Abstract
Objectives:
The usefulness of the implantable loop recorder (ILR) with improved atrial fibrillation (AF) detection capability (Reveal XT) and the factors associated with AF in the setting of unexplained stroke were investigated.
Methods:
A cohort study is reported of 51 patients in whom ILRs were implanted for the investigation of ischemic stroke for which no cause had been found (cryptogenic) following appropriate vascular and cardiac imaging and at least 24 hours of cardiac rhythm monitoring.
Results:
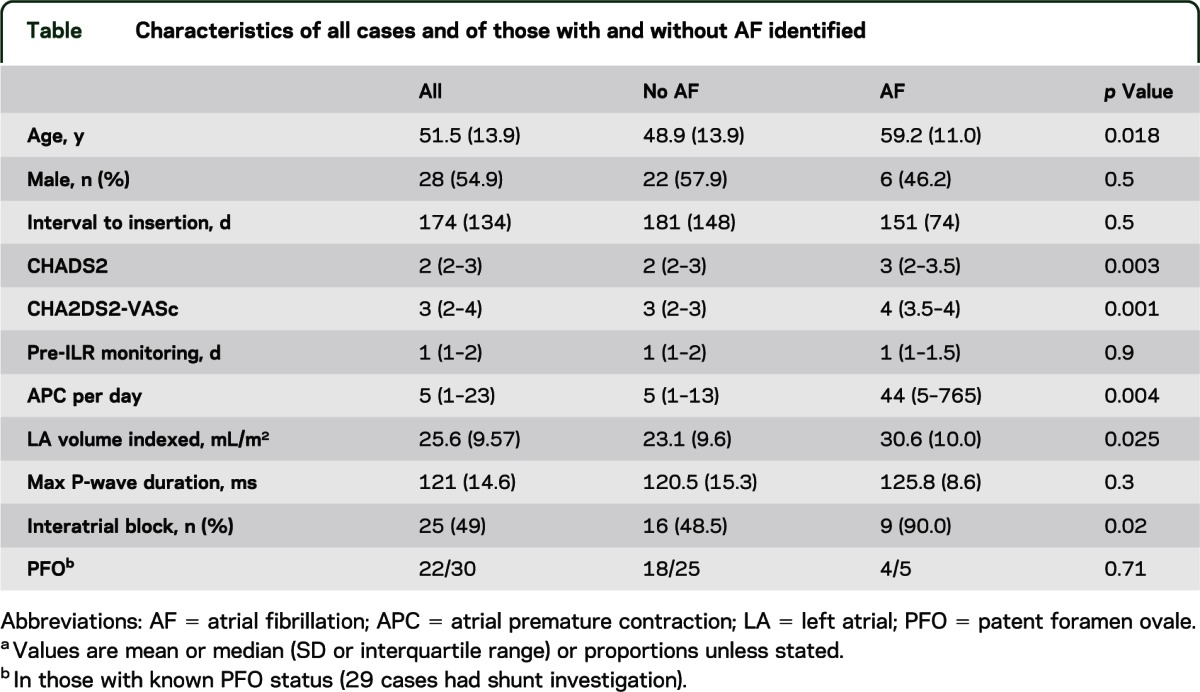
The patients were aged from 17 to 73 (median 52) years. Of the 30 patients with a shunt investigation, 22 had a patent foramen ovale (73.3%; 95% confidence interval [CI] 56.5%–90.1%). AF was identified in 13 (25.5%; 95% CI 13.1%–37.9%) cases. AF was associated with increasing age (p = 0.018), interatrial conduction block (p = 0.02), left atrial volume (p = 0.025), and the occurrence of atrial premature contractions on preceding external monitoring (p = 0.004). The median (range) of monitoring prior to AF detection was 48 (0–154) days.
Conclusion:
In patients with unexplained stroke, AF was detected by ILR in 25.5%. Predictors of AF were identified, which may help to target investigations. ILRs may have a central role in the future in the investigation of patients with unexplained stroke.
Atrial fibrillation (AF) is a common cause of stroke, accounting for about 25% of all infarcts.1 A significant proportion of strokes remain unexplained despite thorough investigation, in particular in younger people,2 and a proportion of these have undiagnosed paroxysmal AF (PAF).
