Abstract
OBJECTIVE
Human blood glucose levels have likely evolved toward their current point of stability over hundreds of thousands of years. The robust population stability of this trait is called canalization. It has been represented by a hyperbolic function of two variables: insulin sensitivity and insulin response. Environmental changes due to global migration may have pushed some human subpopulations to different points of stability. We hypothesized that there may be ethnic differences in the optimal states in the relationship between insulin sensitivity and insulin response.
RESEARCH DESIGN AND METHODS
We identified studies that measured the insulin sensitivity index (SI) and acute insulin response to glucose (AIRg) in three major ethnic groups: Africans, Caucasians, and East Asians. We identified 74 study cohorts comprising 3,813 individuals (19 African cohorts, 31 Caucasian, and 24 East Asian). We calculated the hyperbolic relationship using the mean values of SI and AIRg in the healthy cohorts with normal glucose tolerance.
RESULTS
We found that Caucasian subpopulations were located around the middle point of the hyperbola, while African and East Asian subpopulations are located around unstable extreme points, where a small change in one variable is associated with a large nonlinear change in the other variable.
CONCLUSIONS
Our findings suggest that the genetic background of Africans and East Asians makes them more and differentially susceptible to diabetes than Caucasians. This ethnic stratification could be implicated in the different natural courses of diabetes onset.
Canalization is the way in which organisms develop phenotypic robustness as a response to genetic or environmental perturbations. This process ensures the stability of critical biological processes like blood glucose regulation. Canalization of this trait can be represented by a hyperbolic function of two underlying variables: insulin sensitivity and insulin response, as primarily described by Kahn et al. (1,2).
Global migration in the early history of Homo sapiens placed people in new environments, resulting in novel diets, food scarcity, different climates, and exposure to novel pathogens. These changes may have shifted population averages of factors that influence insulin sensitivity and secretion. They include body size, body composition, energy expenditure, storage, and heat production. As these factors changed, they may have disclosed cryptic genetic variation or adopted novel mutations, leading to disruption of the unique point of stable equilibrium of ancestral populations. As this process continued over hundreds of millennia, specific genetic and environmental perturbations may have pushed some subpopulations to different points of stability (1,3–5).
We hypothesized that there may be ethnic differences in the optimal states in the relationship between insulin sensitivity and insulin response and that these differences may depend on a population’s genetic or evolutionary history. To assess this hypothesis, we performed a systematic review and a meta-analysis of studies of the insulin sensitivity index (SI) and the acute insulin response to glucose (AIRg). Our analysis was done in cohorts in any of the three major ethnic groups: Africans, Caucasians, and East Asians. We found significant differences between the groups.
RESEARCH DESIGN AND METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used as a guidance for our meta-analytic study (6).
Literature search
We searched PubMed in October 2011 for articles measuring SI and AIRg with frequently sampled intravenous glucose tolerance tests (FSIGT) in Africans, Caucasians, or East Asians (Supplementary Data). Our keywords included (“acute insulin response” AND (“insulin sensitivity index” OR “Caucasian” OR “African” OR “Chinese” OR “Japanese” OR “Korean”)) OR (“FSIGT” AND (“Caucasian” OR “African” OR “Chinese” OR “Japanese” OR “Korean”)). In addition, we reviewed the reference lists of the articles we found to find more studies. The language of the studies was restricted to English.
Inclusion and exclusion criteria
A study article that met the following criteria was included: 1) It determined individual SI and AIRg by FSIGT with minimal model (MINMOD) analysis (7,8). 2) It specified the measurement units of SI and AIRg. 3) It investigated a cohort composed of a single ethnicity. If there were multiple publications for the same study, we used the article with the most detailed information. We then classified the study cohorts into three groups: normal glucose tolerance (NGT), impaired glucose regulation (IGR), or type 2 diabetes (T2D). We used diagnoses made by the original authors; these diagnoses were based on recommendations of the World Health Organization, the American Diabetes Association, or the Japan Diabetes Society at the time of the study (9–11).
The NGT cohorts were further limited as follows. A cohort was included if it was composed of apparently healthy subjects (no evidence of systemic diseases influencing glycemic control). A cohort was excluded if it was composed of 1) (mainly) obese subjects (mean of BMI >30 kg/m2), 2) subjects with a history of gestational diabetes mellitus, or 3) subjects with a family history of T2D.
Data extraction
Two investigators (K.K. and D.T.) independently reviewed the abstracts and the full text of all articles retrieved from PubMed. They made decisions about including or excluding them. Discrepancies in eligibility were discussed until agreement was achieved. Data were extracted independently by K.K. and D.T. Relevant data included the key author’s name; year of publication; ethnicity of the population studied; number of participants; participants’ mean age, BMI, and fasting glucose; and participants’ mean ± SD SI and AIRg. We converted different SI units (e.g., ×10−4 ⋅ min−1/μU/mL) to the units used in Kahn’s original study (×10−5 ⋅ min−1/pmol/L) (2). We also recalculated the values of AIRg as the mean increment above basal level of insulin concentrations during the first peak and converted all units to those in Kahn’s original study (pmol/L) (2). Differences in abstracted data were resolved by discussion and consensus with the other authors.
