Abstract
Resolution of inflammation is a coordinated and active process aimed at restoration of tissue integrity and function. This review integrates the key molecular and cellular mechanisms of resolution. We describe how abrogation of chemokine signalling blocks continued neutrophil tissue infiltration and how apoptotic neutrophils attract monocytes and macrophages to induce their clearance. Uptake of apoptotic neutrophils by macrophages reprograms macrophages towards a resolving phenotype, a key event to restore tissue homeostasis. Finally, we highlight the therapeutic potential that derives from understanding the mechanisms of resolution.
Keywords: apoptosis, efferocytosis, macrophage reprogramming, pro-resolving drugs, tissue homeostasis
Introduction
Inflammation is a pathophysiological response to infection or tissue damage. In order to neutralize the causing agent, the innate immune system launches a program that unfolds in several phases (Soehnlein & Lindbom, 2010). Initially, tissue-resident cells of the innate immune system detect the damaging insult and alarm circulating neutrophils. These migrate to the inflamed tissue, promote recruitment of inflammatory monocytes and potentiate the pro-inflammatory environment allowing to appropriately deal with the inflammogen (Mantovani et al, 2011). Under normal conditions, neutrophils undergo apoptosis after performing their action at the inflamed site (Fox et al, 2010) and macrophages ingest apoptotic neutrophils. Clearance of apoptotic neutrophils prompts a switch from a pro- to an anti-inflammatory macrophage phenotype (Fadok et al, 1998; Michlewska et al, 2009), which is a prerequisite for macrophage egress via the lymphatic vessels favouring return to tissue homeostasis (Fig 1).
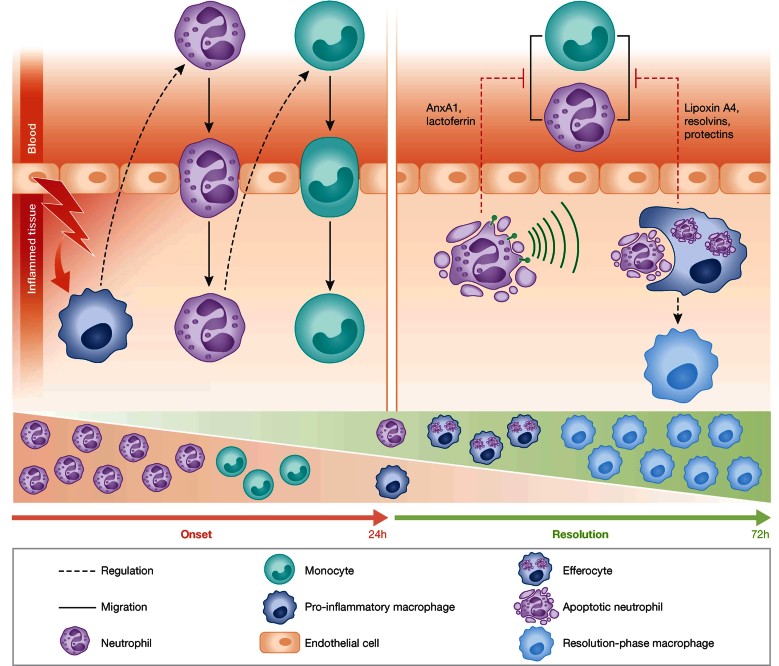
Figure 1.
Cellular interplay during resolution of inflammation. Overview of cellular processes during onset (left) and resolution (right) of inflammation. During early phases of inflammation tissue-resident cells sense damage and launch the release of signals that induce rapid neutrophil and delayed monocyte emigration. Resolution is initiated when neutrophils become apoptotic thus secreting mediators that inhibit continued neutrophil infiltration. Ingestion of apoptotic neutrophils changes the macrophage phenotype towards a resolution-phase macrophage, which promotes return to tissue homeostasis. A switch in tissue (stromal) cells can also contribute to generate the initial signals for resolution to start.
The past few years have witnessed an appreciation of the complex resolution of inflammation process, characterized by molecular and cellular events that ultimately assure tissue repair and regain of physiological function. A crucial point is the concept that resolution is an active phenomenon, aimed at actively suppressing and extinguishing a vibrant inflammatory reaction once its main objective (e.g. clearance of bacteria) is attained. In this review we highlight the essential mechanisms underlying resolution of inflammation and discuss how these mechanisms can be targeted to accelerate return to tissue integrity.
Chemokine depletion sets the brakes on neutrophil tissue infiltration
Chemokines are molecular cues that orchestrate leukocyte migration to sites of inflammation. Abrogation of neutrophil influx is a prerequisite for resolution of inflammation and mechanisms such as chemokine cleavage by proteolysis and chemokine sequestration are necessary to attain a resolving environment (Fig 2A). Macrophage-specific MMP12 cleaves CXC chemokines in the ELR motif, which is crucial for receptor binding, thus rendering them unable to recruit neutrophils (Dean et al, 2008). MMP-dependent chemokine cleavage also depletes CC-chemokines that preferentially attract classical monocytes. For instance, cleaved CCL7 continues to bind CCR1, CCR2 and CCR3, but fails to induce downstream signalling and chemotaxis, thus acting as a general antagonist dampening inflammation (McQuibban et al, 2000, 2002).
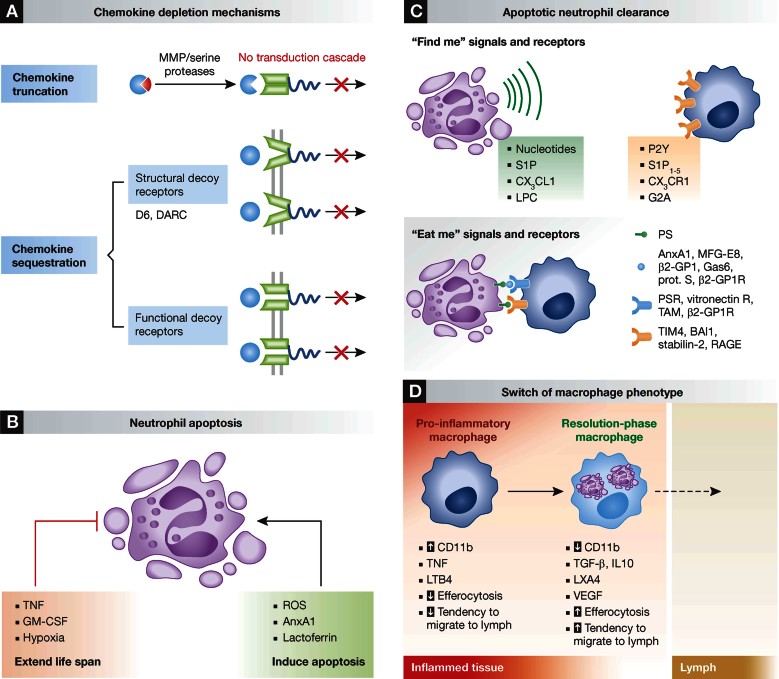
Figure 2.
