Abstract
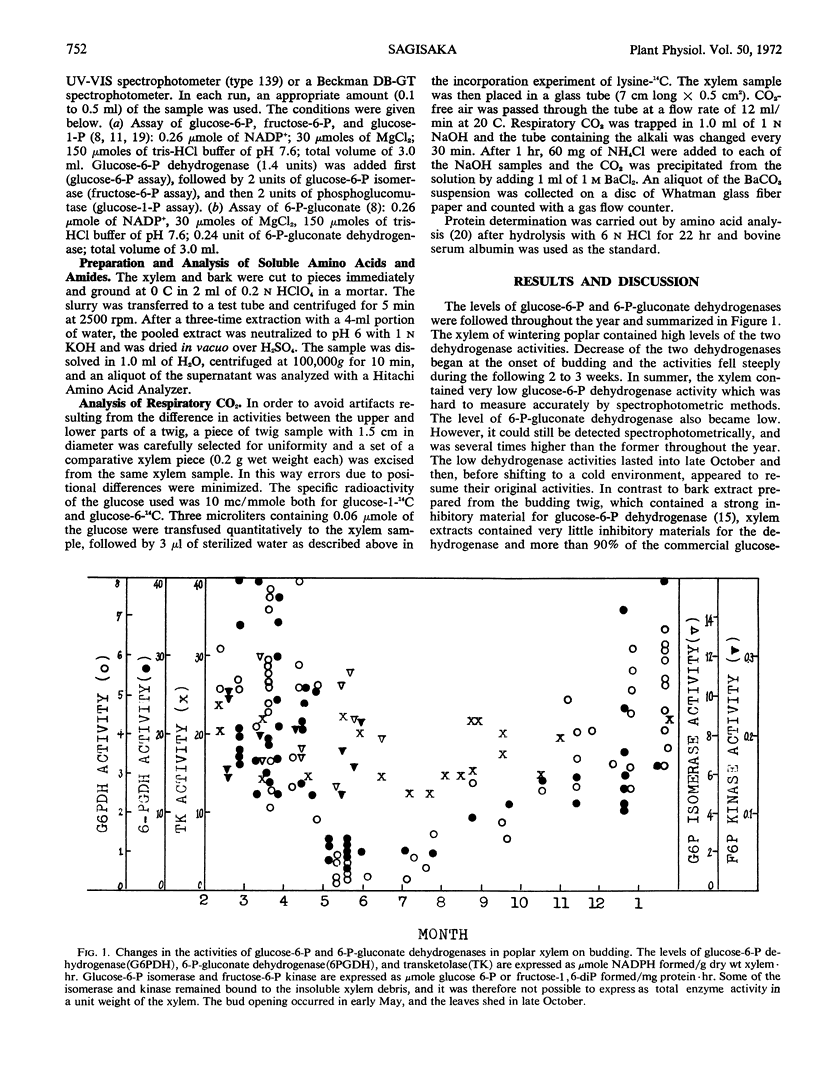
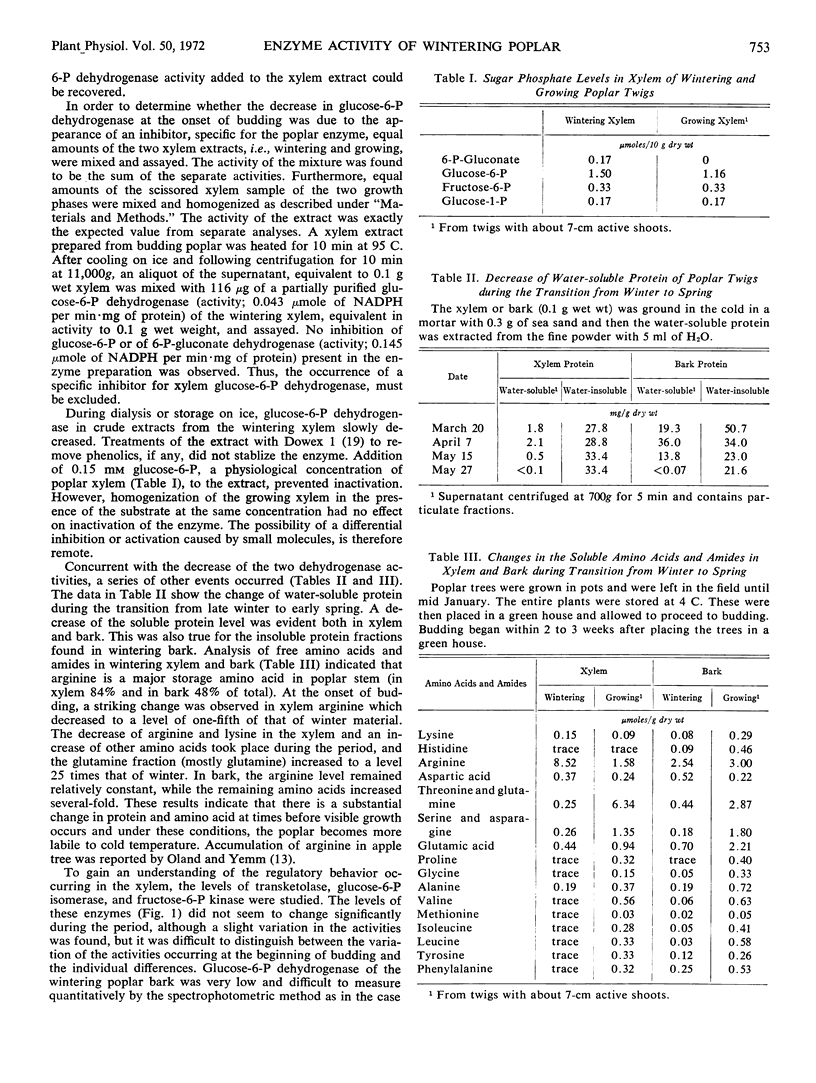
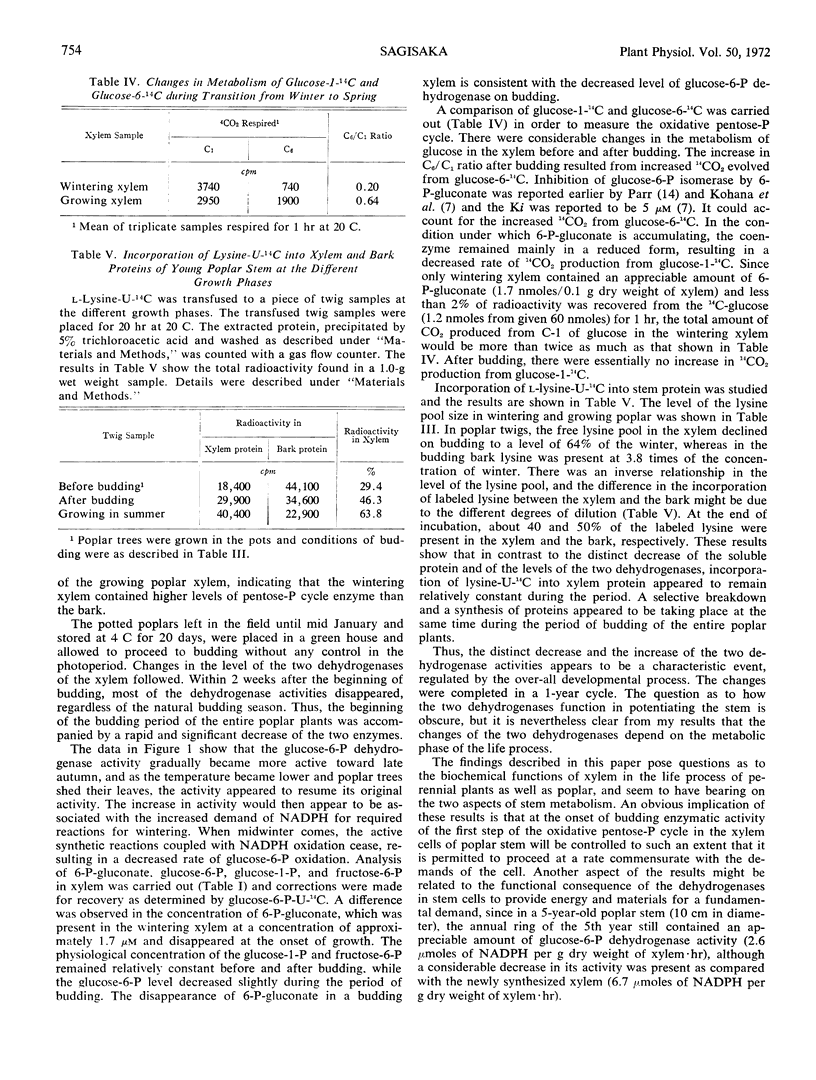
The activities of glucose 6-phosphate and 6-phosphogluconate dehydrogenases, transketolase, phosphoglucose isomerase, and fructose 6-phosphate kinase were studied in extracts of wintering poplar (Populus gelrica) xylem. The xylem of wintering poplar showed high levels of transketolase, glucose 6-phosphate, and 6-phosphogluconate dehydrogenases. On recommencement of growth, the two dehydrogenase activities decreased. The three remaining enzymes appeared to be unchanged. In spring and early summer, glucose 6-phosphate dehydrogenase of the xylem was extremely low. On the other hand, 6-phosphogluconate dehydrogenase, which also became lower during the metabolic shift from winter to spring, was readily detected, and was several times higher than glucose 6-phosphate dehydrogenase throughout the year. The low dehydrogenase activities lasted into late October and then appeared to resume their original activity. A shift of metabolism at the beginning of growth was also observed by