Abstract
Hemoglobin (Hb) has multiple pathophysiologic effects when released into the intravascular space during hemolysis. The extracellular effects of Hb have resulted in novel models of toxicity, which help to explain endothelial dysfunction and cardiovascular complications that accompany genetic hemolytic anemias, malaria, blood transfusion, and atherosclerosis. The majority of models focus on nitric oxide (NO) depletion; however, in local tissue environments, Hb can also act as a pro-oxidant and inflammatory agent. This can alter cellular differentiation with the potential to deviate immune responses. The understanding of these mechanisms set in the context of natural scavenger and detoxification systems may accelerate the development of novel treatment strategies.
Free hemoglobin, released during hemolysis, can act as a toxin and adversely influence the severity of some conditions (e.g., massive transfusions and genetic/acquired anemias). Hemoglobin scavenger proteins may be useful as therapeutics.
The capability of hemoglobin (Hb) to efficiently load and transport oxygen is inevitably linked to a broad reactivity pattern with alternative ligands, such as carbon monoxide (CO), nitric oxide (NO), hydrogen peroxide (H2O2), and many others. The biochemistry of these reactions has been the focus of Hb research for decades. More recently, however, these Hb reactions were examined as a potential cause of adverse pathophysiologic processes that accompany red blood cell (RBC) destruction (i.e., hemolysis) and the accumulation of extracellular free Hb (Baek et al. 2012; Gladwin et al. 2012). Other biologic activities of extracellular free Hb can be traced back either to direct interactions of its globin or heme components with specific cellular receptors and signaling pathways, or to the secondary effects of heme breakdown by the heme oxygenases. The evolving picture suggests that Hb as a toxin can adversely affect the outcome of diverse conditions, including the hemolytic anemias, sepsis, malaria, blood transfusion, and atherosclerosis, in which local accumulation of extracellular Hb causes oxidative stress and changes macrophage polarization in the atherosclerotic plaque microenvironment. The recognition that Hb is a disease-modifying compound, and concurrent research on protective Hb scavenger proteins have provided a framework for novel pathophysiologic models and may lead the way toward a new era of targeted treatment strategies. The toxic effects of free Hb appear to depend on the amounts in the extracellular space, anatomic location, and the activity of scavenger and detoxification pathways. These factors may vary considerably among different disease states and, therefore, extrapolations or application of a general Hb toxicity model to heterogeneous conditions must be considered cautiously. In this article we will summarize current evidence that supports Hb’s role as a disease modifier in hemolytic anemias, malaria, blood transfusion, and atherosclerosis, and how scavenger protein-based therapeutics could be used to attenuate the underlying pathophysiologic processes.
DISEASE STATES THAT ARE MODULATED BY THE TOXICITY OF EXTRACELLULAR HEMOGLOBIN
Sickle Cell Disease and Hemoglobin-Based Oxygen Carriers
Two areas of research, sickle cell disease (SCD), which represents a condition of chronic low-level plasma Hb exposure (3–10 μm plasma heme), and Hb-based oxygen carrier (HBOC) therapy, which represents a condition of acute high-level Hb exposure (>500 μm plasma heme), have driven the evaluation of pathophysiologic models to better understand the roles of extracellular Hb toxicity as a general disease process (Buehler et al. 2010). A pronounced systemic, and in some animal models a pulmonary hypertensive, response is observed within seconds of exposure to cell-free Hb or HBOCs (Buehler et al. 2010). This acute response is likely related to the interaction of Hb with NO and is suspected to be a cause of acute myocardial infarction and stroke in certain subjects receiving HBOCs (Natanson et al. 2008; Silverman and Weiskopf 2009). HBOCs are typically transfused in large quantities reaching millimolar plasma concentrations of extracellular Hb. These dosing levels are required to meet O2 delivery and volume replacement needs in patients with severe hemorrhage.