There are several methods of noninvasive cardiac rhythm monitoring,3 all of which have a low sensitivity for AF detection (30%–65%) in patients with PAF, even when longer duration and repeated monitoring is used.4 Holter monitoring in particular has a low yield for AF detection after stroke (3%–5%) and is costly per AF event detected.5 There is evidence that many patients with AF have long intervals of sinus rhythm, making short duration noncontinuous monitoring suboptimal for excluding AF.6
Implantable loop recorders (ILRs) allow continuous cardiac monitoring for up to 3 years. A previous study investigated the use of ILRs for AF detection following stroke, and reported a very low yield. However, the device used did not have specific capability to detect AF.7 A newer ILR device (Reveal XT, Medtronic, Minneapolis, MN) has a specific AF detection algorithm. The aim of this study is to report the results of the use of ILRs in a cohort of selected patients with unexplained stroke, and to investigate the factors associated with AF detection.
METHODS
Participants.
The study is a cohort study of patients who underwent ILR implantation for investigation of ischemic strokes that were, at the time of implantation, unexplained. All were patients at Cambridge University Hospital NHS Foundation Trust and underwent device implantation between August 2010 and October 2011. Patients with TIA and those with a documented cause of stroke before ILR implantation were excluded.
All had brain imaging (MRI ± CT n = 44, CT alone n = 7) showing infarcts whose topography would be consistent with embolism, such as cortical and striatocapsular infarcts. Those whose symptomatic infarcts were more suggestive of intrinsic small-vessel disease (single, deep branch artery stroke) were excluded. Arterial imaging had been performed in every patient and those with a clearly relevant arterial lesion (atheromatous stenosis >50% or dissection) were excluded. Structural cardiac imaging (transthoracic echo n = 51, transesophageal echo n = 30) had excluded a high-risk cardiac embolic source in all cases. Transthoracic echo with bubble contrast (n = 30) was performed in younger patients (usually ≤55 years) as a routine part of investigation. Isolated patent foramen ovale (PFO) and PFO with accompanying atrial septal aneurysm (ASA) were considered low risk and not a definite cause of stroke (as per A-S-C-O [phenotypic] criteria8). All patients had undergone rhythm monitoring with standard EKG and at least 24 hours of Holter monitoring and no AF had been detected.
Thus, at the time of ILR implantation, all patients would have fulfilled criteria for cryptogenic brain infarcts according to the A-S-C-O criteria (no level 1 evidence, no level 2 evidence excluding PFO with ASA) and the Trial of ORG 10172 in Acute Stroke Treatment (TOAST)9 (except for those with isolated PFO [n = 22] and PFO plus ASA [n = 9], whose relevance is uncertain). In particular, no participant had any prior history of atrial arrhythmia. All cases were discussed at a cardiac-neurovascular multidisciplinary meeting to make the determination of cryptogenic stroke and decision on device implantation.
Implant procedure.
The ILR was implanted in a left parasternal position. Optimal orientation of the device was determined in advance, using the Vector Check Tool (Medtronic), which enables testing for the highest R-wave amplitude obtained from a single lead recording in different locations and orientations on the body surface. The device was inserted into the subcutaneous tissue with limited prior pocket preparation to ensure close tissue-device contact. Sensitivity was programmed according to the manufacturer's recommendation to 0.05 mV. Devices were programmed to detect AF with standard bradycardia and tachycardia detection limits (<40 bpm, >150 bpm).
Arrhythmia recognition and recording.
The AF detection algorithm operates through continuous assessment of the regularity of R-R intervals within a 2-minute time window. It requires 2 minutes of AF for the device to recognize the rhythm as AF (shorter episodes may be captured if patient activated). When AF is detected, it is recorded as a sustained AF episode within the automatic episode counter, showing date and time of occurrence and episode duration. For each detected episode, the device has capacity to store 2 minutes as electrogram, with total storage capacity of 49.5 minutes. When storage capacity is met, older electrograms are overwritten with newer ones.
An additional hand-held tool (“Patient Assistant”) allows patients to assess whether an arrhythmia event has been detected. The patient holds the device over the ILR and presses a button. If an episode of AF has been recorded, a signal on the Patient Assistant lights up and the patient is directed to contact the hospital for device interrogation.
Interrogation and follow-up.
Follow-up device interrogation was recommended at 1-month intervals, either by hospital visit or via the manufacturer's remote monitoring system (CareLink), which enables device interrogation from the hospital clinic over the telephone line. For patients able to use the Patient Assistant, daily device assessment was recommended. Manual analysis was performed by a cardiologist of the electrogram of episodes classified by the ILR as AF, and all cases of AF were independently verified by a second cardiologist. The definition of AF for manual analysis was an irregularly irregular R-R interval and the absence of distinct P waves on the electrogram. A diagnosis of AF was accepted when manual analysis confirmed the ILR classification.
Analysis.
