doi: 10.1104/pp.45.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai Y. F., Thompson J. E. The preparation and properties of an isolated plant membrane fraction enriched in (Na plus-K plus)-stimulated ATPase. Biochim Biophys Acta. 1971 Mar 9;233(1):84–90. doi: 10.1016/0005-2736(71)90360-9. [DOI] [PubMed] [Google Scholar]
- Longo C. P. Evidence for de novo synthesis of isocitratase and malate synthesis in germinating peanut cotyledons. Plant Physiol. 1968 Apr;43(4):660–664. doi: 10.1104/pp.43.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]