Sickle cell anemia is a chronic low-level hemolytic disease; however, some of the sequelae mimic those of HBOC administration. The typical complications of SCD are vasculopathies, stroke, pulmonary hypertension (PH), and renal failure, which suggest a pathophysiology of unopposed constriction within the vasculature and, therefore, may be related to an Hb-induced reduction in NO bioavailability. The NO depletion hypothesis is based on the findings that plasma from patients with SCD had elevated levels of free Hb, and accordingly, that the plasma from these patients had higher ex vivo NO-depleting activity (Reiter et al. 2002). In other studies, positive correlations were found between surrogate markers of hemolysis, PH, and disease-related mortality (Gladwin et al. 2004). PH, measured by Doppler echocardiography, was estimated to occur in up to 30% of patients with SCD and was therefore hypothesized to be a paradigmatic effect of NO depletion that could accompany chronic hemolytic diseases in general (Rother et al. 2005). The NO depletion hypothesis, however, has been challenged by other studies that found a lower, but still relevant, prevalence of PH when assessed by pulmonary artery catheterization (the gold standard for measuring blood pressure within the pulmonary circulation) (Parent et al. 2011). Even fewer patients in this cohort were found to have precapillary PH, which would be expected if Hb-mediated NO depletion within the pulmonary vasculature were highly relevant. Further concerns with the NO depletion hypothesis in SCD are related to the validity of hemolytic surrogate markers, such as plasma lactic acid dehydrogenase (LDH), and to the generally low plasma Hb concentrations (in the low micromolar range) detected in SCD patients, even during vasoocclusive crisis, when free Hb concentrations remain orders of magnitude below those associated with some hyperhemolytic states or HBOC administration (Bunn et al. 2010). Other proposed mechanisms of Hb toxicity during SCD or HBOC administration are related to the pro-oxidative and proinflammatory properties of Hb/heme, which can damage the endothelium or activate components of the coagulation and innate immune systems (Jeney et al. 2002; Belcher et al. 2003). The history of research on Hb toxicity in SCD and HBOC therapy illustrates that multiple pathways of toxicity for free Hb can theoretically coexist and that emphasis on one pathway may not fully define the extent and complexity of Hb-induced pathophysiology in conditions where it may contribute to disease processes.
Transfusion of Stored Blood
RBC transfusion with acute blood loss or chronic anemia is one of the most common therapeutic interventions in medicine. Over the last two decades, however, more restrictive transfusion practices have been implemented, and transfusion thresholds have been lowered by limiting unnecessary transfusions (Barr and Bailie 2011). Accumulating evidence suggests that the adverse consequences of some transfusions are related to the storage period between blood donation and transfusion (Wang et al. 2012). The maximum allowed storage time is now 42 d with most storage solutions, and current blood bank practices following a “first in, first out” system that favors the distribution of older units first. A 2011 meta-analysis of retrospective data from 409,966 patients estimated that a significant (odds ratio, 1.16; 95% confidence interval, 1.07–1.24) risk of death exists when relatively old, rather than fresh, blood is transfused. The definition of older stored blood in the analyzed studies ranged from 9 d up to 42 d, with a range of 1–10 units of blood transfused per patient (Wang et al. 2012). The RBC transfusion-associated risk of death may be increased in certain high-risk populations, such as critically ill or massively transfused (>10 units of blood within 24 h) patients. It is important to point out that storage time-related morbidity/mortality and potential mechanisms associated with transfusion-related toxicity remains controversial and not all studies support the concept that older blood is detrimental (Middelburg et al. 2012). RBCs may undergo complex biochemical and structural changes, collectively referred to as the “RBC storage lesion.” Damaged or aged RBCs can accumulate over time as a low-quality population within stored blood bags. It is therefore possible that some degree of acute hemolysis occurs when older stored blood is transfused. Current regulations only require that 75% of transfused RBCs survive within the circulation for 24 h after transfusion. This means that up to 25% of RBCs may be lost within the first 24 h after transfusion, owing to either intravascular hemolysis or the trapping of RBCs by macrophages in the spleen or liver. In the most extreme situation, this implies an acute burden of >10 g extra Hb per unit of transfused older RBCs that are either acutely released into the circulation or metabolized by spleen and liver macrophages after erythrophagocytosis.
Small studies in healthy volunteers suggest that extravascular hemolysis and increased RBC-Hb catabolism occur when as little as one unit of blood is transfused after 40 days of storage (Hod et al. 2011). Following single-unit transfusion, other investigators found that intravascular Hb exposure is associated with concomitant elevation in plasma nitrite levels and NO depletion in humans following small volume blood transfusion (Berra et al. 2012).