The details and results of all cardiac investigations undertaken were recorded. Left atrial (LA) volumes from the transthoracic echo were measured by the biplane modified area-length method. The duration of pre-ILR implantation noninvasive cardiac monitoring and the average number of atrial premature contractions (APCs) per day were noted. P-wave duration was measured as previously described.10 Interatrial block was defined as a maximum P-wave duration of ≥120 msec.
Prespecified comparison between the groups with and without AF was undertaken for the occurrence of clinical, electrocardiographic, and echocardiographic factors: age, sex, CHADS2 and CHA2DS2-VASc score, number of APCs per day on external monitoring, P-wave duration, and LA volume. Missing values were excluded from analysis. Multivariate analysis was not undertaken given the sample size. The sample size was based on the number of cases who had undergone ILR implantation and had at least 50 days of monitoring.
Standard protocol approvals, registrations, and patient consents.
The intervention was undertaken as part of clinical care. Surgical consent for the procedure was obtained from each patient. Institutional approval was obtained for the study and reporting of data. A protocol with prespecified endpoints was registered with the institution research and development department. The study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines.
RESULTS
Fifty-four ILRs were inserted at the institution during the study period for stroke investigation. Two cases were excluded as they did not meet the definition of cryptogenic stroke for inclusion (both A0S2C3O0), and 51 cases had 50 days of continuous monitoring and were included. Demographics and characteristics of the cases are detailed in the table. The median duration of rhythm monitoring prior to ILR implantation was 1 day (interquartile range [IQR] 1–2 days; range 1–7 days).
Table.
Characteristics of all cases and of those with and without AF identified
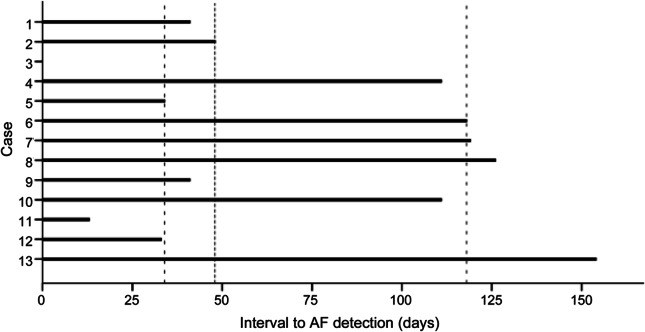
AF was detected in 13 cases (25.5%; 95% confidence interval [CI] 13.1%–37.9%). The median duration of recording prior to first episode of AF was 48 days (IQR 34–118; range 0–154) (figure). The median duration of first detected AF was 6 minutes (range 1–4,320 minutes). The mean (SD) duration of ILR monitoring in those without AF was 229 (116) days. One episode was symptomatic and patient activated; all other episodes were detected automatically by the device.
Figure. Bar graph of time to atrial fibrillation (AF) detection in cases where AF was detected.
Dashed lines represent median and interquartile range. *Case 3 had a delay of 0 days (AF on postimplantation device check).
The characteristics of the groups with and without AF are displayed in the table. Those in whom AF was detected were older than those with no AF (48.9 vs 59.2 years; t = 2.45, p = 0.018). Interatrial block was more prevalent in those with AF identified (χ2 = 5.44; p = 0.02; minimum 95 milliseconds, maximum 159 milliseconds). APCs were more frequent in the preceding rhythm monitoring in those who subsequently developed AF (Z = 2.89; p = 0.004). Those with AF had larger indexed LA volumes (t = −2.31; p = 0.025). The CHADS2 score (Z = −2.95; p = 0.003) and CHA2DS2-VASc score (Z = −3.20; p = 0.001) were both significantly greater in those with AF.
A shunt investigation was undertaken in 30 cases, of whom 22 had a PFO (73.3%; 95% CI 56.5%–90.1%). AF was detected in 4 cases with a PFO (2 isolated PFO and 2 PFO with an ASA). Of those who had had a shunt investigation, AF was not more prevalent in those with a PFO than those without (4/22 vs 1/8; p = 0.71).
DISCUSSION
This study reports the use of ILRs with a dedicated AF detection algorithm in the investigation of cryptogenic stroke patients. The detection rate of AF in this cohort was 25% and provided a firm indication for a switch from antiplatelet therapy to therapeutic anticoagulation in each of these patients.
A previous study of the use of ILRs in the setting of unexplained stroke reported very low detection rates.7 In the 24 patients (age 19–74), just one episode of AF was detected. The authors noted that a limitation of the study was the ILR device used (which did not have a specific AF detection algorithm).