Data correction
FSIGT with MINMOD analysis was conducted to evaluate SI using one of the following three methods: no modification (regular), additional insulin administration at 20 min (insulin modified), or tolbutamide administration at 20 min (tolbutamide boosted). Two studies (12,13) have reported that SI measured by the insulin-modified protocol was systematically lower (16–29%) than that measured using tolbutamide, while another reported no significant difference between SI (insulin) and SI (regular) (14). Most of the studies in our meta-analysis used the insulin-modified or regular protocol (Supplementary Table 1). The tolbutamide-boosted protocol was used in eight study cohorts for all of the participants (2 African and 6 Caucasian) and in four cohorts for the participants (1 African and 3 Caucasian) in the NGT category. It was used in two cohorts for all of the participants (2 Caucasian) in the IGR and for none in the T2D. To correct the effects of the tolbutamide studies, we converted their values of SI (tolbutamide) to the approximate estimates of SI (insulin) using the average rate of decline (25.9%) calculated from the data in the two references noted above (12,13).
Quality assessment for individual studies and overall quality of evidence
To evaluate quality and the risk of bias in each included study, we designed a scoring system for test results (Supplementary Data). Our system is a modification of the Newcastle-Ottawa Quality Assessment Scale, which was originally developed to assess the quality of nonrandomized studies (15). Our scale (the Modified Newcastle–Ottawa Quality Assessment Scale [MNOS]) awards a maximum of 9 points to each included study: up to 4 points for an adequate selection of a study population, up to 2 points for comparability of cohorts in the study [i.e., absence of obvious confounding factor(s)], and up to 3 points for the adequate measurement and presentation of study results. We defined studies as high quality if they scored the maximum 9 points on our scale; studies of medium quality scored 7–8 points, and studies that scored ≤6 points were defined as low quality.
We rated the overall quality of our evidence for each ethnic group by considering the quality (as measured by MNOS), the imprecision of results, or the possibility of publication bias according to recommendations by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group (16).
Statistical analysis
The relationship between mean values of SI and AIRg across three ethnic groups was assessed using power (log-log) regression analysis. Additionally, we calculated combined mean values (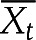 ) and variances (Ut) of SI and AIRg for all cohorts in each ethnic group (NGT, IGR, or T2D) as follows:
) and variances (Ut) of SI and AIRg for all cohorts in each ethnic group (NGT, IGR, or T2D) as follows:
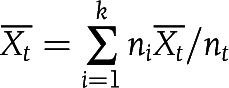 |
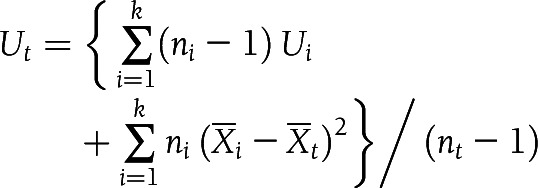 |
In this calculation, k was the number of cohorts in each ethnic group, ni was the sample size of cohort i in each ethnic group, nt was the total number of samples in each ethnic group, Xi was the mean value of cohort i in each ethnic group, and Ui was the variance of cohort i in each ethnic group. We made comparisons between multiple-group means with a Bonferroni corrected t test for pairwise group comparisons. A two-tailed P value <0.017 (0.05/3) was considered to indicate statistical significance (3 comparisons). All analyzed data are represented as means ± SE unless otherwise noted.