Animal models of RBC transfusion have been designed to explore the possible roles of intravascular hemolysis and cell-free Hb in the pathophysiologic consequences of the massive transfusion of older stored blood in guinea pigs (Baek et al. 2012). These studies suggest that high-volume transfusions of older blood, but not fresh blood, result in posttransfusion intravascular and extravascular hemolysis that approaches a 15%–20% loss of transfused RBCs within 24 h. Transfusion of older, rather than fresh, blood caused an acute hypertensive response, hemoglobinuria, acute renal failure, and vascular injury (Fig. 1) (Baek et al. 2012). Infusion of transfused old blood with the specific Hb scavenger haptoglobin (Hp) attenuated several of the transfusion-related adverse effects. These antagonist experiments proved that free Hb was a contributor to the observed adverse effects. Additionally, animal studies focused on the acute hemodynamic effects of stored blood have suggested that NO donors could be a therapeutic option to limit transfusion-related and hemolysis-induced pulmonary and/or systemic hypertensive responses in mice, rats, and sheep (Yu et al. 2008, 2009, 2012; Donadee et al. 2011; Baron et al. 2012). One of these studies identified Hb and Hb-containing RBC membrane microparticles released in vitro before transfusion as an additional factor that may enhance the adverse posttransfusion effects (Donadee et al. 2011). Blood transfusion leading to stimulation of the heme breakdown pathway and accumulation of heme catabolites was associated with anti-inflammatory and immunosuppressive effects in multiple animal models, and such effects could contribute to the immune-compromised state of severely ill and massively transfused patients (Yazdanbakhsh et al. 2011). On the other hand, proinflammatory effects may be triggered by free heme through the stimulation of toll-like receptor 4 (TLR-4), by reaction intermediates emanating from Hb/heme peroxidative reactions with phospholipids, or by Hb/heme breakdown products (D’Agnillo and Alayash 2001; Jeney et al. 2002; Jia et al. 2007; Lin et al. 2010; Yazdanbakhsh et al. 2011). In a mouse blood transfusion model, older stored RBCs induced an acute phase cytokine release response that accompanied the clearance of transfused RBCs in the spleen (Hod et al. 2010).
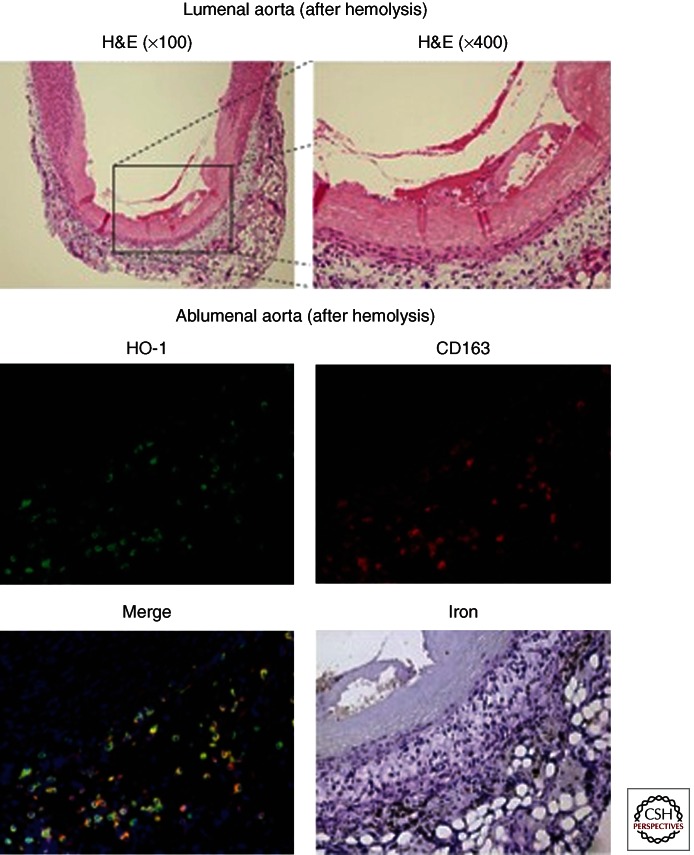
Figure 1.
