Abstract
Vision is a sensation that is created from complex processes and provides us with a representation of the world around us. There are many important aspects of vision, but visual acuity was judged to be the most appropriate vision assessment for the NIH Toolbox for Assessment of Neurological and Behavioral Function, both because of its central role in visual health and because acuity testing is common and relatively inexpensive to implement broadly. The impact of visual impairments on health-related quality of life also was viewed as important to assess, in order to gain a broad view of one's visual function. To test visual acuity, an easy-to-use software program was developed, based on the protocol used by the E-ETDRS. Children younger than 7 years were administered a version with only the letters H, O, T, and V. Reliability and validity of the Toolbox visual acuity test were very good. A 53-item vision-targeted, health-related quality of life survey was also developed.
Vision is a complex sensation that provides us with a personal representation of our surrounding environment. The process resulting in vision begins when the cornea and lens refract light from objects and surfaces in the world to form a panoramic, hemispheric image on the retina, the thin layer of nerve cells that lines the inside surface of the eye. Like pixels in a digital camera, photoreceptor cells sample the light in the retinal image and photochemically transform the absorbed light energy into neural signals that are processed first by neurons in the retina and then by neurons in the visual parts of the brain.
The important aspects of visual sensation that may vary in the general population or that can be impaired by diseases or disorders of the visual system include resolution of detail, field of view, appearance of contrast, appearance of colors, appearance of motion, resolution of depth, seeing in dim light, and seeing in glaringly bright light.
Vision was identified as the highest-ranking sensory subdomain for inclusion in the NIH Toolbox for Assessment of Neurological and Behavioral Function (NIH Toolbox) on an online request for information from the expert research community (primarily NIH-funded investigators actively involved in neurologic or behavioral research; n = 232). Reasons that experts ranked vision highly included its impact on independence, function, safety, and well-being for individuals of all ages, and the fact that vision may affect the quality of other senses. Two aspects of vision that were prioritized for testing in the NIH Toolbox vision subdomain were visual acuity and the impact of visual function on health-related quality of life. The selection criteria emphasized tests that would be reliable, valid, cost- and time-efficient, portable, and appropriate for a broad age range (3–85 years), and would not overly burden the test participants. Visual acuity was selected for testing in the NIH Toolbox because loss of visual acuity is the most common and important form of visual impairment that may reduce a person's ability to complete daily activities such as reading or driving, as well as other important aspects of life including mobility and social interaction. Visual acuity tests are used to measure impairments in visual resolution that can be caused by blurring of the retinal image, neural processing disorders, or damage to neurons in the retina or other parts of the visual pathway. The impact of visual impairment on vision-targeted health-related quality of life was also considered a priority for inclusion in the NIH Toolbox because of potential impacts of different types of visual impairments. Areas of important vision-related functional abilities include reading, mobility (which includes driving), visual information processing (also called “seeing”), and visually guided motor behavior (also called “manipulation”). Loss of vision may affect one or more of these aspects of vision-related function.
Visual acuity testing.