measuring the amount of sugar phosphates, soluble amino acids and amides, and proteins in the xylem. In contrast to the decrease of the two dehydrogenases and soluble proteins at the time of budding, incorporation of lysine-U-14C into the xylem protein ramained constant. A method to transfuse radioactive compounds into a section of stem was described.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., SALTMAN P., BANDURSKI R. S., BAKER R. S. Phosphonexokinase in higher plants. J Biol Chem. 1952 May;197(1):89–96. [PubMed] [Google Scholar]
- DE LA HABA G., LEDER I. G., RACKER E. Crystalline transketolase from bakers' yeast: isolation and properties. J Biol Chem. 1955 May;214(1):409–426. [PubMed] [Google Scholar]
- KAHANA S. E., LOWRY O. H., SCHULZ D. W., PASSONNEAU J. V., CRAWFORD E. J. The kinetics of phosphoglucoisomerase. J Biol Chem. 1960 Aug;235:2178–2184. [PubMed] [Google Scholar]
- Lam T. H., Shaw M. Removal of phenolics from plant extracts by grinding with anion exchange resin. Biochem Biophys Res Commun. 1970 Jun 5;39(5):965–968. doi: 10.1016/0006-291x(70)90418-3. [DOI] [PubMed] [Google Scholar]
- Nevins D. J., English P. D., Albersheim P. The specific nature of plant cell wall polysaccharides. Plant Physiol. 1967 Jul;42(7):900–906. doi: 10.1104/pp.42.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARR C. W. Inhibition of phosphoglucose isomerase. Nature. 1956 Dec 22;178(4547):1401–1401. doi: 10.1038/1781401a0. [DOI] [PubMed] [Google Scholar]
- SLEIN M. W. Phosphomannose isomerase. J Biol Chem. 1950 Oct;186(2):753–761. [PubMed] [Google Scholar]
- Siminovitch D., Wilson C. M., Briggs D. R. Studies on the Chemistry of the Living Bark of the Black Locust in Relation to Its Frost Hardiness. V. Seasonal Transformations and Variations in the Carbohydrates: Starch-Sucrose Interconversions. Plant Physiol. 1953 Jul;28(3):383–400. doi: 10.1104/pp.28.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABACHNICK M., SRERE P. A., COOPER J., RACKER E. The oxidative pentose phosphate cycle. III. The interconversion of ribose 5-phosphate, ribulose 5-phosphate and xylulose 5-phosphate. Arch Biochem Biophys. 1958 Apr;74(2):315–325. doi: 10.1016/0003-9861(58)90003-1. [DOI] [PubMed] [Google Scholar]