RESULTS
Search results
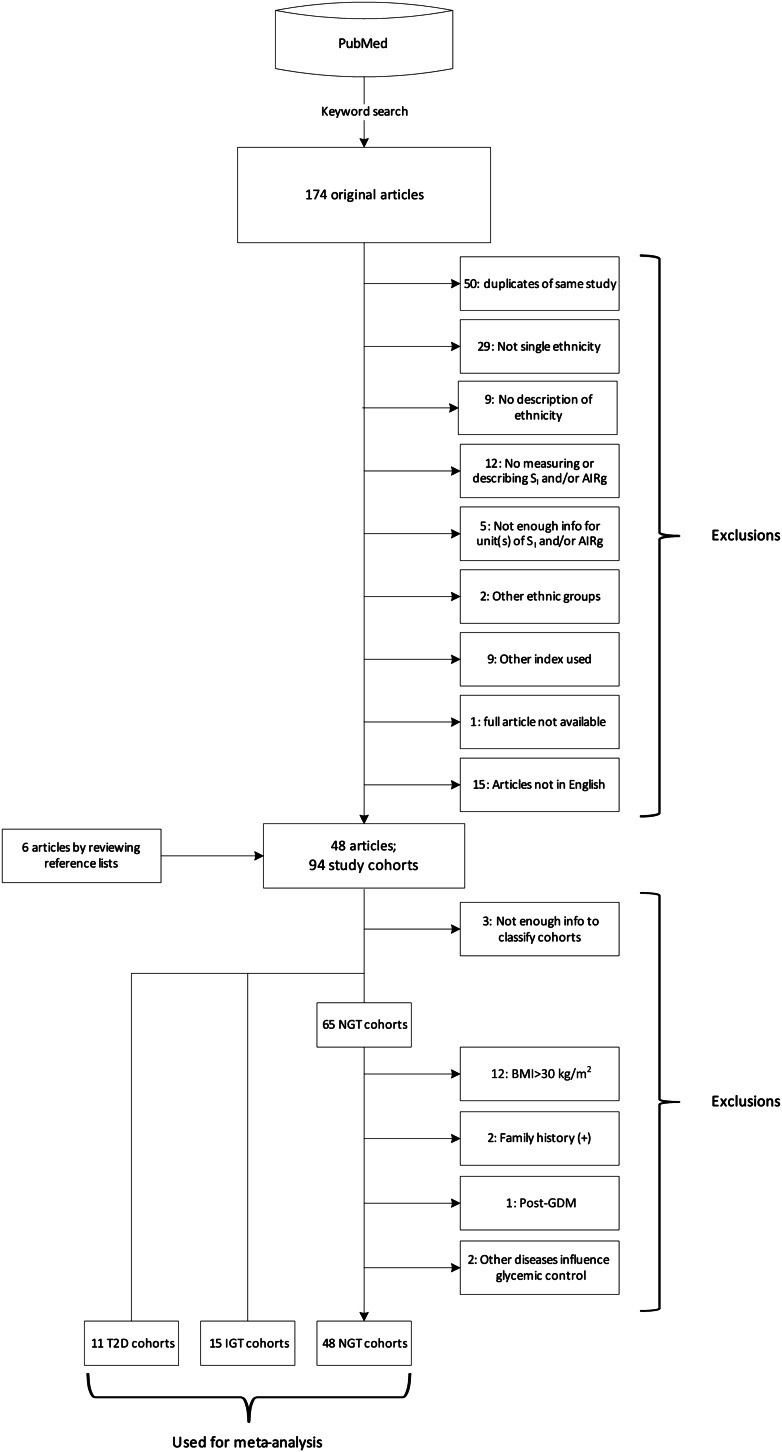
Fig. 1 is a flowchart showing how we identified study cohorts. Our PubMed search yielded 174 journal articles. After review of the full text and reference lists of each article, 48 studies including 94 cohorts met our selection criteria and were subjected to further screening. These cohorts were classified as NGT, IGR, and T2D, and the NGT group was further restricted according to our predetermined criteria. Eventually, we identified 74 ethnically homogeneous cohorts in which SI and AIRg were measured. There were 48 NGT (13 African, 23 Caucasian, and 12 East Asian), 15 IGR (3 African, 5 Caucasian, and 7 East Asian), and 11 T2D (3 African, 3 Caucasian, and 5 East Asian). They included 3,813 individuals (Supplementary Table 1).
Figure 1.
Flow diagram of literature search and cohort identification.
Risk of bias assessment
We used our MNOS scale to assess the quality of the individual studies for each ethnic group classified by degree of glucose tolerance. In NGT, 39 studies were deemed to be high quality (11 African, 19 Caucasian, and 9 East Asian), 8 were medium quality (2 African, 3 Caucasian, and 3 East Asian), and 1 was low quality (1 Caucasian; MNOS score: 5 points). Eleven IGR studies were of high quality (3 African, 3 Caucasian, and 5 East Asian), four were medium quality (2 Caucasian and 2 East Asian), and none were low quality. In T2D, eight studies were high quality (3 African, 1 Caucasian, and 4 East Asian), two were medium quality (1 Caucasian and 1 East Asian), and one was low quality (1 Caucasian; MNOS score: 6 points) (Supplementary Table 1). The studies defined as medium all lost points in the selection or comparability categories. The low-quality studies lost two points in a selection category and additional point(s) for other potential bias in either a comparability or an outcome category.