Software was developed for the NIH Toolbox to allow for computerized static visual acuity (SVA) testing in research studies whose investigators may not have expertise in vision testing. The SVA was designed to be an inexpensive and easy-to-use tool to screen for deficits in central visual acuity. A more detailed description of this tool has been published previously.1 The computerized SVA facilitates testing by using a menu-display system to collect and store quantitative data for use in epidemiologic studies. The software runs on a standard personal computer with images (either ETDRS or HOTV optotype presentation, depending on age) displayed on a 19-inch computer screen. ETDRS (letter charts originally designed for the Early Treatment for Diabetic Retinopathy Study) images are used for participants 7 years of age and older, while HOTV images (the 4 letters H, O, T, and V) can be used for participants aged 3 through 6 years. Although some previous research has studied HOTV down to age 5,2 several experienced consultants to the NIH Toolbox believed that HOTV could be used effectively down to age 3, as required for NIH Toolbox. Visual acuity testing using ETDRS charts for adults is now considered the gold standard for visual acuity testing3 and is recommended by the National Academy of Sciences and by the American National Standards Institute. Visual acuity testing using HOTV for children 5–12 years of age has been used in place of the ETDRS chart in some studies in an attempt to improve testability in younger children.2 However, ETDRS continues to be used in recent studies of 5- to 12-year-olds.4,5 Use of pictures for visual acuity testing in young children, as in the Lea charts, has been used with high testability but has been shown to overestimate visual acuity in some children with visual impairment.6 Recognition acuity tests such as HOTV are also known to overestimate visual acuity compared with ETDRS, especially when visual acuity is poor.4
For Toolbox visual acuity testing, participants were asked to wear any corrective lenses for distance vision that they normally wear. This approach was used because the focus of Toolbox is on everyday functioning, and “corrected” visual acuity will more accurately assess this than “uncorrected.” In addition, participants were tested using both eyes at the same time (binocular), both in the interest of time and, again, to best assess current functioning. The SVA software was developed based on the E-ETDRS protocol,7 which is an electronic or computerized version of the ETDRS chart testing. An initial screening is performed via computer to obtain an approximation of the subject's visual acuity threshold. Next, a testing phase is performed to obtain the visual acuity score. For the screening phase, the software starts at size 20/50 and presents a single random letter per line size (sizes range from 20/10 to 20/800), going either smaller until the subject makes an error or larger until the subject answers correctly. For the testing phase, 5 letters per line size are presented, starting with the smallest size answered correctly from the screening phase. If any letter is identified incorrectly at the largest size seen, the next larger level is added to the test. If 3 or more letters are correct at the smallest size seen, the next smaller level is added to the test. The subject always sees all 5 letters for each size added to the test. Letters from the largest level in the test are always shown first, working down to the smallest level. Testing stops when 3 or more letters are missed at the smallest level, or the subject reaches the 20/10 level. The software records the history of all optotypes displayed and the participants' responses. The examiner records whether the participant's response was correct or incorrect on the software display screen.
Reliability and validity evaluation of the SVA.
The reliability and validity of the SVA scores were evaluated in pediatric and adult participants at test centers at the University of North Florida, the University of Southern California, and The Johns Hopkins University. A total of 318 individuals were tested, of whom 99 were 3–6 years of age, 141 were 7–12 years of age, and 78 were 13–75 years of age. For children 3–12 years of age, both Lea symbols8 (simple outline drawings of easily identifiable shapes) and HOTV letters were tested to allow comparison of testability and agreement across ages. Lea symbols were originally considered for inclusion in computerized SVA if a high percentage of young children had been found not to recognize the HOTV letters; however, if most young children were able to recognize HOTV letters (the standard test for visual acuity in young children), this would be the preferred set of optotypes. For participants 7 years of age and older, computerized SVA was completed using ETDRS optotypes. Children 7–12 years of age therefore completed testing for all 3 optotypes; testing was done using a randomized block design so that the fatigue effect on any particular optotype was controlled. Reliability of computerized SVA was evaluated by retesting on the same day. Computerized SVA scores were compared to visual acuity scores obtained from standard ETDRS lightbox charts (for individuals 7 years of age and older).
Of the 318 tested individuals, 81% were white, 4% were African American, 3% were Asian, and 3% were mixed race/ethnicity (9% did not report their race/ethnicity); 7% of all participants described themselves as Hispanic. Among the 3-year-olds, a high proportion of children could complete the computerized SVA test using either Lea symbols (81%) or HOTV (76%); by age 4, this percentage had increased to 97% for Lea and 91% for HOTV. All children could complete both the Lea and HOTV examinations by 5 years of age.
Reliability of the computerized SVA for HOTV and ETDRS was assessed by retesting 292 of the 318 individuals on the same day. The intraclass correlation (ICC) for HOTV among 3- to 12-year-old participants was high (ICC = 0.91); the ICC for ETDRS for participants 7 years of age and older was similar (ICC = 0.89). Computerized SVA using ETDRS optotypes was compared to visual acuity scores from standard lightbox ETDRS charts. For participants 7–17 years of age (n = 143), a strong and statistically significant correlation was found (Pearson product-moment correlation coefficient = 0.80; p < 0.001); similarly, the correlation for participants 18 years of age and older (n = 36) was also strong (r = 0.80; p < 0.001).
