Abstract
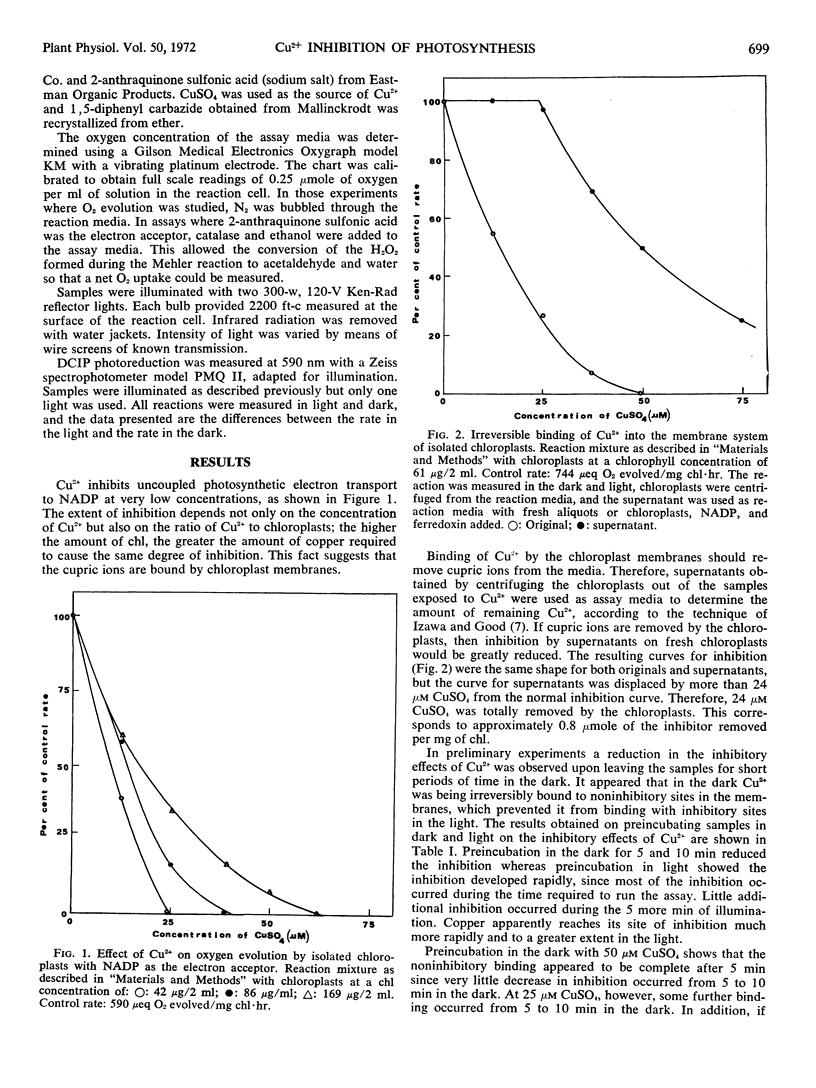
Strong inhibition of uncoupled photosynthetic electron transport by Cu2+ in isolated spinach chloroplasts was observed by measuring changes in O2 concentration in the reaction medium. Inhibition was dependent not only on the concentration of the inhibitor, but also on the ratio of chlorophyll to inhibitor. Binding of Cu2+ to the chloroplast membranes resulted in removal of Cu2+ from solution. When chloroplasts were exposed to preincubation in light, there was increased inhibition as a result of Cu2+ binding to inhibitory sites. Preincubation in the dark resulted in Cu2+ binding to noninhibitory sites and decreased inhibition. The degree of inhibition was lower at low light intensities than at high light intensities.