Ethnic differences in the relationship between SI and AIRg in NGT cohorts
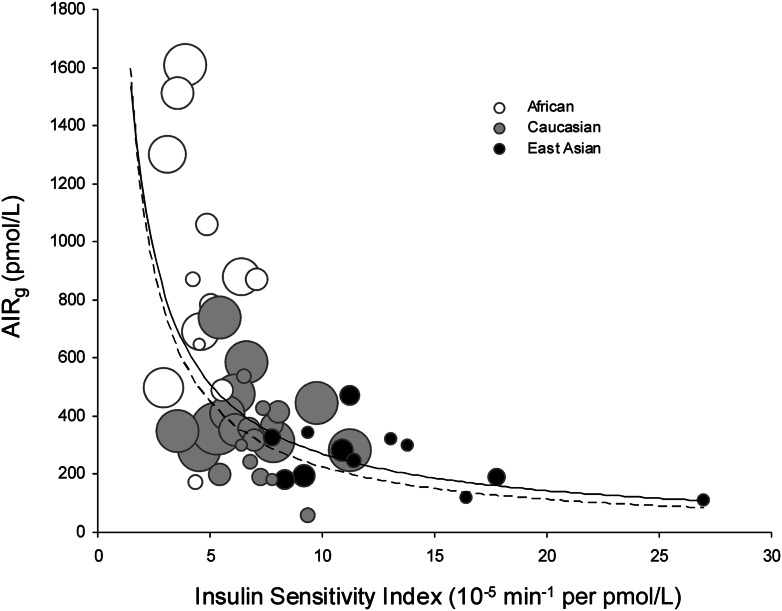
We used the method of Kahn et al. (2) to calculate the hyperbolic relationship between insulin sensitivity and insulin response. We used mean values of SI and AIRg in the NGT (healthy) cohorts and then stratified distributions by ethnicity (Fig. 2). Caucasian subpopulations clustered around the middle of the hyperbola, while African and East Asian subpopulations were located in unstable extreme points. In these areas, a small change in one variable is associated with a large nonlinear change in the other variable.
Figure 2.
Ethnic differences in the relationship between insulin sensitivity and insulin response in NGT cohorts. Scatter plot of SI vs. AIRg measured in NGT (healthy) African, Caucasian, and East Asian cohorts. Each circle represents one study cohort. Circle area is proportional to cohort sample size. The solid line is the curve calculated in our meta-analysis [ln(AIRg) = –0.915 × ln(SI) – 2.82]. The dashed line is the curve of Kahn et al. (2) describing healthy individuals who were primarily Caucasian [ln(AIRg) = –1.0 × ln(SI) – 3.80].
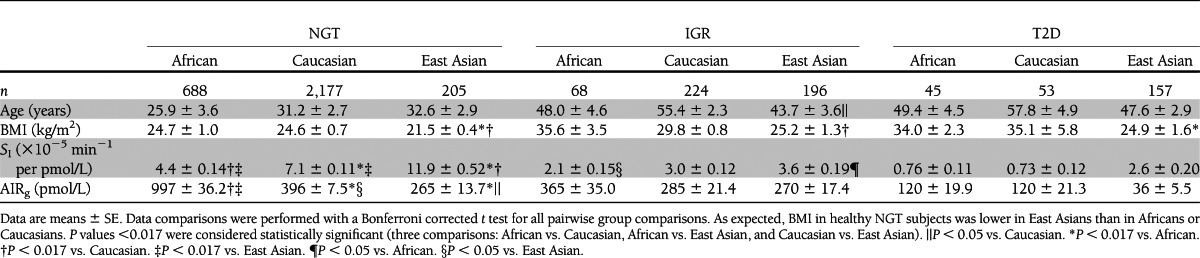
We then compared the mean values of these parameters between the two different ethnic groups using a two-tailed Welch t test. We found that Africans had significantly lower insulin sensitivity (SI) and higher insulin response (AIRg) than the other two groups. Insulin sensitivity (SI) in East Asians was higher than the other two groups, while insulin response (AIRg) was much lower than that of Africans and lower than Caucasians [SI (×10−5 min−1/pmol/L), African 4.4 ± 0.14, Caucasian 7.1 ± 0.11, East Asian 11.9 ± 0.52, African vs. Caucasian P = 5.3 × 10−5, African vs. East Asian P = 1.9 × 10−4, and Caucasian vs. East Asian P = 0.002; AIRg (pmol/L), African 997 ± 36.2, Caucasian 396 ± 7.5, East Asian 265 ± 13.7, African vs. Caucasian P = 6.4 × 10−4, African vs. East Asian P = 1.4 × 10−4, and Caucasian vs. East Asian P = 0.028] (Table 1).
Table 1.