Vascular effects of old blood transfusion-associated hemolysis. The top panel shows regions of vascular necrosis appearing to initiate within the vascular lumen and progress to the tunica media. These observations were captured 24 h after massive transfusion to guinea pigs at 100× and 400× magnification. The injury in isolated regions of the aorta in transfused animals was consistent with coagulative necrosis and may be the result of hemoglobin and/or damaged red blood cells. The lower panels indicate macrophage accumulation to hemoglobin-rich regions within the adventitia consistent with HO-1, CD163, and nonheme iron accumulation.
In summary, intravascular release and enhanced turnover of Hb after transfusion of older stored blood could contribute to cardiovascular and renal dysfunction, as well as inflammation and enhanced susceptibility to infection in severely ill patients. However, similar to interpretation of retrospective human data, the translation of animal-based transfusion model data to the human situation is nearly impossible. In animal studies, RBCs are often maximally stressed and RBCs of animals and humans behave with varying degrees of difference both under storage and following transfusion. As a result it is unlikely that any model will provide a definitive understanding of the potential complications associated with human blood storage. The best that animal studies can be expected to provide are suggestions for pathophysiologic mechanisms that may help to explain poor outcomes previously reported in clinical settings.
Malaria
Malaria is a paradigmatic hemolytic condition and considered a driving force in human evolution. Several common hemoglobinopathies, such as SCD or the thalassemias, have been proposed to result, in part, from the coevolutionary interactions between the plasmodium parasite and its human host (Rosenthal 2011). Animal studies in mice have been used to construct hypothetic models of the pathophysiologic role of the Hb molecule in malarial disease progression and tolerance. In the acute phase of severe malaria, hemolysis appears to exaggerate inflammatory processes and endothelial damage through NO depletion, oxidation, and inflammation (Gramaglia et al. 2006; Pamplona et al. 2007). The appearance of Hb and heme in plasma has been linked to the development of cerebral malaria, which remains the most severe and difficult to treat complication of the infection. In contrast, chronic hemolysis (before malaria infection) in SCD induces a state of malaria tolerance by enhancing the basal activity of heme defense systems, such as heme oxygenase 1 (HO-1) up-regulation, which drives the generation of metabolites with anti-inflammatory effects (i.e., CO, bilirubin, and ferritin) (Ferreira et al. 2011). Thus, active hemolysis before infection does not alter the course of parasitemia per se, but may prevent progression to fatal disease complications. However, similar mechanisms that confer a state of relative tolerance to severe malaria may also enhance host susceptibility to concurrent nonplasmodium infections. Infection with nontyphoid Salmonella during the acute hemolytic phase of plasmodium infection was associated with the appearance of an immature granulocyte population with reduced oxidative burst capacity and, consequently, a reduced capacity to kill phagocytosed Salmonella (Cunnington et al. 2011). This effect was mimicked experimentally by heme injection and attenuated when HO-1 was inhibited. As a result, animals that were coinfected with malaria were much more susceptible to fatal Salmonella infection than control animals that were not heme stressed.
It is overly simplistic to reduce the pathophysiology of a complex parasite infection with diverse host–pathogen interaction pathways to the level of the Hb molecule as a single mechanism of pathology. However, based on the discussed animal studies, the impact of hemolysis in malaria could be a relevant component. Low-level hemolysis before plasmodium infection may induce a state of tolerance, attenuating the deleterious effects of Hb/heme in the acute phase. Acute Hb and heme exposure exaggerate inflammatory and oxidative tissue damage in nontolerant individuals and may enhance susceptibility to superinfection by nonmalaria pathogens. An important lesson to take away from these studies is that Hb and heme may have diverse pathophysiology in patients with inflammatory diseases and infection. The net impact of extracellular Hb in a given disease state can be protective or deleterious, and the effect is likely determined by the anatomic site, timing, and degree of hemolysis on one hand, and availability of scavenger systems on the other hand.
Atherosclerosis—A Paradigm for Hb as a Locally Active Disease Modifier
As exemplified in the sections above, the pathophysiology of extracellular Hb has largely been studied on a systemic level. It is likely, however, that Hb also acts as an important modulator of local disease processes, where RBCs extravasate and are lysed within tissues. Under such conditions, very high extracellular concentrations of Hb and heme (in the millimolar range) could affect cell viability, activation state, and differentiation (Boyle et al. 2009).