Mechanisms of neutrophil- and macrophage-driven resolution. A. Depletion of chemokines during resolution. MMPs cleave CC and CXC chemokines rendering them non-functional. Structural decoy receptors such as D6 and DARC sequester chemokines without subsequent signal transduction. Functional decoy receptors are classical chemokine receptors with repressed signalling. B. Factors controlling neutrophil life span at sites of inflammation. Interestingly, the pro-apoptotic stimuli can often override those that augment neutrophil life span. C. Upon apoptosis find me signals such as nucleotides, S1P, CX3CL1 and LPC are released that attract scavengers. These recognize apoptotic cells via eat me signals exposed on the cell surface. Clearance is mediated by direct cell–cell contact or by involvement of bridging molecules. D. In response to local mediators and upon efferocytosis, pro-inflammatory macrophages switch to resolution-phase macrophages.
Chemokine receptors possess a conserved DRY motif in the second intracellular loop which is involved in coupling to G-proteins. Receptors lacking the DRY motif sequester chemokines without launching a signalling cascade and are hence termed decoy receptors (Mantovani et al, 2006). Prominent members of this subfamily are duffy antigen receptor for chemokines (DARC) and D6. DARC is predominantly expressed by endothelial cells near sites of leukocyte extravasation and can sequester CC and CXC pro-inflammatory chemokines (Gardner et al, 2004). The lack of this atypical chemokine receptor leads to increased neutrophil influx into lung and liver upon application of lipopolysaccharide (LPS; Dawson et al, 2000; Luo et al, 2000) hence supporting its importance in termination of neutrophil recruitment. D6 on the other hand, binds a broad range of CC chemokines including most agonists of CCR1, CCR2 and CCR5 (Bonecchi et al, 2004). D6-deficient mice exhibit a dysregulated inflammatory reaction with impaired clearance of chemokines, resulting in tissue accumulation of neutrophils (Jamieson et al, 2005). After myocardial infarction, D6 prevents excessive infiltration of classical monocytes and neutrophils by scavenging CCL2 (Cochain et al, 2012) a phenomenon functionally associated with adverse remodelling and reduced ejection fraction. Another atypical chemokine receptor, CCX-CKR, scavenges constitutive chemokines such as CCL19, CCL21 and CCL25 which control homeostatic leukocyte trafficking. Therefore, an overriding function of this receptor in the resolution of inflammation appears rather unlikely (Bunting et al, 2013).
A sophisticated mechanism for chemokine entrapment is the generation of functional decoy receptors from classical chemokine receptors. In an environment rich in microbial peptides or pro-inflammatory cytokines, IL10 inhibits down-regulation of CCR1, CCR2 and CCR5, but these receptors are now unable to translate ligand binding into cell migration, thus trapping pro-inflammatory chemokines (D'Amico et al, 2000). A similar mechanism is centred on the up-regulation of CCR5 on apoptotic neutrophils leading to depletion of pro-inflammatory chemokines during the resolution phase of acute murine peritonitis (Ariel et al, 2006).
Neutrophil apoptosis is central to resolution
Aborted neutrophil recruitment is but one of the steps required to reconstitute tissue homeostasis, clearance of neutrophils must also be achieved in due time. Despite the recent controversy regarding the life span of neutrophils (Dixon et al, 2012; Pillay et al, 2010), they are in general considered to be short-lived. Once emigrated, the lifespan of neutrophils can be modified by the local inflammatory environment (Fox et al, 2010; Geering & Simon, 2011; Fig 2B). For instance, macrophages secrete death receptor ligands such as Fas-ligand and TNF to promote neutrophil survival at low concentrations and neutrophil death at higher concentrations (van den Berg et al, 2001). In this regulatory pathway, phosphoinositide 3-kinase (PI3K)-mediated reactive oxygen species (ROS) production represents the key event in limiting cell survival and triggering apoptosis (Geering et al, 2011). Furthermore, Btk, a negative regulator of NADPH activation, is associated with neutrophil survival. Thus, Btk-deficient neutrophils feature high levels of ROS production, driven by excessive NADPH and PI3K activation (Honda et al, 2012). Other environmental signals such as hypoxia extend neutrophil lifespan; neutrophilic inflammation occurs in hypoxic environments, where hypoxia-inducible factor-1α (HIF1α) interferes with neutrophil survival (Walmsley et al, 2011). Finally, GM-CSF, a survival factor for neutrophils, triggers ERK activation and interference with ERK or other life span prolonging signalling pathways may allow for induction of neutrophil apoptosis.
Intriguingly, apoptotic neutrophils may attenuate inflammation through distinct mechanisms. Dying neutrophils secrete mediators that inhibit further neutrophil recruitment. One is annexin A1 (AnxA1; 37 kDa), a protein abundant in the cytosol of resting neutrophils. AnxA1 translocates to the plasma membrane when activated, where it interacts with the formyl peptide receptor 2 (FPR2/ALX) to moderate leukocyte adhesion and migration (Dalli et al, 2008; Perretti et al, 2002). It also promotes neutrophil apoptosis and clearance of dead neutrophils by macrophages (Perretti & Solito, 2004; Scannell et al, 2007). Another neutrophil-derived protein with similar activities is lactoferrin. It is contained within the secondary granules of neutrophils and when released, lactoferrin binds to specific receptors that trigger MAPK-mediated intracellular signalling, which is crucial in the regulation of cytoskeletal remodelling and cell adhesion (Bournazou et al, 2009). In a model of acute lung injury, lactoferrin application prevents neutrophil tissue infiltration, oedema formation and improves lung function (Table 1; Li et al, 2012). Nonetheless, lactoferrin exerts survival effects on neutrophils in rheumatoid arthritis (Wong et al, 2009) depending on its iron saturation level (Francis et al, 2011). Thus, the survival/apoptosis balance in neutrophils is monitored by an intricate and complex network of signalling factors which might present opposite effects depending on their concentration (e.g. TNF) or iron saturation state (e.g. lactoferrin).
Table 1.