Characteristics of NGT, IGR, or T2D cohort subjects
Ethnic differences in the relationship between SI and AIRg across glucose tolerance subgroups
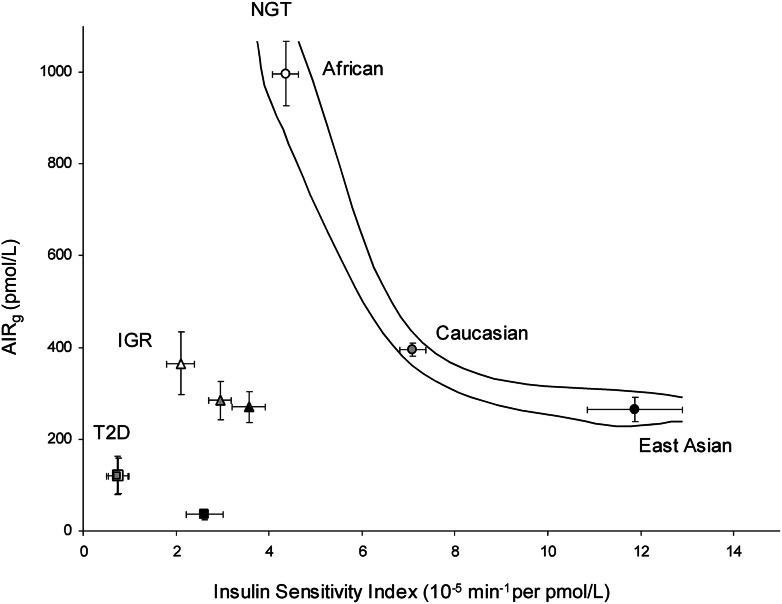
Our findings raised the question of whether there was ethnic variation in the relationship between insulin sensitivity and insulin response across three glucose tolerance subgroups. To address this question, we plotted the mean and 95% CI values of SI against AIRg in subjects with NGT, IGR, and T2D from each ethnic group. We found ethnic variation in the distribution of SI and AIRg across the three glucose tolerance subgroups (Table 1 and Fig. 3). These results suggest that in Africans, insulin response may be strikingly reduced in the course from NGT to T2D via IGR. In the same groups of Caucasians, decreased insulin sensitivity could be more predominant than decreased insulin response. In East Asians, insulin sensitivity may be strikingly reduced, while insulin response was not changed in the course from NGT to IGR. However, insulin response could be rapidly reduced from IGR to T2D.
Figure 3.
Ethnic differences in the relationship between insulin sensitivity and insulin response across glucose tolerance subgroups. Plot of mean ± 95% CI values of SI vs. AIRg measured in NGT (circles), IGR (triangles), or T2D (squares) subjects across three ethnic cohorts.
Publication bias
We assessed the possibility of publication bias in our included studies for each ethnic group in NGT using the method of Macaskill, Walter, and Irwig (17). This method uses a funnel-plot regression of AIRg or SI on the sample size, weighted by the inverse variance. We found no evidence of publication bias (AIRg, African P = 0.18, Caucasian P = 0.78, and East Asian P = 0.24; SI, African P = 0.14, Caucasian P = 0.25, and East Asian P = 0.42 [Supplementary Fig. 1]). We did not examine possible publication bias of the results on IGR and T2D group, as guidelines do not recommend testing for funnel-plot asymmetry in analyses of <10 studies (18).
Overall quality of evidence
We assessed the overall quality of the evidence for each ethnic group classified by degree of glucose tolerance (NGT, IGR, or T2D), with reference to the GRADE system (16). In NGT, we considered our results for all of the three ethnic groups to be of high quality, as most studies in this category had received maximum MNOS points. In IGR, the results of Caucasians and East Asians were deemed to be of high quality, while results for Africans were of moderate quality. In T2D, we judged evidence for East Asians to be of high quality. It was of moderate quality for Africans and low quality for Caucasians. We graded the overall evidence quality for IGR and T2D Africans as moderate because there were only three studies (<100 subjects) included in our meta-analysis. However, all of these studies had scored the maximum of 9 MNOS points. There were only three studies of T2D Caucasians (<100 subjects) in our meta-analysis, and the quality scores for two were medium and low quality on the MNOS. Therefore, we graded the overall quality of evidence on the T2D Caucasians as low. Overall, we regarded our results on the differences in the relationship between SI and AIRg in NGT across three ethnic groups to be generalizable to other populations in the same ethnic groups. However, we think that the results on the IGR and T2D categories could be partially changed by further research because these categories included one or two ethnic groups rated as low or moderate quality of evidence.
CONCLUSIONS
We have confirmed that there is a hyperbolic relationship between insulin sensitivity and insulin response in African, Caucasian, and East Asian NGT cohorts. We discovered ethnic differences in the stabilization points of insulin sensitivity and insulin response to maintain the normal blood glucose levels (canalization).