One of the paradigmatic conditions in which local Hb/heme effects have been studied is atherosclerosis. The principal findings of these investigations may similarly apply to other clinical situations, such as wound healing or hemorrhagic stroke. Microscopic hemorrhage from fragile neovessels is a hallmark of advanced plaques, and a predictor of catastrophic clinical events such as myocardial infarction and stroke (Kolodgie et al. 2003). Besides the cholesterol and phospholipid-rich RBC membrane components that are deposited in the areas of microbleeding, Hb is the most abundant and biologically reactive compound within these lesions (Schaer et al. 2006).
The potential effects of Hb are diverse. Heme can oxidize low-density lipoprotein (LDL) and other lipids, and transform these compounds into proinflammatory and cytotoxic species (e.g., oxidized LDL) that have an established impact on atherosclerotic plaque progression (Nagy et al. 2010). Hb-derived free heme, as well as heme- and globin-degradation products, have a cytotoxic impact on endothelial and vascular smooth muscle cells, and may act as proinflammatory mediators that perpetuate inflammatory cell recruitment through the stimulation of chemokine (e.g., IL-8) expression (Boyle et al. 2009; Kaempfer et al. 2011). Free Hb in the extracellular environment may also polarize the differentiation plasticity of certain cell types. The observation that phenotypically distinct macrophage subpopulations cluster within hemorrhagic and nonhemorrhagic plaque areas has stimulated research exploring whether Hb and/or heme are the factors reshaping monocyte-macrophage lineage differentiation in these lesions (Boyle et al. 2009).
The specific interest in macrophages is twofold. On the one hand, macrophages are the principal inflammatory cell type within atherosclerotic plaques, and primary mediators of disease progression. On the other hand, they are the only cell type that expresses the Hb scavenger receptor CD163, and possess a highly specialized physiology to handle and detoxify extracellular Hb. Because of this unique function, macrophages in Hb-rich environments have very high expression levels of HO-1, ferritin, and the iron exporter ferroportin. In vitro and in the coronary arteries of patients, Hb/heme-exposed macrophages were described as a distinct phenotype with high expression of the Hb detoxification pathway (CD163high HO-1high), high mannose receptor expression, and profoundly suppressed expression of HLA class 2 (Boyle et al. 2009; Kaempfer et al. 2011; Finn et al. 2012). This nonfoamy macrophage subtype localizes to the postbleeding plaque areas, has enhanced antioxidative and iron export functions, and is resistant to cholesterol loading as a result of up-regulated ATP-binding cassette transporters (ABCA1 and ABCG1) and down-regulated scavenger receptors (SR-A/B and CD36). The available data suggest that these Hb/heme-driven macrophages constitute a unique phenotype (referred to as M-hem or M-Hb), with distinct and nonoverlapping features, within the known spectrum of macrophage phenotypes extending from proinflammatory (M1) to anti-inflammatory (M2) macrophages. The Hb/heme-driven macrophage phenotype polarization appears to be triggered by increased intracellular heme levels via a pathway involving signaling by Nrf-2 and the activating transcription factor 1 (ATF1), with subsequent coinduction of HO-1 and liver-X-receptor β (LXRβ) (Kaempfer et al. 2011; Boyle et al. 2012). The functional significance of the profoundly suppressed levels of HLA class 2 in these macrophages has not been evaluated, but the spectrum of functional consequences of this intriguing Hb/heme-induced condition may include enhanced control of autoimmunity and/or increased susceptibility to infection. Accumulating evidence now suggests that Hb/heme can impact multiple levels of innate and acquired immunity. Hb should therefore be considered a novel intrinsic alarm molecule that serves to signal bleeding and tissue destruction.
In summary, Hb may play a dual role in atherosclerosis. The proinflammatory and oxidative effects of heme within the extracellular milieu initiate and promote plaque formation, whereas Hb/heme drives macrophage polarization toward a protective, nonfoamy, and antioxidative macrophage phenotype that halts lesion progression at later stages of the disease. It remains to be explored how these opposing processes could be shifted favorably by pharmacological interventions.
MECHANISMS OF HEMOGLOBIN-MEDIATED ADVERSE EFFECTS
The primary acute pathophysiologic responses to extracellular Hb in plasma are blood pressure elevation (Rother et al. 2005) and pro-oxidative toxicity occurring in vascular and renal tissues (Boretti et al. 2009; Baek et al. 2012). The processes driving Hb-induced toxicity are depicted in Figure 2 and likely involve a complex interaction between NO balance, oxidation, and inflammation.