In vivo approaches to induce resolution of inflammation
| Disease model | Drug | Effect on resolution | Refs. |
|---|---|---|---|
| Inhibition of leukocyte recruitment | |||
| Air pouch model in mice | AnxA1 of human neutrophil-derived microparticles | Decrease of leukocyte infiltration | Dalli et al (2008) |
| Rheumatoid arthritis in mice | LXA4 | Decrease of oedema, neutrophil influx, and mRNA levels of CXCL1, LTB4, and TNF after LXA4 treatment | Conte et al (2010) |
| Peritonitis in mice | Nanoparticles containing aspirin-triggered RvD1 or a lipoxinA4 analogue | Reduced neutrophil influx into the peritoneum, and resolution intervals | Norling et al (2011) |
| LPS-induced ALI | lactoferrin | Reduced lung neutrophil infiltration with less oedema and plasma leakage | Li et al (2012) |
| Air pouch and skin inflammation in mice | LXA4 analogue | Reduced neutrophil infiltration | Clish et al (1999) |
| Peritonitis in mice | RvE1 | Reduced leukocyte infiltration into the peritoneum | Arita et al (2005) |
| Colitis in mice | RvE1 | Increased survival rates, sustained body weight, improvement of histologic scores, reduced serum anti-2,4,6-trinitrobenzene sulfonic acid IgG and reduction of leukocyte infiltration and pro-inflammatory gene expression | Arita et al (2005) |
| Peritonitis in mice | Chemerin C15 | Reduction of neutrophil and monocyte infiltration into the peritoneum, enhancement of apoptotic cell phagocytosis of by macrophages and reduction of pro-inflammatory mediators | Cash et al (2008) |
| Induction of neutrophil apoptosis | |||
| Acute pleurisy, lung injury and arthritis in mice | R-roscovitine (CDK inhibitor) | Reduced presence of inflammatory cells (neutrophil and monocytes/macrophages) and amelioration of disease according to clinical scores | Rossi et al (2006) |
| Acute lung injury mice | 15-epi-LXA4 | Increased neutrophil apoptosis and efferocytosis | El Kebir et al (2009) |
| Lung inflammation in rats | Ectoine | Restoration of normal neutrophil apoptosis rates | (Sydlik et al 2013) |
| Pleurisy in rats | PD98059 (ERK inhibitor) | Decrease of total number macrophages and neutrophils in the pleural cavity, increased rate of neutrophil apoptosis | Sawatzky et al (2006) |
| Acute pleurisy in mice | Ac2-26 (AnxA1-active N-terminal peptide) | Reduction of neutrophils by induction of neutrophil apoptosis | Vago et al (2012) |
| Peritonitis in mice | Histone deacetylase inhibitors | AnxA1-dependent decrease in neutrophil, monocyte and macrophage infiltration | Montero-Melendez et al (2013) |
| Enhancement of efferocytosis and macrophage reprogramming | |||
| Microbial peritonitis/air pouch model in mice | RvD1, RvD5, PD1s | Enhancement of bacterial killing, neutrophil efferocytosis and prevention of hypothermia. Acceleration of resolution in combination with antibiotics | Chiang et al (2012) |
| Allergic airway inflammation model in mice | RvE1 | Suppression of the production of IL17, IL23 and IL6 and increased concentration of IFN-γ and LXA4 in brocheoalveolar lavage fluid, decrease of resolution intervals, acceleration of resolution of allergic airway pathology and hyper-responsiveness | Haworth et al (2008) |
| Sepsis model in rats | LXA4 | Increase of animal survival, decrease of blood bacterial load and pro-inflammatory mediators | Walker et al (2011) |
| Peritonitis in mice | RGD-AnxA5 | Enhancement of engulfment of apoptotic cells by macrophages and increase of secretion of IL-10 during efferocytosis | Schutters et al (2013) |
| CGD in mice | IFN-γ | Enhanced engulfment of apoptotic cells by macrophages | Fernandez-Boyanapalli et al (2010) |
| Peritonitis in CGD mice | IFN-γ | Enhanced engulfment of apoptotic cells by macrophages | Fernandez-Boyanapalli et al (2010) |
| Early renal fibrosis in rats | LXA4/benzoLXA4 | Prevention of collagen deposition and renal apoptosis. Inhibition of TNF production and stimulation of IL-10 | Borgeson et al (2011) |
| CGD in mice | IL4 | Normalized CGD macrophage efferocytosis | Fernandez-Boyanapalli et al (2009) |
| CGD in mice | PS | Restored IL4-dependent macrophage reprogramming and efferocytosis | Fernandez-Boyanapalli et al (2009) |
| Stimulation of tissue repair | |||
| Mucosal injury and ulceration in mice | AnxA1 | Promotion of intestinal epithelial migration trough activation of FPR1-, Rac1- and NOX1-dependent redox signalling to induce wound repair | Leoni et al (2013) |
| Rheumatoid arthritis model in mice | Flavopiridol (CDK inhibitor) | Suppression of synovial hyperplasia and joint destruction | Sekine et al (2008) |
| Rheumatoid arthritis model in mice | CDK4/6-selective inhibitor | Suppression of synovial hyperplasia and joint destruction | Sekine et al (2008) |
| Peritoneal and lung inflammation in mice | PS/PC liposomes | Induction of TGF-β secretion, resulting in accelerated resolution of inflammation | Huynh et al (2002) |
| Myocardial infarction in rats | PS/PC liposomes | Induction of angiogenesis, preservation of small scars, prevention of ventricular dilatation and remodelling | Harel-Adar et al (2011) |
| Retinal ischaemia in mice | PS/PC liposomes | Reduced expression of pro-inflammatory genes, reduced neuronal death in the retina after ischaemia-reperfusion | Dvoriantchikova et al (2009) |
| Liver cirrhosis in rats | Vitamin A-coupled liposomes carrying siRNAgp46 | Decrease in collagen recreation from HS cells and abrogation of hepatic stellate cells in fibrotic tissue through apoptosis, resulting in reduced fibrotic area in rat kidneys | Sato et al (2008) |
ALI, acute lung injury; Anx, annexin; CGD, chronic glomerular disease; LXA4, IFN, interferon; Lipoxin A4; PC, phosphatidylcholine; PS, phosphatidlyserine; Rv, resolving.
Apoptotic neutrophils are cleared by macrophages via efferocytosis. Apoptotic neutrophils promote their own clearance by expressing find me and eat me signals (Fig 2C). Find me signals are secreted factors that attract scavengers. To date, four major find me signals have been described: lysophosphatidylcholine (LPC), sphingosine 1-phosphate (S1P), fractalkine (CX3CL1), and the nucleotides ATP and UTP (Elliott et al, 2009; Gude et al, 2008; Lauber et al, 2003; Truman et al, 2008). Find me signal gradients guide the efferocyte towards the dead cell through the involvement of G2A, S1P1-5, CX3CR1, and P2Y2 receptors, respectively. Eat me signals are surface markers which allow the identification of a dying cell. These signals can be either molecules exposed de novo at the cell membrane or existing ones that undergo modifications during apoptosis, as for instance peptides derived from the AnxA1 N-terminal domain (Blume et al, 2012). Phosphatidylserine (PS) on the outer membrane of the apoptotic cell is the best-known eat me signal. Direct recognition of PS by the efferocyte is mediated by a variety of receptors including TIM4, BAI1, stabilin-2 and the receptor for advanced glycation end products (RAGE; He et al, 2011; Ravichandran, 2011). In contrast, other receptors are engaged via soluble bridging molecules, one example being AnxA1 that links PS to the PS receptor (Arur et al, 2003). Similarly, MFG-E8 links PS and the vitronectin receptor, Gas 6 or Protein S facilitate contact between PS and TAM receptors, and β2-glycoprotein-I with its receptor and PS (Stitt et al, 1995). How these various receptors signal intracellularly to mediate cytoskeletal rearrangement thereby facilitating corpse uptake remains to be understood.
A functional macrophage switch governs the return to tissue homeostasis
Macrophages possess a striking functional and phenotypic plasticity that becomes apparent during the resolution phase of inflammation. Upon apoptotic cell efferocytosis, macrophages turn off production of pro-inflammatory cytokines and lipid mediators and launch an anti-inflammatory transcriptional program characterized by the release of IL10 and TGF-β (Fadok et al, 1998; Fig 2D), quite reminiscent of the M2 alternative macrophage activation pattern. Transcriptomic analyses of murine resolution-phase macrophages shed light on the main features of macrophages during resolution in vivo (Stables et al, 2011). Resolution-phase macrophages are rich in molecules important for antigen processing and presentation and secrete T- and B-cell chemoattractants (XCL1, CCL5 and CXCL13). Consequently, the lymphocytes that repopulate sites of resolving inflammation comprise B1, NK, γδT, CD4+CD25+ and B2 cells which might exert a protective effect in the post-resolution phase (Rajakariar et al, 2008). In addition, resolution-phase macrophages express TIM4 and TGF-β, key molecules in the clearance of inflammatory cells and the return to tissue homeostasis. Functional characterization of resolving macrophages revealed lower levels of CD11b, enhanced capacity to engulf dead neutrophils, reduced responsiveness to TLR4 ligands, thus possibly leading to a “satiated” state with the ultimate departure through the lymph (Schif-Zuck et al, 2011). Clearly, the latter two studies indicate heterogeneity of resolution-phase macrophages and call for a standardization of the classification criteria. A chemokine scavenging-independent role for D6 in promoting macrophage-mediated resolution has recently been outlined. D6-deficient macrophages display a defect in conversion to a resolution phase macrophage. Furthermore, senescent human neutrophils and resolution-phase murine neutrophils present an increased expression of D6, suggesting that D6 might play a role in the interactions of apoptotic neutrophils with macrophages, as well as satiation of these macrophages (Pashover-Schallinger et al, 2012).