It has been reported that average values of height, weight, BMI, body composition, and fat distribution differ by ethnic group. Among healthy subjects, Africans have less visceral fat area but more skeletal muscle and bone mineral mass than Caucasians, while Africans and Caucasians tend to have similar body sizes (19–22). East Asians have smaller mean height, weight, and BMI and less mean total visceral fat volume than Africans and Caucasians (23–26). In fact, the BMI cutoffs for normal weight, overweight, and obesity proposed for Japanese and Singaporean people (who are majority Chinese) are lower than international classification criteria. For example, the Japan Society for the Study of Obesity defines obese class 1 as BMI ≥25 kg/m2, whereas the International Obesity Task Force defines it as BMI ≥30 kg/m2 (27–29).
The AIRg reflects pancreatic insulin secretion as well as hepatic insulin clearance. It is possible that lower AIRg in healthy East Asians in our meta-analysis may be affected by either 1) high insulin sensitivity due to low visceral fat content in healthy subjects or 2) lower baseline function of insulin secretion or higher of insulin clearance sufficient to maintain smaller body size compared with the other two ethnic groups. Some researchers have reported that the relationship between insulin sensitivity and insulin responses might be linear rather than hyperbolic in the Japanese population (30,31). It is possible that these research groups made this suggestion because they had observed only the part of the hyperbolic occupied by the Japanese population and misread it as a straight correlation line.
On the other hand, when we compared healthy Africans with healthy Caucasians, we found that the insulin response function was higher in Africans and that their insulin sensitivity was lower, as previously observed in many single studies (32–34). This finding was despite the fact that Africans have less visceral fat than Caucasians (19). It is paradoxical that Africans have less visceral fat and yet are more insulin resistant (and have greater insulin secretion and lesser insulin clearance) than other ethnic groups (35,36). This finding contradicts the expected positive association between visceral fat and insulin resistance. However, it suggests that insulin resistance in healthy Africans may be influenced by other factors. It is possible that Africans’ large amount of muscle and bone mass affects insulin sensitivity determinants, resulting in potential insulin resistance and more insulin response. In addition, a higher baseline insulin secretion or lower insulin clearance may be also needed to maintain and grow the large amount of muscle and bone in this group (21,22).
In general, as obesity progresses and insulin sensitivity decreases, the insulin response increases to maintain NGT. This process can be seen as “moving up” the hyperbolic curve (canalization). β-Cell exhaustion can induce failure of this compensation cycle, resulting in a deviation from NGT that precipitates the development of IGR and T2D. This process can be seen as “falling off” the curve (decanalization) (1,37).
We found that the stabilization points in the hyperbolic relationship between insulin sensitivity and insulin response in NGT Africans and East Asians are located around unstable extreme points in the curve in Fig. 2. At these points, a small change in one variable is associated with a large nonlinear change in the other variable. This fact allows us to speculate that in Africans, even a small increase in insulin resistance in modern life could cause a rapid increase in the amount of insulin secretion required to maintain NGT. We assume that this unstable feedback loop may be implicated in this group’s vulnerability of canalization of blood glucose levels. In fact, African Americans have a twofold higher risk of developing T2D compared with Caucasians (38).
It is thought that East Asians have a limited innate capacity of insulin secretion (39). This capacity may be decreased by aging or β-cell exhaustion due to continuous insulin resistance in many modern humans. We can therefore speculate that in East Asians, even a small decrease in insulin secretory function may lead to a rapid decrease in the threshold level of insulin resistance above which T2D occurs. This possible instability and vulnerability of canalization due to lower β-cell function may contribute to the increased prevalence of diabetes in East Asia in recent decades.
This systematic review and meta-analysis has strength and some limitations. An important strength of this work is that it is the first study to explicitly examine the relationship between insulin sensitivity and insulin response in three major ethnic groups, across different states of glucose tolerance, and on the basis of a comprehensive literature search. The major limitation in our meta-analysis is that we included only a few study cohorts (<100 subjects) in some groups in the IGR and T2D categories (IGR Africans, T2D Africans, and T2D Caucasians), even if most of these studies included were rated as high quality in our MNOS scoring system. We acknowledge that the results concerning these groups may be slightly changed by further experiments.
Another limitation was that the definition of degrees of glucose tolerance was not precisely the same in all studies, primarily because the original authors used different criteria (World Health Organization, American Diabetes Association, or Japan Diabetes Society recommendations) (9–11). Additionally, we cannot exclude the possibility that some NGT cohorts may have included participants with either a family history of T2D or a history of gestational diabetes mellitus when the original authors did not indicate the exclusion criteria for those participants. These may have reduced the comparability of each group in our meta-analysis.