Figure 2.
Schematic of hemoglobin-mediated toxicity initiated by intravascular hemolysis. (I) NO reactions with hemoglobin—Red blood cells (RBC) do not enter the vascular luminal RBC-free zone; however, free hemoglobin can enter the RBC-free zone and gain access to endothelial and perivascular spaces. When free oxyhemoglobin (red) enters the perivascular space it can react with NO rapidly via dioxygenation forming ferric hemoglobin (brown) and nitrate (NO3−). Additionally, deoxyhemoglobin (purple) can react with NO to form iron nitrosyl hemoglobin. Additionally, ferric hemoglobin can react with NO, but at much slower rates. It remains unknown whether this reaction is of any biologic significance. (II) Hemoglobin peroxidative reactions—Oxyhemoglobin (red) undergoes peroxidation to ferryl hemoglobin (green) followed by a redox cycle that is driven by hydrogen peroxide. In the presence of large quantities of reducing agents ferryl stability in this reaction is reduced to an extent that the species cannot accumulate, and in most cases cannot be directly detected. The most prevalent oxidized Hb species in vivo is therefore ferric Hb, which can result from peroxidation but also from autoxidation and NO dioxygenation depicted above. In vitro, the result of redox cycling mediated by H2O2 can be release of heme, globin chain radicals, and oxidation of amino acids within the hemoglobin molecule. The protein may also degrade to precipitate formed by globin-heme adducts and cross-links that physically damage endothelial cells leading to inflammation. Additionally, free radical transfer from ferryl radical oxidizes lipid, leading to potential tissue oxidation as well as inflammation.
Nitric Oxide Consumption—Systemic and PH
The most accepted hypothesis for Hb-induced hypertension involves the interactions of NO with both oxy- and deoxyhemoglobin (Doherty et al. 1998; Olson et al. 2004). The pathophysiologic response to Hb and its consumption of NO is observed primarily as an increase in systolic/diastolic and mean arterial blood pressure (MAP) with cardiac output either unchanged (Boretti et al. 2009) or decreased (Minneci et al. 2005). This consistently causes an increase in systemic vascular resistance, or vasoconstriction (Reiter et al. 2002; Minneci et al. 2005; Boretti et al. 2009). In addition, heart rate and stroke volume are often deceased with the aggregate hemodynamic response resulting in decreased perfusion to some organ systems, particularly the kidneys.
Following infusion of a bolus of Hb into the vascular space, the MAP response appears to be related to the pharmacokinetics of the protein in circulation (Fig. 3), with rapid blood pressure normalization and parallel renal clearance of free Hb. However, in animal models of RBC transfusion-related hemolysis and cases in which an HBOC with a long intravascular circulatory time is administered, there is a clear disconnect between pharmacodynamics (blood pressure response and duration) and pharmacokinetics (maximal concentration and total exposure) (Buehler et al. 2007). This suggests that some degree of tolerance to extracellular Hb in circulation occurs with longer-term exposures. If analyses of Hb-mediated vascular wall injury are considered, however, the duration of sustained intravascular Hb exposure appears to be a critical determinant of the cumulative toxicity (Baek et al. 2012).