In addition, rapidly generated lipid mediators play a key role in orchestration of inflammation and its resolution (Serhan et al, 2008). In particular, the arachidonic acid pathway synthesizes pro-inflammatory lipids such as prostaglandin (PG) E2 and D2, and, during the resolution phase, pro-resolving bioactive lipid mediators including lipoxins and the ω3-unsaturated fatty acid-derivatives termed resolvins and protectins. Intriguingly, differential gene regulation of arachidonate metabolism-related enzymes has been reported in M1- and M2-polarized human macrophages (Martinez et al, 2006). M1 macrophages display a marked induction of COX2, with down-regulation of COX1, leukotriene A4 hydrolase, thromboxane A synthase 1 and arachidonate 5-lipoxygenase. Conversely, M2 macrophages show up-regulation of arachidonate 15-lipoxygenase and COX1. Furthermore, microsomal PGE synthase, the key enzyme in PGE2 production, is induced in macrophages by inflammatory M1 signals such as LPS and is functionally coupled to COX2 expression. In contrast, M2 stimuli such as IL4 and IL13 down-regulate mPGES expression in macrophages.
Functionally, lipoxin A4 (LXA4) inhibits neutrophil entry to tissue sites while promoting monocyte migration (Maddox et al, 1997). Cell-type specific signalling pathways downstream of FPR2/ALX activation by LXA4 can explain this differential effect (Chiang et al, 2006). Changes in cytoskeletal protein phosphorylation with consequent cell arrest occur in neutrophils exposed to LXA4, whilst mobilization of intracellular Ca2+ occurs in monocytes and macrophages, thus promoting chemotaxis. LXA4, at low nanomolar concentrations, generally decreases neutrophil activity, with lower levels of CD11b/CD18 expression, ROS formation and NFκB activity, as well as decreased synthesis of pro-inflammatory chemokines and cytokines. In monocytes and macrophages, LXA4 also promotes non-phlogistic phagocytosis of apoptotic neutrophils with reduced release of CXCL8 (Jozsef et al, 2002). Resolvins are another class of pro-resolving lipid mediators that were initially identified in exudates from mouse air-pouch models during the spontaneous resolution of inflammation. Resolvin E1 binds ChemR23 on monocytes, macrophages and dendritic cells to attenuate TNF-mediated NFκB activation, thus forming an anti-inflammatory signalling pathway (Arita et al, 2007). Furthermore, resolvin E1 reduces neutrophil infiltration in murine peritonitis and shortens the resolution interval while modifying the expression of miRNAs that target genes involved in resolution (Recchiuti et al, 2011). This effect appears to involve specific G protein-coupled receptors, namely ALX/FPR2 and GPR32 (Krishnamoorthy et al, 2012).
Restoration of tissue functionality
The functional recovery of homeostasis after an inflammatory injury requires tissue repair and reestablishment of tissue functionality. The underlying mechanisms are complex and tissue-dependent but require a tight interplay between macrophages, stem and progenitor cells, together with stromal cells to prevent fibrosis or scar formation, a pathophysiological condition that leads to ineffective and inappropriate tissue function. Landmark studies have shown that macrophages orchestrate these reparative processes (Leibovich & Ross, 1975; Polverini et al, 1977). Recent work points towards a critical role of alternatively activated macrophages (Lucas et al, 2010; Saclier et al, 2013; Sindrilaru et al, 2011), which secrete anti-inflammatory and reparative mediators including IL1 receptor antagonist, IL10, TGF-β and VEGF. The generation of growth factors promotes cell proliferation and protein synthesis in neighbouring cells (Rappolee et al, 1988), while the production of proteases and their inhibitors regulate extracellular matrix (ECM) composition and remodelling. Macrophage-derived TGF-β contributes to tissue regeneration and wound repair by promoting (i) fibroblast differentiation into myofibroblasts, (ii) expression of tissue inhibitors of metalloproteinases (TIMPs) that regulate ECM remodelling and (iii) synthesis of interstitial fibrillar collagens by myofibroblasts. Macrophages also produce the majority of wound-associated VEGF, assuring angiogenesis and restoration of oxygen supply (Knighton et al, 1983). M2 macrophages also produce MMPs and TIMPs that control ECM turnover engulf and digest various ECM components that would promote tissue-damaging M1 macrophage responses (Atabai et al, 2009), and secrete specific chemokines that recruit fibroblasts and regulatory T (TReg) cells (Curiel et al, 2004).
There is scattered evidence supporting the hypothesis that macrophages interact with progenitor or stem cells, and that this interplay may contribute to repair and remodelling. Mesenchymal stem cells (MSCs) are candidates for cellular therapies aimed at promoting tissue repair or immunoregulation (Uccelli et al, 2008). MSCs engage in a bidirectional interaction with cells of the macrophage lineage. M2-like macrophages and their mediators promote growth of human MSCs (Freytes et al, 2013) and can also stimulate MSC motility (Anton et al, 2012). Conversely, MSCs profoundly influence macrophage function by inducing a IL-10high IL12low alternative activation phenotype (Kim & Hematti, 2009). Injection of MSCs is associated with promotion of functional recovery after spinal cord injury, including axonal preservation and reduced scar formation (Nakajima et al, 2012). Neuroprotection in this system is attributed to MSC-induced shift in macrophage polarization from M1 to M2.
The fate of infiltrating macrophages is not fully understood. It has been postulated that after performing their central tasks in resolution, macrophages emigrate to the draining lymph node, where they may play an important role in the presentation of antigens from the inflamed site (Bellingan et al, 1996). MMP-mediated shedding of β2 integrin seems important for macrophage egress from the injured tissue (Cao et al, 2005; Gomez et al, 2012). A failure in macrophage egression results in accumulation and may potentially lead to chronic inflammatory diseases, such as atherosclerosis (Llodra et al, 2004).
Suppressive immune cells as emerging players in resolution
Although neutrophils and macrophages have traditionally been looked upon as dominant cell types during the resolution phase, accessory cells such as myeloid-derived suppressor cells (MDSCs) and TReg have more recently emerged as important players during resolution and may link innate and adaptive immune systems (Fig 3).