Further, there may be some points that could affect estimation of SI values in Africans. For example, our criteria excluding obese cohorts from the NGT category may have inadvertently excluded insulin-sensitive individuals. There is evidence that overweight or obese insulin-sensitive subjects are predisposed to further weight gain (40,41). We may have missed individuals with relatively high SI and high AIRg (predisposed to weight gain), especially in the African obese cohorts. Another potential issue is an artifact of the MINMOD analysis. This model was originally developed in dogs and was not intended to be used outside of a narrow range of insulin and glucose values (7). This range may be exceeded by the high AIRg of NGT Africans. Several researchers have published studies concerning artifacts of MINMOD or the possibility of underestimation of SI in the higher peak of insulin concentrations (42–44). We assume that the SI values may have been underestimated in the individuals with high AIRg in NGT Africans. These issues may have slightly shifted the combined data of SI in NGT Africans in our meta-analysis.
Also, we acknowledge that most African subjects in our meta-analysis were unlikely to be of pure African ancestry, given that most of them were African Americans and may have some European ancestors. However, given the relative stability of Caucasian subjects here, this fact may mean that people of pure African descent could be even further up the hyperbolic curve than is indicated here.
Other factors that may have affected our results are related to methods used to evaluate SI. There were three methods: no modification (regular), insulin modified, or tolbutamide boosted. Two studies have reported that SI (insulin) was systematically and significantly lower than SI (tolbutamide) (12,13), while another reported that SI (insulin) was similar to SI (regular) (14). Of the 74 study cohorts in our meta-analysis, 10 used a tolbutamide protocol for all participants in the cohorts and 4 for the part of participants. We adjusted errors that can occur owing to different measurement methods in the 12 studies according to previously reported data (12,13). However, we could not correct the SI values in the remaining two cohorts because the ratio of participant-administrated tolbutamide was not specified. This would have changed the results of the individual studies and, hence, slightly affected the results of combined data.
Finally, we acknowledge that our literature search may have missed studies involving FSIGT, AIRg, SI, and our three ethnic groups. PubMed searches only titles and abstracts and not the full text of articles. If the titles or abstracts of relevant studies did not include our keywords (Supplementary Data), we would not have identified them for inclusion in our meta-analysis. Our search was also limited to publications in English, and we may have missed articles in other languages. For resolution of these limitations, we hope that a simultaneous multinational survey with a unified method and criteria will be conducted in the future.
In spite of these limitations, our results have significant implications for public health and clinical care. We have demonstrated that there is a hyperbolic relationship between insulin sensitivity and insulin response in healthy NGT cohorts across three ethnic groups (African, Caucasian, and East Asian). We also found an ethnically inherent difference in the optimal points in the canalization of normal blood glucose levels. In addition, we also found ethnic differences in the distribution of insulin sensitivity and insulin response in the course from NGT to T2D (decanalization). Our findings imply these scenarios: Africans have evolved to develop very robust β-cell function to maintain NGT despite having relatively higher insulin resistance. East Asians have evolved to be very insulin sensitive and thus require less robust β-cells. However, there are some Africans who cannot increase insulin secretion further, and they are prone to developing IGR and T2D with further decrease in insulin sensitivity. There are also some East Asians who, because they have especially vulnerable β-cells, despite relatively good insulin sensitivity, are unable to increase insulin secretion further if there is even a slight decrease in insulin sensitivity. These people thus develop IGR and T2D. In both scenarios, environmental factors that contribute to decreased insulin sensitivity would play roles in decanalization. Our findings suggest that it is necessary to consider a patient’s ethnic background when addressing prevention, surveillance, and treatment of IGR and T2D.
Acknowledgments
This work was supported by grants from the Howard Hughes Medical Institute, the National Library of Medicine (R01 LM009719), and the Lucile Packard Foundation for Children's Health.
No potential conflicts of interest relevant to this article were reported.
K.K. designed the research; performed the literature search, independent review of articles, and data extraction and analysis; wrote the manuscript; participated in data interpretation; provided critical review of the draft; and approved the final version. D.T. performed the literature search, independent review of articles, and data extraction and analysis; participated in data interpretation; provided critical review of the draft; and approved the final version. S.Y., K.T., and C.J.P. participated in data interpretation, provided critical review of the manuscript, and approved the final version. A.J.B. designed the research, participated in data interpretation, provided critical review of the manuscript, and approved the final version. K.K. and A.J.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. R. Chen in the Division of Systems Medicine, Stanford University School of Medicine, for helpful comments on the study.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1235/-/DC1.