Noninvasive rhythm monitoring has significant limitations in the search for (and exclusion of) AF following stroke. While 24 hours of Holter monitoring is commonly used, the yield is low at less than 5%.5 Longer monitoring improves the yield, with a doubling of diagnosis by increasing to 7 days.11 Prolonged monitoring with external loop recorders, event recorders, and outpatient telemetry may improve detection rates.3 However, data extrapolated from implanted devices have suggested the yield of AF detection continues to increase with increasing duration of monitoring.12 In a substudy of TRENDS, a single 24-hour Holter would have diagnosed AF in 3%, whereas 7, 21, or 30 days of monitoring would have led to diagnosis of AF in 6%, 9%, or 11%, respectively.12 Similarly, the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) reported a high yield of subclinical AF with a median delay to first episode of 38 days.6 In keeping with these findings, a recent article reported a new diagnosis of PAF in 11% of a cohort of cryptogenic stroke with 30-day monitoring.13 It is interesting to note that the median delay to AF detection in the current article was longer than in this 30-day study, suggesting that the yield may continue beyond this 30-day window.
To date, ILRs have been primarily used for the investigation of syncope, and until recently did not have a dedicated AF detection capability. The device used in this study has an AF detection algorithm based on the incoherence of R-R intervals, and does so by constructing a Lorenz plot of R-R interval variation.14 When compared with Holter monitoring, the Reveal XT device has a sensitivity and specificity for AF detection of 96.1% and 85.4%, respectively.14 The specificity is further improved in practice by manual review of the recorded electrograms. The undetected episodes were those less than 2 minutes duration recorded by the Holter monitor.
Based on current evidence, a clinical decision was made at the institution to implant ILRs with the ability to detect AF in those with unexplained stroke despite complete investigation (i.e., genuine cryptogenic stroke). The ILR was implanted after a variable period of noncontinuous monitoring, which may have involved sequential Holter monitors. A consequence of this approach is that there was a long delay from the event to the ILR implant. It has been shown that earlier monitoring is associated with a higher yield of AF, and efforts to reduce the delay to implantation with improved algorithms may be beneficial.3,15
This report describes the yield of AF and time to AF with duration of first episode. The Reveal XT will, in addition, give a measure of AF burden. In this study, the AF burden is not described, as data were not collected for study purposes beyond the first detection of AF. It is likely that the AF burden will be very low in most cases due to the long time to first episode. The duration of AF episodes in this study was in general brief and shorter than may be considered significant in other patient groups. In studies of pacemaker data, longer duration of AF (more than 5 minutes16 and more than 1 day17) is associated with a higher stroke risk. Therefore it is not possible to be certain as to the stroke risk conferred by the shorter detected episodes of AF in this study. However, the cases included had all had a definite cerebral infarct and no other cause of stroke and therefore differ from cohorts in the pacemaker studies. They all had a CHADS2 of at least 2 (with a median of 3), and therefore the relevance of transient episodes of AF may be greater in this cohort than AF detected incidentally in other patient groups. Other groups have reported short episodes of AF as significant in a poststroke setting.18
In the reported series, easily identifiable factors associated with AF were observed. While the small numbers limit the ability to investigate the independence of these features (in particular when controlled for age), they suggest that, with larger numbers, a predictive model may allow identification of those at highest risk of AF. This would allow optimum targeting of resources, with cost benefits.
Patients with atrial septal abnormalities were included in this cohort. It is important to include this group and reflects the current understanding (or lack of understanding) of the reason underlying the strong association between PFO and unexplained stroke in younger people.19 While AF was not more frequent in those with a PFO, the finding of AF (a high-risk cardiac abnormality) in 4 patients with atrial septal abnormalities (low-risk cardiac abnormalities) is noteworthy. This adds to the uncertainty regarding the relevance of such anomalies in ischemic stroke and suggests that the finding of a low-risk atrial septal anomaly should not prevent a search for a recognized high-risk pathology.
The current report has the strength of demonstrating the utility of ILRs in clinical practice for stroke investigation. In similar cohorts of unexplained stroke, a similar proportion may be anticipated. A limitation of the study is the small sample size. The true population yield of the test is estimated at between 13.1% and 37.9%. At even the lower bound of the CI, the proportion of patients with undetected AF by traditional noncontinuous monitoring is important. It is encouraging that significant difference in the prespecified endpoints was noted. The independence of predictive factors could not be assessed as there were insufficient numbers to allow a meaningful multivariate model to be constructed. A further limitation of the study is that the total population of stroke presentations from whom this cohort was recruited is not known. It would improve the generalizability of the findings if this were known. This limitation is minimized by the clear definition of the population and of cryptogenic stroke, suggesting the results may be generalized to others with similar characteristics.