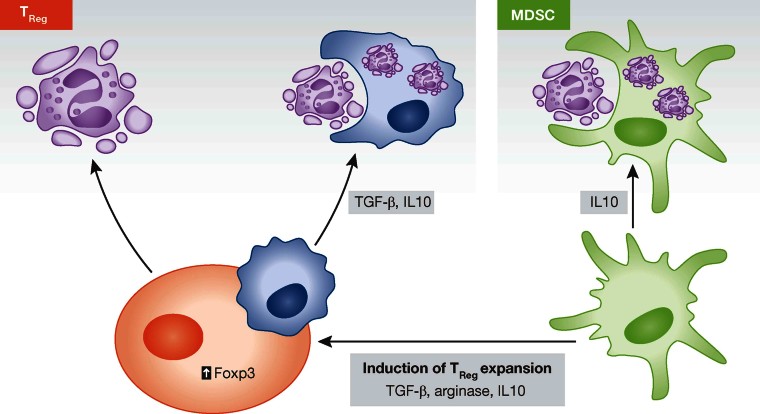
Figure 3.
Role of accessory cells in resolution. MDSCs clear apoptotic neutrophils, release anti-inflammatory IL10 and contribute to the expansion of TReg. TReg stimulate neutrophil apoptosis, enhance the efferocytosis capacity of macrophages and secrete resolving cytokines like IL10 and TGF-β in a contact-dependent manner.
TReg, a subset of CD4+CD25+ T lymphocytes that co-express the transcription factor Foxp3 (Hori et al, 2003), regulate immune responses through the secretion of immunosuppressive and pro-resolving cytokines such as IL10 and TGF-β, thus being key players in immune homeostasis (Asseman et al, 1999; Fahlen et al, 2005). To date, beneficial roles of TReg have emerged in a number of inflammatory diseases including rheumatoid arthritis (Cao et al, 2003), multiple sclerosis (Viglietta et al, 2004) and atherosclerosis (Ait-Oufella et al, 2006). Traditionally, TReg are activated in an antigen-dependent manner to inhibit self-reactive or pathogenic T cells. However, TReg may also exert functions independent of inhibition of lymphocyte function, as was shown in a model of LPS-induced acute lung injury (D'Alessio et al, 2009). The transfer of wild type TReg to Rag1−/− mice lacking mature T and B cells caused abrogation of lung inflammation, accompanied by increased apoptosis of alveolar neutrophils and macrophage efferocytosis, together with reduced pro-inflammatory cytokines and doubling of TGF-β levels. TReg abrogated TNF production and boosted TGF-β release in isolated alveolar macrophages in a contact-dependent manner. These results are in line with a previous study demonstrating that anti-inflammatory macrophages induce differentiation of TReg via TGF-β (Savage et al, 2008). These findings highlight the cross-talk between innate and adaptive immunity where cells of both systems regulate each other to coordinate resolution.
MDSCs represent a heterogenic population of immature myeloid cells expressing Gr1 and CD11b in mice and suppress T-cell functions (Bronte et al, 2000; Ribechini et al, 2010). In healthy individuals immature myeloid cells migrate to different peripheral organs, where they differentiate into dendritic cells, macrophages and/or granulocytes. However, under pathological conditions such as acute or chronic infection, sepsis, trauma or tumours, their differentiation is partially blocked leading to the generation and accumulation of MDSC. One of the original features of MDSCs is the production of arginase I (ARG1) or inducible nitric oxidase synthase (iNOS) that initiate the release of nitric oxide (NO) and ROS involved in programmed cell death or apoptosis and other immunosuppressive mechanisms (Gabrilovich & Nagaraj, 2009). The transcription factor STAT1 is of key importance in the signalling pathway leading to increased expression of ARG1 and iNOS. Indeed, MDSCs from Stat1−/− mice fail to up-regulate these enzymes and therefore do not inhibit T-cell responses (Kusmartsev et al, 2005). In contrast to MDSCs, neutrophils create a pro-inflammatory environment upon activation that further activates T cells and can induce their differentiation by release of IL12, IL4 or IL6 (Muller et al, 2009).
MDSCs exert beneficial activities in inflammatory conditions such as sepsis, inflammatory bowel disease, autoimmune encephalomyelitis and multiple sclerosis (Delano et al, 2007; Haile et al, 2008; Zhu et al, 2007). MDSCs also phagocytose apoptotic neutrophils and produce large amounts of IL10 contributing to resolution in bacterial pneumonia (Poe et al, 2013). Interestingly, MDSCs induce expansion of TReg by increasing their Foxp3 expression in a IL10, TGF-β and arginase dependent manner (Huang et al, 2006; Serafini et al, 2008). Although research on the function of MDSCs in resolution is still in its infancy, the latter findings suggest that MDSCs might exert a regulatory role in resolution by establishing a link with the adaptive immune system.
Therapeutic stimulation of resolution
Agents that target pro-inflammation mediators have dominated drug research for inflammatory diseases for the last decades. Current anti-inflammatory therapies control cardinal signs of inflammation, mostly antagonizing specific pathways that are engaged when acute inflammation sets in. To transfer of concepts of resolution from bench to bedside requires a shift in emphasis from inhibitory therapy to replacement therapy, i.e. from antagonism to agonism. The advantage of immunoresolvents would be to limit continued neutrophil infiltration, counter-regulate pro-inflammatory mediators, enhance the containment and phagocytosis of cellular debris and apoptotic neutrophils, and promote restoration of tissue homeostasis (Box 1). Therapies that actively promote resolution may also have the advantage of enhancing innate immune responses to bacterial infections (Chiang et al, 2012), whereas established anti-inflammatory therapies such as anti-TNF strategies may be immunosuppressive (Bruns et al, 2009).
Box 1: Criteria for resolution-inducing mediators
Resolution, the return to normal inflammatory conditions, does not merely consist in catabolism of inflammatory mediators and abrogation of inflammatory processes. Instead, resolution is an active and coordinated anti-inflammatory, pro-resolving programme aimed at restoration of tissue homeostasis, integrity and function. Pro-resolving mediators should ideally fulfill the following criteria:
Stop of inflammatory cell recruitment—the abrogation of neutrophil influx to block delivery of tissue-toxic proteases and oxygen radicals is of crucial importance in resolution.
Induction of neutrophil apoptosis and clearance (efferocytosis)—removal of apoptotic neutrophils is of dual importance: it induces reprogramming of macrophages and prevents spilling of potentially toxic contents from the neutrophil cytoplasm as they become necrotic.
Egress of immune cells—following efferocytosis, macrophages and dendritic cells leave the site of inflammation.
Positive modulation of the immune response—instruction of suppressive immune cells and the adaptive immune response to help dealing with subsequent encounters.
Induction of tissue repair—return to homeostasis without fibrosis or scar formation marks the final step of resolution.
| ↓Recruitment | ↑Apoptosis/efferocytosis | ↑Egress | ↑Immune response | ↑Tissue repair | Refs. | |
|---|---|---|---|---|---|---|
| Annexin A1 | + | + | N.D. | + | + | Perretti & D'Acquisto (2009) |
| Chemerin C15 | + | + | N.D. | N.D. | N.D. | Cash et al (2008), Cash et al (2010) |
| Lipoxins | + | + | + | + | + | Serhan et al (2008) |
| Resolvins | + | + | + | No effect | + | Ariel & Serhan (2007) |
| Galectin-1 | + | N.D. | N.D. | − | + | Ilarregui et al (2009) |
| Glucocorticoids | + | + | N.D. | − | − | Perretti & D'Acquisto (2009) |
| Adenosine | + | + | N.D. | N.D. | + | Csoka et al (2012), Koroskenyi et al (2011) |
| Melanocortins | + | + | N.D. | N.D. | + | Patel et al (2011) |
+, stimulation; −, inhibition; N.D., not defined.