References
- 1.Gibson G. Decanalization and the origin of complex disease. Nat Rev Genet 2009;10:134–140 [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 3.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet 2008;9:403–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R, Corona E, Sikora M, et al. Type 2 diabetes risk alleles demonstrate extreme directional differentiation among human populations, compared to other diseases. PLoS Genet 2012;8:e1002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruff CB. Variation in human body size and shape. Annu Rev Anthropol 2002;31:211–232 [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 8.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986;23:113–122 [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27(Suppl. 1):S5–S10 [DOI] [PubMed] [Google Scholar]
- 11.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. Journal of Diabetes Investigation 2010;1:212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saad MF, Steil GM, Kades WW, et al. Differences between the tolbutamide-boosted and the insulin-modified minimal model protocols. Diabetes 1997;46:1167–1171 [DOI] [PubMed] [Google Scholar]
- 13.Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 1990;71:1508–1518 [DOI] [PubMed] [Google Scholar]
- 14.Pacini G, Tonolo G, Sambataro M, et al. Insulin sensitivity and glucose effectiveness: minimal model analysis of regular and insulin-modified FSIGT. Am J Physiol 1998;274:E592–E599 [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, O'Connell D, Pearson J, Welch V, Lossos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [article online], 2011. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed 3 September 2012
- 16.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med 2001;20:641–654 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S; Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions, version 5.1.0 [article online], 2011. Available from www.cochrane-handbook.org Accessed 28 August 2012
- 19.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism 1996;45:1119–1124 [DOI] [PubMed] [Google Scholar]
- 20.Van Horn LV, Ballew C, Liu K, et al. Diet, body size, and plasma lipids-lipoproteins in young adults: differences by race and sex. The Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Epidemiol 1991;133:9–23 [DOI] [PubMed] [Google Scholar]
- 21.Ortiz O, Russell M, Daley TL, et al. Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. Am J Clin Nutr 1992;55:8–13 [DOI] [PubMed] [Google Scholar]
- 22.Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res 2001;16:1343–1352 [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 1994;60:23–28 [DOI] [PubMed] [Google Scholar]
- 24.Kagawa M, Kerr D, Uchida H, Binns CW. Differences in the relationship between BMI and percentage body fat between Japanese and Australian-Caucasian young men. Br J Nutr 2006;95:1002–1007 [DOI] [PubMed] [Google Scholar]
- 25.Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol 2003;40(Suppl. 1):S302–S304 [DOI] [PubMed] [Google Scholar]
- 26.Kadowaki T, Sekikawa A, Murata K, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond) 2006;30:1163–1165 [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. Asia Pac J Clin Nutr 2002;11(Suppl. 8):S732–S737 [DOI] [PubMed] [Google Scholar]
- 28.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet 2002;360:235. [DOI] [PubMed] [Google Scholar]
- 29.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i–xii, 1–253 [PubMed] [Google Scholar]
- 30.Asano T, Yoshida R, Ogata H, et al. Beta-cell function is a major contributor to oral glucose disposition in obese Japanese students. Endocr J 2007;54:903–910 [DOI] [PubMed] [Google Scholar]
- 31.Sakaue S, Ishimaru S, Ikeda D, et al. Estimation of beta-cell function from the data of the oral glucose tolerance test. Am J Physiol Endocrinol Metab 2007;292:E1575–E1580 [DOI] [PubMed] [Google Scholar]
- 32.Festa A, Williams K, D’Agostino R, Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 2006;55:1114–1120 [DOI] [PubMed] [Google Scholar]
- 33.Goedecke JH, Dave JA, Faulenbach MV, et al. Insulin response in relation to insulin sensitivity: an appropriate beta-cell response in black South African women. Diabetes Care 2009;32:860–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goree LLT, Darnell BE, Oster RA, Brown MA, Gower BA. Associations of free fatty acids with insulin secretion and action among African-American and European-American girls and women. Obesity (Silver Spring) 2010;18:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med 1994;11:755–762 [DOI] [PubMed] [Google Scholar]
- 36.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab 2002;87:2218–2224 [DOI] [PubMed] [Google Scholar]
- 37.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 38.Haffner SM. Epidemiology of type 2 diabetes: risk factors. Diabetes Care 1998;21(Suppl. 3):C3–C6 [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto WY. Overview of non-insulin-dependent diabetes mellitus (NIDDM) in different population groups. Diabet Med 1996;13(Suppl. 6):S7–S10 [PubMed] [Google Scholar]
- 40.Yost TJ, Jensen DR, Eckel RH. Weight regain following sustained weight reduction is predicted by relative insulin sensitivity. Obes Res 1995;3:583–587 [DOI] [PubMed] [Google Scholar]
- 41.Swinburn BA, Nyomba BL, Saad MF, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest 1991;88:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quon MJ, Cochran C, Taylor SI, Eastman RC. Non-insulin-mediated glucose disappearance in subjects with IDDM. Discordance between experimental results and minimal model analysis. Diabetes 1994;43:890–896 [DOI] [PubMed] [Google Scholar]
- 43.Finegood DT, Tzur D. Reduced glucose effectiveness associated with reduced insulin release: an artifact of the minimal-model method. Am J Physiol 1996;271:E485–E495 [DOI] [PubMed] [Google Scholar]
- 44.Prigeon RL, Røder ME, Porte D, Jr, Kahn SE. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Invest 1996;97:501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]