With the use of ILRs in unexplained stroke, AF can be identified in a substantial proportion of patients despite normal traditional monitoring methods. This may have a major impact on the secondary prevention of stroke in these patients. Identification of predictive factors may allow targeting of implantation with greater cost-effectiveness.
Supplementary Material
GLOSSARY
- AF
atrial fibrillation
- ASA
atrial septal aneurysm
- CI
confidence interval
- ILR
implantable loop recorder
- IQR
interquartile range
- LA
left atrial
- PAF
paroxysmal atrial fibrillation
- PFO
patent foramen ovale
Footnotes
Editorial, page 1542
AUTHOR CONTRIBUTIONS
Paul E. Cotter was involved in the conception and design of the study, the analysis and interpretation of the data, and drafting or revising the manuscript for intellectual content. He also contributed to the acquisition of data, statistical analysis, and study coordination. Peter J. Martin was involved in the conception and design of the study, the interpretation of the data, and drafting and revising the manuscript for intellectual content. He also contributed to study supervision. Liam Ring was involved in the analysis and interpretation of the data. He also contributed to the acquisition of data. Elizabeth A. Warburton contributed to the conception and design of the study and revising the manuscript for intellectual content. Mark Belham participated in the conception and design of the study, the interpretation of the data, and drafting and revising the manuscript for intellectual content. He also contributed to study supervision and coordination. Peter J. Pugh was involved in the conception and design of the study, the interpretation of the data, and revising the manuscript for intellectual content. He also contributed to statistical analysis and study supervision and coordination.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
P. Cotter received a speaker honorarium from Medtronic and funding for conference travel from Boehringer Ingelheim. He was funded by the UK NIHR (National Institute for Health Research), Cambridge Biomedical Research Centre. P. Martin and L. Ring report no disclosures. E. Warburton's research is supported by salary support from the UK NIHR and grants from the British Heart Foundation (BHF) and UK Medical Research Council (MRC). She has received honoraria as a member of a scientific advisory board for Boehringer Ingelheim. M. Belham received speaker honoraria and funding for conference travel from Medtronic. P. Pugh reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke 2005;36:1115–1119 [DOI] [PubMed] [Google Scholar]
- 2.Cotter PE, Belham M, Martin PJ. Stroke in younger patients: the heart of the matter. J Neurol 2010;257:1777–1787 [DOI] [PubMed] [Google Scholar]
- 3.Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 2011;124:477–486 [DOI] [PubMed] [Google Scholar]
- 4.Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm 2006;3:1445–1452 [DOI] [PubMed] [Google Scholar]
- 5.Liao J, Khalid Z, Scallan C, Morillo C, O'Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke 2007;38:2935–2940 [DOI] [PubMed] [Google Scholar]
- 6.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129 [DOI] [PubMed] [Google Scholar]
- 7.Dion F, Saudeau D, Bonnaud I, et al. Unexpected low prevalence of atrial fibrillation in cryptogenic ischemic stroke: a prospective study. J Interv Card Electrophysiol 2010;28:101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis 2009;27:502–508 [DOI] [PubMed] [Google Scholar]
- 9.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 10.Cotter PE, Martin PJ, Pugh PJ, Warburton EA, Cheriyan J, Belham M. Increased incidence of interatrial block in younger adults with cryptogenic stroke and patent foramen ovale. Cerebrovasc Dis Extra 2011;1:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke 2004;35:1647–1651 [DOI] [PubMed] [Google Scholar]
- 12.Ziegler PD, Glotzer TV, Daoud EG, et al. Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke 2010;41:256–260 [DOI] [PubMed] [Google Scholar]
- 13.Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: the Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke 2012;43:2788–2790 [DOI] [PubMed] [Google Scholar]
- 14.Hindricks G, Pokushalov E, Urban L, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol 2010;3:141–147 [DOI] [PubMed] [Google Scholar]
- 15.Rizos T, Rasch C, Jenetzky E, et al. Detection of paroxysmal atrial fibrillation in acute stroke patients. Cerebrovasc Dis 2010;30:410–417 [DOI] [PubMed] [Google Scholar]
- 16.Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20:241–248 [DOI] [PubMed] [Google Scholar]
- 17.Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol 2005;46:1913–1920 [DOI] [PubMed] [Google Scholar]
- 18.Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008;71:1696–1701 [DOI] [PubMed] [Google Scholar]
- 19.Cotter PE, Belham M, Martin PJ. Towards understanding the cause of stroke in young adults utilising a new stroke classification system (A-S-C-O). Cerebrovasc Dis 2012;33:123–127 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.