Inhibition of leukocyte recruitment
The inhibition of continued leukocyte recruitment is essential to favour the return to homeostasis. However, the therapeutic targeting of cell adhesion molecules or chemokines to limit inflammatory cell recruitment has so far been largely unsuccessful. Pro-resolving AnxA1 dampens neutrophil tissue accumulation by several mechanisms including downregulation of transendothelial migration (Perretti et al, 1996), promotion of neutrophil apoptosis (Perretti & Solito, 2004), and stimulation of the removal of dead neutrophils (Scannell et al, 2007). The combination of these mechanisms results in potent pro-resolving effects in in vivo models of inflammation (Table 1; Dalli et al, 2008; Vago et al, 2012). Similar activities have also been ascribed to chemerin-derived peptides. While chemerin primarily attracts antigen-presenting cells, C-terminal peptides released from chemerin by cysteine proteases have opposite effects—they block neutrophil tissue infiltration and the release of pro-inflammatory mediators from classically activated macrophages (Cash et al, 2008). In addition, chemerin-derived peptides promote clearance of dead neutrophils by macrophages (Cash et al, 2010). Among the resolution-inducing agents, resolving lipid mediators like LXA4, resolvin E1 and D1, and protectins exert multiple pro-resolving effects, including inhibition of neutrophil tissue infiltration, induction of neutrophil apoptosis and efferocytosis and stimulation of tissue repair (Serhan et al, 2011). In animal models of inflammation, resolvin E1 is protective in a model of colitis as shown by decreased neutrophil tissue infiltration, pro-inflammatory gene expression and improved survival (Arita et al, 2005). Similarly, resolvin E1 reduces leukocyte infiltration in a mouse model of asthma and ultimately improves lung function (Haworth et al, 2008). Although LXA4 is equally potent in inducing resolution, due to rapid degradation in the blood stream, various delivery strategies have evolved, including the use of stable forms such as fluorinated analogues (Clish et al, 1999). Nanoparticles—efficient delivery microstructures—can be enriched with aspirin-triggered resolvin D1 or a LXA4 analogue to accelerate resolution in experimental peritonitis (Norling et al, 2011).
Pending issues
Integrated understanding of the complex interaction between pro- and anti-resolution mediators.
Existence of positive networks in resolution, where one pro-resolving mediator would induce another one.
Further investigation on the role of new cellular players in resolution.
Design of drugs integrating the key aspects that define a potent resolution-inducing mediator that incites resolution rather than simply buffering inflammation.
Discernment of tissue-specific resolution mechanisms.
Induction of neutrophil apoptosis
Given the central role of neutrophil apoptosis in resolution, strategies aiming at the promotion of neutrophil apoptosis have emerged (Table 1; Geering & Simon, 2011). Despite being end-differentiated cells, the use of cyclin dependent kinase inhibitors such as R-roscovitine has been demonstrated to promote neutrophil apoptosis, leading to resolution in a wide range of inflammatory models (Gherardi et al, 2004; Rossi et al, 2006; Sekine et al, 2008; Zoja et al, 2007). However, a long-term treatment that induces neutrophil apoptosis may attenuate the innate immune response; hence molecules targeting the inflammation-induced prolongation of neutrophil life span are preferable. 5-epi-LXA4 induces resolution in a mouse model of lung injury by counteracting the myeloperoxidase-mediated suppression of neutrophil apoptosis (El Kebir et al, 2009). Ectoine, a compatible solute with anti-inflammatory properties, is able to prevent the anti-apoptotic mechanisms that extend neutrophil life span in an inflammatory microenvironment. Importantly, ectoine does not affect apoptosis of non-activated neutrophils (Sydlik et al, 2013). Finally, PD098059, an ERK inhibitor, was shown to counteract GM-CSF-induced neutrophil survival and to promote resolution in a rat model of carrageenan-induced pleurisy (Sawatzky et al, 2006). #box2
Enhancement of efferocytosis and macrophage reprogramming
An increase of neutrophil apoptosis without a “disposal strategy” (i.e. efferocytosis) for the cell remnants perpetuates inflammation. AnxA1, resolvins and LXA4 were all shown to induce efferocytosis, which partially explains their resolution-inducing activities in vivo (Table 1). In addition, in a model of chronic granulomatous disease, IL4 administration induces alternative macrophage activation with enhanced ability to clear apoptotic neutrophils (Fernandez-Boyanapalli et al, 2009). A recent study used an alternative strategy to enhance the surface expression of eat me signals on apoptotic cells and ensure clearance; delivery of AnxA5-RGD complexes that bind to PS on apoptotic cells and on the vitronectin receptor on the efferocyte would promote cell clearance and secretion of IL-10 (Schutters et al, 2013). Beyond their use as vehicles to deliver resolving compounds (Metselaar et al, 2004), liposomes may also be used to mimic apoptotic cells to induce macrophage reprogramming. For instance, PS-containing liposomes can mimic the phagocyte stimulation achieved by apoptotic cells and induce secretion of pro-resolution mediators (Table 1). In addition, PS-liposomes are preferentially ingested by macrophages (Geelen et al, 2012), highlighting their potential in targeting macrophage-driven resolution mechanisms.
Clinical trials of pro-resolving drugs
In addition to the pharmacological activities demonstrated in animal models, several on-going clinical trials are yielding positive results. Resolvin E1 (RX-10001) and a synthetic analogue (RX-100045) are being tested in numerous inflammatory diseases including dry eye, retinal disease, asthma, inflammatory bowel diseases, rheumatic arthritis and cardiovascular diseases. RX-100045 has successfully completed the phase II study in dry eye patients and is scheduled to enter a phase III randomized, placebo-controlled, multi-centre study (Lee, 2012). In another clinical study, a LXA4-based compound was tested for the topical treatment of eczema (Wu et al, 2013). Albeit in a small number of patients, the drug reduced the severity of eczema to a similar extent as steroid therapy in a double-blind placebo-controlled setting, thus indicating efficacy and safety of resolving mediators in a clinical study. AP214, a melanocortin agonist developed by Action Pharma AS (recently acquired by Abbott), elicits pro-resolving and tissue-protective effects in experimental systems (Montero-Melendez et al, 2011); in clinical settings, it effectively reduces acute kidney injury associated with major cardiac surgery. This study offers positive expectations as it has presented phase 2b top-line results in the evaluation of the efficacy, safety and tolerability of the compound. A second phase 2b study is scheduled for the end of 2012.
Conclusion
Our understanding of resolution as an active process has come a long way since the early observations by Elie Metchnikoff who observed that neutrophils are phagocytosed by macrophages and how this clearance resolves tissue inflammation (Mechnikov, 1988). Resolution is now looked upon as a complex process where apoptosis of neutrophils and their subsequent clearance herald potent anti-inflammatory, tissue-restoring mechanisms. To fully appreciate the complexity of such processes and to design resolution-based therapeutic strategies, future work is needed to discern differences in the mechanisms of resolution in acute and chronic inflammatory disorders as well as decipher the tissue-specific resolution networks.