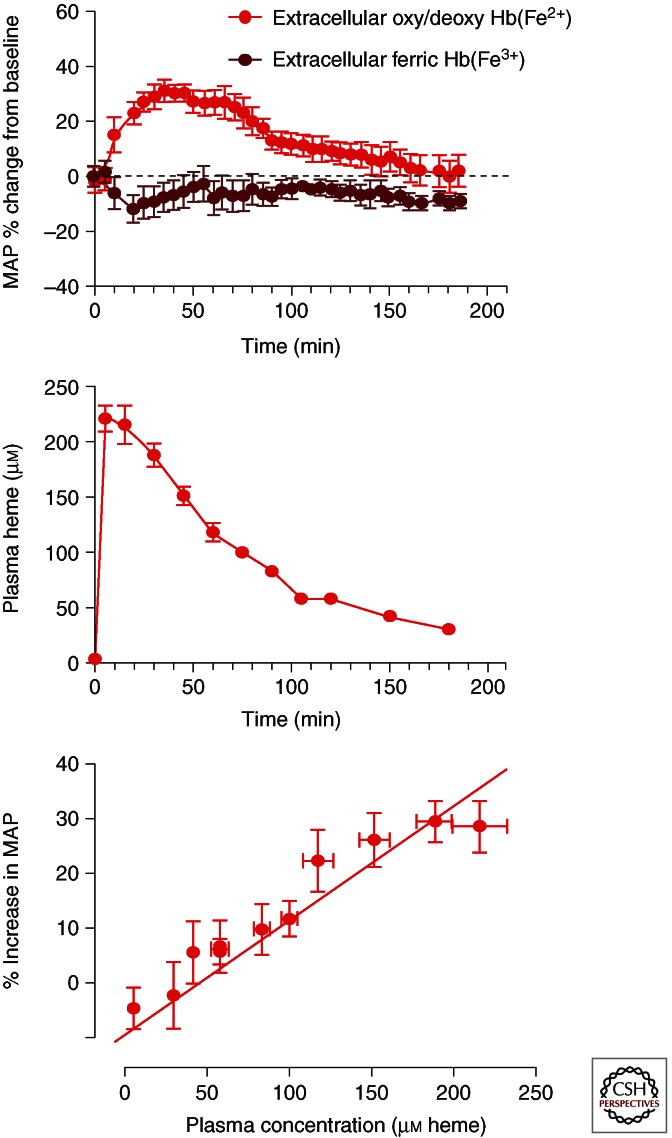
Figure 3.
Plasma concentration blood pressure relationships. (Top) The blood pressure response to ferrous (red) and ferric (brown) hemoglobin following a 20% top load to conscious guinea pigs. Ferric hemoglobin shows no response, whereas ferrous hemoglobin shows a rapid and transient increase in mean arterial pressure. This is consistent with the hemoglobin plasma concentration time course shown (middle). These two time effects can be better visualized by plotting plasma concentration versus percent mean arterial blood pressure change from baseline (bottom).
Within the context of potential pathology, Hb access to NO progresses via two primary reactions: (1) NO dioxygenation, and (2) iron nitrosylation of NO deoxy-Hb:
-
(1)
Hb-Fe2+(O2) + NO → {Hb(Fe3+OONO•)} → Hb-Fe3+ + NO3−,
-
(2)
Hb-Fe2+ + NO → Hb(NO).
Our understanding of the factors controlling these processes is largely owing to the extensive study of HBOCs and their effects on systemic and pulmonary hemodynamics (Doherty et al. 1998; Olson et al. 2004). Targeted mutagenesis to limit interactions with NO or chemical modification of human Hb attenuated the systemic vascular response, presumably via reduced NO consumption within sites of NO generation or decreased access to sites of NO production. Hb exposure also stimulates other vasoconstrictive physiologic systems and effectors such as angiotensin, endothelin, and prostaglandins (Buehler et al. 2010).
Pulmonary arterial pressure (PAP) is regulated by NO, prostacyclin, endothelin, as well as thromboxane; several of these regulators have been studied in association with Hb (Heller et al. 1998; Farber and Loscalzo 2004; Yu et al. 2009). The primary cause for PAP elevation induced by extracellular Hb has also been attributed to NO consumption. Elevated pulmonary vascular resistance was shown in dogs (Minneci et al. 2005) and PH was shown in rodents and sheep following exposure to Hb (Yu et al. 2008). These studies suggest a causal relationship between Hb and NO, based on the use of NO synthase inhibitors and reversal of PH via NO supplementation before Hb exposure. In other acute Hb exposures, such as transfusion-related hemolysis, PH is reported to occur more readily in animals with endothelial dysfunction compared with normal animals (Yu et al. 2012). Pretransfusion NO inhalation was shown to attenuate this pathology (Baron et al. 2012). Certain animal species show PH in response to HBOCs; however, it is unknown whether this effect regularly occurs following HBOC infusion in humans and whether a short-term increase in PAP could contribute to adverse events following extracellular Hb exposure or HBOC administration. Publicly available data on the incidence of PH from HBOC clinical trials show one event among 504 subjects treated with diaspirin-cross-linked Hb (DCLHb, HemAssist) (no events among 505 control subjects) and three events among 708 subjects treated with HBOC-201 (no events among 618 control subjects). It is unknown whether subjects who experienced acute transient PAP elevation developed clinically relevant events.