Vision-targeted health-related quality of life.
A self-reported, vision-targeted health-related quality of life instrument was developed for the NIH Toolbox to examine visual function in ages 18 years and above. The new instrument was developed based on a review of 12 existing measures (see the table for a list of existing questionnaires).
Table.
Existing vision quality-of-life questionnaires
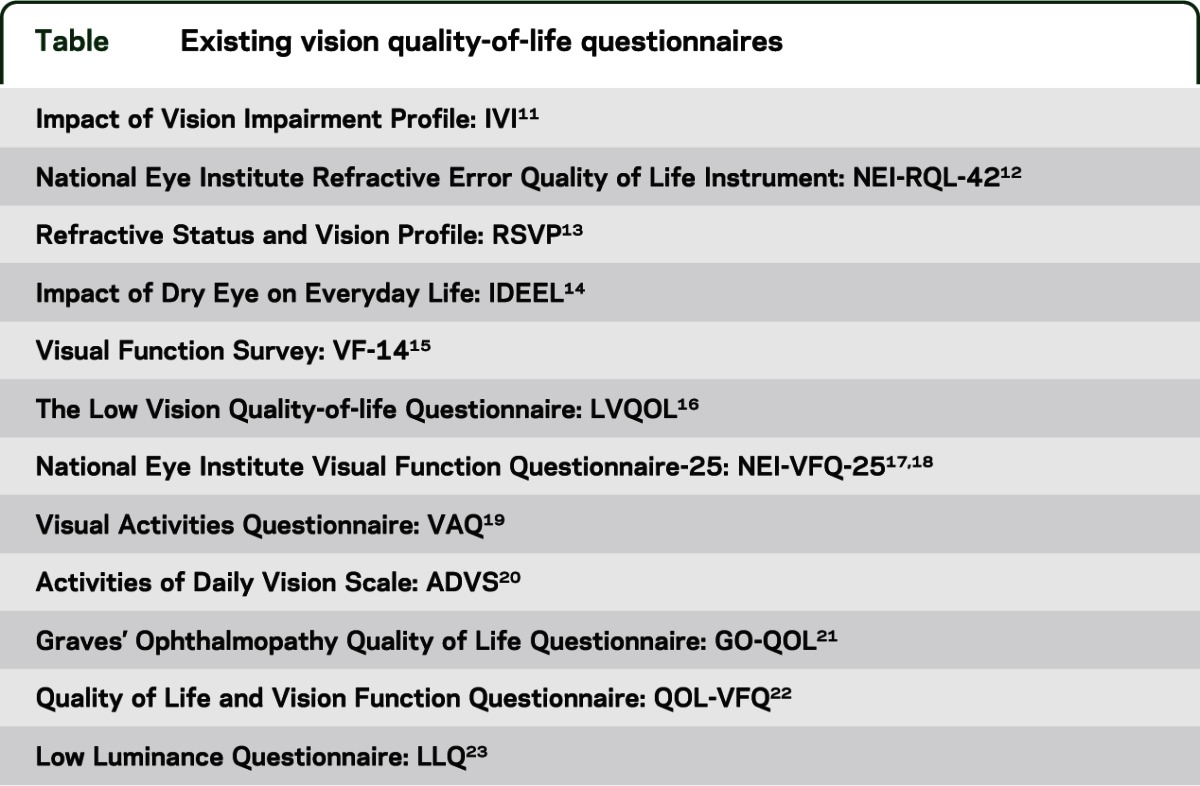
Items from existing questionnaires were reviewed, sorted, and evaluated for overlap in content. Based on this review, 53 items were written for the new instrument to assess 6 domains of vision-targeted functioning and well-being, including 1) color vision; 2) distance vision; 3) near vision; 4) ocular symptoms; 5) psycho/social functioning; and 6) role performance. Nonredundant items from the original questionnaires were selected and revised to improve clarity and to create consistent wording across items when possible. Response choices were written to be similar across items. Five response options were used to rate difficulty, including 1) not difficult at all, 2) a little difficult, 3) somewhat difficult, 4) very difficult, and 5) unable to do because of eyesight. A recall interval of 7 days was selected to improve consistency, as people tend to focus on more recent events rather than those that happened over an extended period of time.9,10 Stems for most questions started with “How much,” “To what extent,” or “How much of a problem,” with revision of wording to obtain more uniformity across the instrument.