Acknowledgments
O.S. is supported by the NWO (VIDI project 91712303), the DFG (SO876/3-1, SO876/6-1, FOR809, SFB914 TPB08), the German-Israeli Foundation, and the Else Kröner Fresenius Stiftung. M.P. is supported by the Wellcome Trust (program 086867/Z/08), the Arthritis Research UK, the British Heart Foundation (PG/09/060 and PG/11/48/28981) and the Medical Research Council.
The authors declare that they have no conflict of interest.
Glossary
- Annexin A1
A glucocorticoid-regulated protein, highly abundant in myeloid cells such as neutrophils and macrophages, with profound effects on several phases of the resolution of inflammation.
- Chemokines
Low molecular weight cytokines with chemotactic effects on leukocytes.
- Decoy receptor
Receptor for cytokines that, while irreversibly binding to its ligands, does not trigger activation of the corresponding intracellular signalling pathway, hence acting as a scavenger of these molecules.
- Efferocytosis
Phagocytosis and clearance of an apoptotic cell.
- Find me/eat me signals
Group of molecules secreted or exposed at the surface of an apoptotic cell that guide efferocytes to facilitate their disposal.
- Liposome
Spherical particle composed by a lipid bilayer, containing a lumen with the capacity to encapsulate and transport substances in biological systems, thus being a suitable vehicle for drug delivery.
- Myeloid derived suppressor cells
Heterogeneous population of early myeloid progenitors with the ability to suppress T cells activity.
- Pro-resolving lipid mediators
Bioactive lipids secreted in response to an inflammatory state that stimulate molecular and cellular events to achieve resolution.
- Resolution-phase macrophage
Macrophage that, in response to stimuli announcing the conclusion of an acute inflammation, reprograms its phenotype and acts towards the termination of the inflammatory process and return to homeostasis.
- TReg
Naturally immunosuppressive subpopulation of CD4+ T cells characterized by constitutive expression of the transcription factor Foxp3.
For more information
Information about clinical trials:
Information about chemokine receptor nomenclature:
Information about the authors:
http://www.phagocytes.net/groups/soehnlein.html
http://www.whri.qmul.ac.uk/staff-all/staff-research/207-perretti-mauro
References
- Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- Anton K, Banerjee D, Glod J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS ONE. 2012;7:e35036. doi: 10.1371/journal.pone.0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- Blume KE, Soeroes S, Keppeler H, Stevanovic S, Kretschmer D, Rautenberg M, Wesselborg S, Lauber K. Cleavage of annexin A1 by ADAM10 during secondary necrosis generates a monocytic “find-me” signal. J Immunol. 2012;188:135–145. doi: 10.4049/jimmunol.1004073. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Locati M, Galliera E, Vulcano M, Sironi M, Fra AM, Gobbi M, Vecchi A, Sozzani S, Haribabu B, et al. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J Immunol. 2004;172:4972–4976. doi: 10.4049/jimmunol.172.8.4972. [DOI] [PubMed] [Google Scholar]
- Borgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O'Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A(4) and benzo-lipoxin A(4) attenuate experimental renal fibrosis. FASEB J. 2011;25:2967–2979. doi: 10.1096/fj.11-185017. [DOI] [PubMed] [Google Scholar]
- Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB, Rossi AG, Gregory CD. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting MD, Comerford I, Seach N, Hammett MV, Asquith DL, Korner H, Boyd RL, Nibbs RJ, McColl SR. CCX-CKR deficiency alters thymic stroma impairing thymocyte development and promoting autoimmunity. Blood. 2013;121:118–128. doi: 10.1182/blood-2012-06-434886. [DOI] [PubMed] [Google Scholar]
- Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 2005;106:3234–3241. doi: 10.1182/blood-2005-03-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–223. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- Cash JL, Christian AR, Greaves DR. Chemerin peptides promote phagocytosis in a ChemR23- and Syk-dependent manner. J Immunol. 2010;184:5315–5324. doi: 10.4049/jimmunol.0903378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Clish CB, O'Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Recalde A, Zouggari Y, Yin KY, Bruneval P, et al. The chemokine decoy receptor d6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2012;32:2206–2213. doi: 10.1161/ATVBAHA.112.254409. [DOI] [PubMed] [Google Scholar]
- Conte FP, Menezes-de-Lima O, Jr, Verri WA, Jr, Cunha FQ, Penido C, Henriques MG. Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br J Pharmacol. 2010;161:911–924. doi: 10.1111/j.1476-5381.2010.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico G, Frascaroli G, Bianchi G, Transidico P, Doni A, Vecchi A, Sozzani S, Allavena P, Mantovani A. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nat Immunol. 2000;1:387–391. doi: 10.1038/80819. [DOI] [PubMed] [Google Scholar]
- Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- Dawson TC, Lentsch AB, Wang Z, Cowhig JE, Rot A, Maeda N, Peiper SC. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96:1681–1684. [PubMed] [Google Scholar]
- Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon G, Elks PM, Loynes CA, Whyte MK, Renshaw SA. A method for the in vivo measurement of zebrafish tissue neutrophil lifespan. ISRN Hematol. 2012;2012:915868. doi: 10.5402/2012/915868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Agudelo C, Hernandez E, Shestopalov VI, Ivanov D. Phosphatidylserine-containing liposomes promote maximal survival of retinal neurons after ischemic injury. J Cereb Blood Flow Metab. 2009;29:1755–1759. doi: 10.1038/jcbfm.2009.95. [DOI] [PubMed] [Google Scholar]
- El Kebir D, Jozsef L, Pan W, Wang L, Petasis NA, Serhan CN, Filep JG. 15-Epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, Henson PM, Bratton DL. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–2055. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Boyanapalli R, McPhillips KA, Frasch SC, Janssen WJ, Dinauer MC, Riches DW, Henson PM, Byrne A, Bratton DL. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J Immunol. 2010;185:4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S, Leitch AE, Duffin R, Haslett C, Rossi AG. Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun. 2010;2:216–227. doi: 10.1159/000284367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis N, Wong SH, Hampson P, Wang K, Young SP, Deigner HP, Salmon M, Scheel-Toellner D, Lord JM. Lactoferrin inhibits neutrophil apoptosis via blockade of proximal apoptotic signaling events. Biochim Biophys Acta. 2011;1813:1822–1826. doi: 10.1016/j.bbamcr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner L, Patterson AM, Ashton BA, Stone MA, Middleton J. The human Duffy antigen binds selected inflammatory but not homeostatic chemokines. Biochem Biophys Res Commun. 2004;321:306–312. doi: 10.1016/j.bbrc.2004.06.146. [DOI] [PubMed] [Google Scholar]
- Geelen T, Yeo SY, Paulis LE, Starmans LW, Nicolay K, Strijkers GJ. Internalization of paramagnetic phosphatidylserine-containing liposomes by macrophages. J Nanobiotechnol. 2012;10:37. doi: 10.1186/1477-3155-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering B, Gurzeler U, Federzoni E, Kaufmann T, Simon HU. A novel TNFR1-triggered apoptosis pathway mediated by class IA PI3Ks in neutrophils. Blood. 2011;117:5953–5962. doi: 10.1182/blood-2010-11-322206. [DOI] [PubMed] [Google Scholar]
- Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011;18:1457–1469. doi: 10.1038/cdd.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi D, D'Agati V, Chu TH, Barnett A, Gianella-Borradori A, Gelman IH, Nelson PJ. Reversal of collapsing glomerulopathy in mice with the cyclin-dependent kinase inhibitor CYC202. J Am Soc Nephrol: JASN. 2004;15:1212–1222. doi: 10.1097/01.asn.0000124672.41036.f4. [DOI] [PubMed] [Google Scholar]