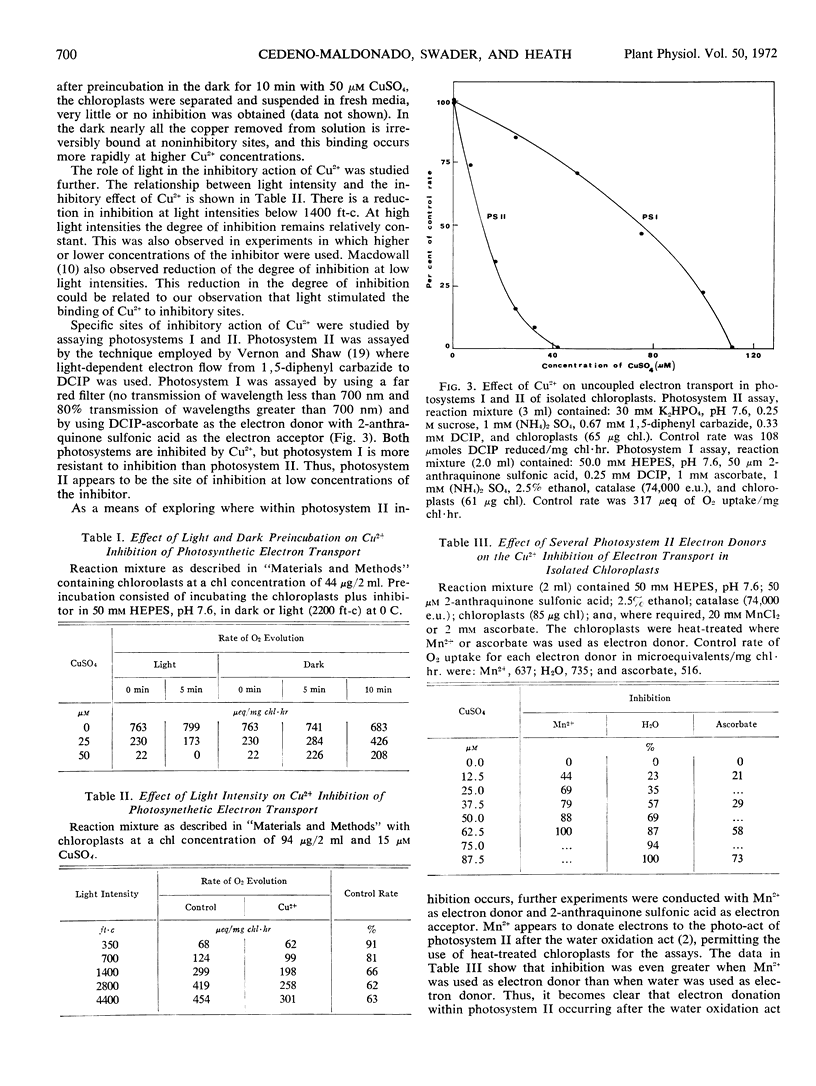
When the photosystems were assayed separately, photosystem I was more resistant to inhibition than photosystem II. The most sensitive site to the inhibitor was the oxidizing side of photosystem II.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hayyim G., Avron M. Mn2+ as electron donor in isolated chloroplasts. Biochim Biophys Acta. 1970 Apr 7;205(1):86–94. doi: 10.1016/0005-2728(70)90064-2. [DOI] [PubMed] [Google Scholar]
- Gross R. E., Pugno P., Dugger W. M. Observations on the mechanism of copper damage in chlorella. Plant Physiol. 1970 Aug;46(2):183–185. doi: 10.1104/pp.46.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann H. M. Reversal of copper inhibition in chloroplast reactions by manganese. Plant Physiol. 1969 Mar;44(3):331–336. doi: 10.1104/pp.44.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkson J. W., Vernon L. P. Comparison of Three Photochemical Activities of Chloroplasts. Plant Physiol. 1959 May;34(3):268–272. doi: 10.1104/pp.34.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Good N. E. The number of sites sensitive to 3-(3,4-dichlorophenyl)-1,1-dimethylurea,3-(4-chlorophenyl)-1,1-dimethylurea and 2-chloro-4-(2-propylamino)-6-ethylamino-s-triazine in isolated chloroplasts. Biochim Biophys Acta. 1965 May 25;102(1):20–38. doi: 10.1016/0926-6585(65)90200-1. [DOI] [PubMed] [Google Scholar]
- KATOH S., SHIRATORI I., TAKAMIYA A. Purification and some properties of spinach plastocyanin. J Biochem. 1962 Jan;51:32–40. doi: 10.1093/oxfordjournals.jbchem.a127497. [DOI] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Stocking C. R. Oxygen evolution and the permeability of the outer envelope of isolated whole chloroplasts. Plant Physiol. 1968 Oct;43(10):1597–1604. doi: 10.1104/pp.43.10.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon L. P., Shaw E. R. Oxidation of 1,5-diphenylcarbazide as a measure of photosystem 2 activity in subchloroplast fragments. Biochem Biophys Res Commun. 1969 Sep 10;36(6):878–884. doi: 10.1016/0006-291x(69)90285-x. [DOI] [PubMed] [Google Scholar]
- WALKER J. B. Inorganic micronutrient requirements of chlorella. I. Requirements for calcium (or strontium), copper, and molybdenum. Arch Biochem Biophys. 1953 Sep;46(1):1–11. doi: 10.1016/0003-9861(53)90163-5. [DOI] [PubMed] [Google Scholar]