Cognitive interviews were completed on a small group of test individuals to obtain feedback on survey item wording, and modifications to some stem and response option wording were made accordingly. The detailed methods used to develop the vision-targeted health-related quality of life instrument and evaluate the reliability and validity of the instrument have been described separately.24 Briefly, the 53 items were administered to a sample of 819 individuals, ages 18–85, to evaluate reliability and validity and to calibrate the items, using item response theory methods. The domain scores had high reliability (coefficient αs ranged from 0.85 to 0.94). Further, there was good correlation (0.59–0.77) between the National Eye Institute Visual Function Questionnaire scales and the NIH Toolbox instrument, and lower mean scores among groups self-reporting eye disease compared to participants without eye disease. These data, along with the selection of original items from existing validated instruments, support the validity of the current instrument. The instrument may be useful for investigators who wish to complete a comprehensive assessment of the impact of eye disease on daily functioning (e.g., reading, walking, driving) and well-being (e.g., social interaction, independence). The survey instrument was designed for adults and is inappropriate for children younger than 18 years because the question contexts are more adult-focused. Development of a pediatric vision-specific health-related quality of life instrument for the NIH Toolbox is considered an important priority for the future.
ACKNOWLEDGMENT
The authors thank the following individuals for their help during the selection and development of the Toolbox Vision instruments: Eileen Birch (Retina Foundation of the Southwest), Karen Cruickshanks (University of Wisconsin), Christopher Johnson (University of Iowa), Paul Lee (Duke University), Maureen Maguire (University of Pennsylvania), Robert Massof (Johns Hopkins University), Cynthia Owsley (University of Alabama-Birmingham), Michael Repka (Johns Hopkins University), and Michael Wall (University of Iowa).
AUTHOR CONTRIBUTIONS
R. Varma: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. R. McKean-Cowdin: study concept and design, acquisition of data, interpretation of data, drafting and critical revision of the manuscript. S. Vitale: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. J. Slotkin: study concept and design, acquisition of data, analysis and interpretation, drafting and critical revision of the manuscript, study supervision. R.D. Hays: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
This study is funded in whole or in part with Federal funds from the Blueprint for Neuroscience Research, NIH, under Contract No. HHS-N-260-2006-00007-C.
DISCLOSURE
R. Varma serves as a consultant for Alcon Laboratories, Allergan, Aquesys, Bausch & Lomb Surgical, Genentech, Inc., Merck & Co., Pfizer Ophthalmics, and Replenish Inc. He receives grant support from Allergan, Aquesys, Pfizer Ophthalmics, and Replenish Inc. He has equity ownership with Aquesys and Replenish, Inc. R. McKean-Cowdin is funded by NIH grants NCI-PC-35016-20, R01-NR0122774-01, U10-EY-014472-01, and U10-EY-11753. S. Vitale and J. Slotkin report no disclosures. R. Hays received research funding from the NIA (AG020679-01, P30AG021684, P30-AG028748), NIAMS (UAR057936A, AR052177), NCMHD (2P20MD000182), and the Agency for Healthcare Research and Quality (U18 HS016980). He also received consulting money from Allergan, UBC, the VA, and SciMetrika. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rine RM, Roberts D, Corbin BA, et al. New portable tool to screen vestibular and visual function: National Institutes of Health Toolbox initiative. J Rehabil Res Dev 2012;49:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice ML, Leske DA, Holmes JM. Comparison of the amblyopia treatment study HOTV and electronic-early treatment of diabetic retinopathy study visual acuity protocols in children aged 5 to 12 years. Am J Ophthalmol 2004;137:278–282 [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL, III, Sperduto RD. Standardized illumination for visual acuity testing in clinical research. Am J Ophthalmol 1982;94:97–98 [DOI] [PubMed] [Google Scholar]