- Gomez IG, Tang J, Wilson CL, Yan W, Heinecke JW, Harlan JM, Raines EW. Metalloproteinase-mediated Shedding of Integrin beta2 promotes macrophage efflux from inflammatory sites. J Biol Chem. 2012;287:4581–4589. doi: 10.1074/jbc.M111.321182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, Barbour SE, Milstien S, Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. doi: 10.1053/j.gastro.2008.06.032. 881 e871–875. [DOI] [PubMed] [Google Scholar]
- Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA. 2011;108:1827–1832. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, Yamada M, Yamaya M, Maekawa T, Yamamoto Y, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12:358–364. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, Takagi M, Mizutani S, Morio T. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat Immunol. 2012;13:369–378. doi: 10.1038/ni.2234. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci USA. 2002;99:13266–13271. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Koroskenyi K, Duro E, Pallai A, Sarang Z, Kloor D, Ucker DS, Beceiro S, Castrillo A, Chawla A, Ledent CA, et al. Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation. J Immunol. 2011;186:7144–7155. doi: 10.4049/jimmunol.1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Lee CH. Resolvins as new fascinating drug candidates for inflammatory diseases. Arch Pharm Res. 2012;35:3–7. doi: 10.1007/s12272-012-0121-z. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Liu DP, Chen HL, Pan XH, Kong QY, Pang QF. Lactoferrin protects against lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2012;12:460–464. doi: 10.1016/j.intimp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Luo H, Chaudhuri A, Zbrzezna V, He Y, Pogo AO. Deletion of the murine Duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Mol Cell Biol. 2000;20:3097–3101. doi: 10.1128/mcb.20.9.3097-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- Mechnikov II. Immunity in infective diseases. By Il'ia Il'ich Mechnikov, 1905. Rev Infect Dis. 1988;10:223–227. [PubMed] [Google Scholar]
- Metselaar JM, van den Berg WB, Holthuysen AE, Wauben MH, Storm G, van Lent PL. Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Ann Rheum Dis. 2004;63:348–353. doi: 10.1136/ard.2003.009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- Montero-Melendez T, Dalli J, Perretti M. Gene expression signature-based approach identifies a pro-resolving mechanism of action for histone deacetylase inhibitors. Cell Death Differ. 2013;20:567–575. doi: 10.1038/cdd.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TE, Perretti M. The melanocortin agonist AP214 exerts anti-inflammatory and proresolving properties. Am J Pathol. 2011;179:259–269. doi: 10.1016/j.ajpath.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller I, Munder M, Kropf P, Hansch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms. Trends Immunol. 2009;30:522–530. doi: 10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashover-Schallinger E, Aswad M, Schif-Zuck S, Shapiro H, Singer P, Ariel A. The atypical chemokine receptor D6 controls macrophage efferocytosis and cytokine secretion during the resolution of inflammation. FASEB J. 2012;26:3891–3900. doi: 10.1096/fj.11-194894. [DOI] [PubMed] [Google Scholar]
- Patel HB, Montero-Melendez T, Greco KV, Perretti M. Melanocortin receptors as novel effectors of macrophage responses in inflammation. Front Immunol. 2011;2:41. doi: 10.3389/fimmu.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Perretti M, Solito E. Annexin 1 and neutrophil apoptosis. Biochem Soc Trans. 2004;32:507–510. doi: 10.1042/BST0320507. [DOI] [PubMed] [Google Scholar]
- Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, Levy DE, Lee JS, Mallampalli RK, Chan YR, et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 2013;6:189–199. doi: 10.1038/mi.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverini PJ, Cotran PS, Gimbrone MA, Jr, Unanue ER. Activated macrophages induce vascular proliferation. Nature. 1977;269:804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Rajakariar R, Lawrence T, Bystrom J, Hilliard M, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood. 2008;111:4184–4192. doi: 10.1182/blood-2007-08-108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee DA, Mark D, Banda MJ, Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988;241:708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Med Microbiol Immunol. 2010;199:273–281. doi: 10.1007/s00430-010-0151-4. [DOI] [PubMed] [Google Scholar]
- Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- Savage ND, de Boer T, Walburg KV, Joosten SA, van Meijgaarden K, Geluk A, Ottenhoff TH. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181:2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- Sawatzky DA, Willoughby DA, Colville-Nash PR, Rossi AG. The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo. Am J Pathol. 2006;168:33–41. doi: 10.2353/ajpath.2006.050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell M, Flanagan MB, deStefani A, Wynne KJ, Cagney G, Godson C, Maderna P. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–4605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol. 2011;41:366–379. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutters K, Kusters DH, Chatrou ML, Montero-Melendez T, Donners M, Deckers NM, Krysko DV, Vandenabeele P, Perretti M, Schurgers LJ, et al. Cell surface-expressed phosphatidylserine as therapeutic target to enhance phagocytosis of apoptotic cells. Cell Death Differ. 2013;20:49–56. doi: 10.1038/cdd.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine C, Sugihara T, Miyake S, Hirai H, Yoshida M, Miyasaka N, Kohsaka H. Successful treatment of animal models of rheumatoid arthritis with small-molecule cyclin-dependent kinase inhibitors. J Immunol. 2008;180:1954–1961. doi: 10.4049/jimmunol.180.3.1954. [DOI] [PubMed] [Google Scholar]
- Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–e208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Sydlik U, Peuschel H, Paunel-Gorgulu A, Keymel S, Kramer U, Weissenberg A, Kroker M, Seghrouchni S, Heiss C, Windolf J, et al. Recovery of neutrophil apoptosis by ectoine: a new strategy against lung inflammation. Eur Respir J. 2013;41:433–442. doi: 10.1183/09031936.00132211. [DOI] [PubMed] [Google Scholar]
- Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Vago JP, Nogueira CR, Tavares LP, Soriani FM, Lopes F, Russo RC, Pinho V, Teixeira MM, Sousa LP. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J Leukoc Biol. 2012;92:249–258. doi: 10.1189/jlb.0112008. [DOI] [PubMed] [Google Scholar]
- van den Berg JM, Weyer S, Weening JJ, Roos D, Kuijpers TW. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol. 2001;69:467–473. [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36:410–416. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- Walmsley SR, Chilvers ER, Thompson AA, Vaughan K, Marriott HM, Parker LC, Shaw G, Parmar S, Schneider M, Sabroe I, et al. Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Invest. 2011;121:1053–1063. doi: 10.1172/JCI43273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Francis N, Chahal H, Raza K, Salmon M, Scheel-Toellner D, Lord JM. Lactoferrin is a survival factor for neutrophils in rheumatoid synovial fluid. Rheumatology. 2009;48:39–44. doi: 10.1093/rheumatology/ken412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol. 2013;168:172–178. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- Zoja C, Casiraghi F, Conti S, Corna D, Rottoli D, Cavinato RA, Remuzzi G, Benigni A. Cyclin-dependent kinase inhibition limits glomerulonephritis and extends lifespan of mice with systemic lupus. Arthritis Rheum. 2007;56:1629–1637. doi: 10.1002/art.22593. [DOI] [PubMed] [Google Scholar]