- 4.Birch EE, Strauber SF, Beck RW, Holmes JM; Pediatric Eye Disease Investigator Group Comparison of the amblyopia treatment study HOTV and the electronic-early treatment of diabetic retinopathy study visual acuity protocols in amblyopic children aged 5 to 11 years. J AAPOS 2009;13:75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Treatment for Retinopathy of Prematurity Cooperative Group; Good WV, Hardy RJ, et al. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol 2010;128:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer DL, Gross RD. Modified Allen pictures to assess amblyopia in young children. Ophthalmology 1990;97:827–832 [DOI] [PubMed] [Google Scholar]
- 7.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthalmol 2001;132:903–909 [DOI] [PubMed] [Google Scholar]
- 8.Hyvarinen L, Nasanen R, Laurinen P. New visual acuity test for pre-school children. Acta Ophthalmol 1980;58:507–511 [DOI] [PubMed] [Google Scholar]
- 9.DeWalt DA, Rothrock N, Yount S, Stone AA; PROMIS Cooperative Group Evaluation of item candidates: the PROMIS qualitative item review. Med Care 2007;45:S12–S21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone AA, Broderick JE, Schwartz JE, Schwarz N. Context effects in survey ratings of health, symptoms, and satisfaction. Med Care 2008;46:662–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weih LM, Hassell JB, Keeffe J. Assessment of the impact of vision impairment. Invest Ophthalmol Vis Sci 2002;43:927–935 [PubMed] [Google Scholar]
- 12.Hays RSK. National Eye Institute Refractive Error Quality of Life Instrument (NEI-RQL-42TM), Version 1.0: A Manual for Use and Scoring. Los Angeles: National Eye Institute; 2002 [Google Scholar]
- 13.Vitale S, Schein OD, Meinert CL, Steinberg EP. The refractive status and vision profile: a questionnaire to measure vision-related quality of life in persons with refractive error. Ophthalmology 2000;107:1529–1539 [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan K, Abetz L, Mertzanis P, et al. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health 2005;8:168–174 [DOI] [PubMed] [Google Scholar]
- 15.Steinberg EP, Tielsch JM, Schein OD, et al. The VF-14. An index of functional impairment in patients with cataract. Arch Ophthalmol 1994;112:630–638 [DOI] [PubMed] [Google Scholar]
- 16.Wolffsohn JS, Cochrane AL. Design of the low vision quality-of-life questionnaire (LVQOL) and measuring the outcome of low-vision rehabilitation. Am J Ophthalmol 2000;130:793–802 [DOI] [PubMed] [Google Scholar]
- 17.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050–1058 [DOI] [PubMed] [Google Scholar]
- 18.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ): NEI-VFQ Field Test Investigators. Arch Ophthalmol 1998;116:1496–1504 [DOI] [PubMed] [Google Scholar]
- 19.Sloane M, Ball K, Owsley C, Bruni JR, Roenker DL. The Visual Activities Questionnaire: developing an instrument for assessing problems in everyday visual tasks. Tech Dig Noninvas Assess Vis Sys 1992;1:26–29 [Google Scholar]
- 20.Mangione CM, Phillips RS, Seddon JM, et al. Development of the ‘Activities of Daily Vision Scale': a measure of visual functional status. Med Care 1992;30:1111–1126 [DOI] [PubMed] [Google Scholar]
- 21.Terwee CB, Gerding MN, Dekker FW, Prummel MF, Wiersinga WM. Development of a disease specific quality of life questionnaire for patients with Graves' ophthalmopathy: the GO-QOL. Br J Ophthalmol 1998;82:773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carta A, Braccio L, Belpoliti M, et al. Self-assessment of the quality of vision: association of questionnaire score with objective clinical tests. Curr Eye Res 1998;17:506–511 [DOI] [PubMed] [Google Scholar]
- 23.Owsley C, McGwin G, Jr, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci 2006;47:528–535 [DOI] [PubMed] [Google Scholar]
- 24.Paz SH, Slotkin J, McKean-Cowdin R, et al. Development of a vision-targeted health related quality of life item measure. Qual Life Res. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]