In Vivo Oxidation of Hemoglobin
Hydrogen peroxide and lipid hydroperoxides in tissue compartments are important components influencing the oxidative toxicity of heme proteins (Moore et al. 1998). Intracellular hydrogen peroxide (H2O2) is generated by the dismutation of superoxide (O2•−), either spontaneously or by superoxide dismutase, and is involved in cellular signaling. Its primary fate is intracellular conversion to H2O and O2 by catalases and peroxidases. The remaining H2O2 can be converted to reactive oxygen species or secreted into the extracellular environment, typically in low (1–15 μm) quantities (D’Agnillo et al. 2001). During inflammation, certain cell types, such as granulocytes and macrophages, increase the production and release of H2O2. The production, from H2O2 and chloride (Cl−), and secretion of hypochlorous acid are also increased. The increased levels of pro-oxidants could interact with extracellular Hb within localized tissue compartments and certain vascular sites. The reactions of Hb with peroxides have been extensively studied under “test-tube” conditions, and can be summarized as follows: Deoxy-Hb reacts with H2O2 via the following steps: (1) oxo-ferryl Hb [Hb(Fe4+ = O)] generation, (2) ferric Hb [Hb(Fe3+)] generation, and (3) protein radical generation [•Hb(Fe4+ = O)].
-
(1)
Hb(Fe2+) + H2O2 → Hb(Fe4+ = O) + H2O,
-
(2)
Hb(Fe4+ = O) + H+ → Hb(Fe3+) OH−,
-
(3)
Hb(Fe3+) + H2O2 → •Hb(Fe4+ = O) + H2O.
Ultimately, the globin chain-free radical shown in reaction (3) is available to participate in localized amino acid oxidations (e.g., within Hb) or transfer to LDL (Miller et al. 1997; Vallelian et al. 2008; Pimenova et al. 2010). Peroxidative Hb reactions with production of ferryl Hb and free radicals have been considered an important component of Hb and HBOC toxicity in the past (Alayash 2004). However, despite the extensive biochemical knowledge of oxidative Hb reactions in vitro, it is far less clear to what extent these reactions do occur in vivo and how they could contribute to Hb toxicity. For example, it is not consistently possible to trap ferryl Hb or Hb-associated free radicals in vivo. An explanation for this discrepancy might be that the reactions occur in vivo in a complex environment with different oxidants and in the presence of large quantities of reductants (e.g., ascorbate, urate, and enzymatic reductants). These conditions likely prevent ferryl Hb accumulation above a certain threshold of detection. In vivo evidence for these oxidative Hb processes is therefore largely based on indirect markers such as intramolecular Hb cross-links and globin amino acid oxidations that have been detected in some studies (Moore et al. 1998; Boutaud et al. 2010).
The kidneys are heavily exposed to heme and globin components owing to the extensive and rapid filtration of extracellular Hb. The mechanism of Hb toxicity in the kidneys may be related to redox cycling and ferryl Hb formation (Zager and Gamelin 1989; Moore et al. 1998; Boutaud et al. 2010). During rhabdomyolysis, redox cycling of ferric and ferryl myoglobin, driven by lipid hydroperoxides, enhances tissue oxidation and F2-isoprostane release (Moore et al. 1998; Boutaud et al. 2010). Observations of the excretion of covalently cross-linked dimeric, tetrameric, and polymeric Hb, as well as iron deposition and oxidative tissue modifications, in the kidneys of Hb-infused animals were interpreted as indicators of the peroxidative activity of Hb in vivo (Boretti et al. 2009; Butt et al. 2010). Within the gastrointestinal circulation of rats, an increase in microvascular permeability to fluorescently labeled albumin was reported to occur with several HBOCs (Baldwin et al. 2002). These effects appeared to be causally linked to the rates of oxidation of the individual HBOCs (Baldwin et al. 2002). Coadministration of the antioxidant sodium selenite reduced mesenteric vascular leakage and mucosal epithelial damage (Baldwin et al. 2003).
PHYSIOLOGIC HEMOGLOBIN SCAVENGERS, ANTIOXIDANTS, AND DETOXIFICATION SYSTEMS
The Hb scavenger system is a multiple-component pathway made up of several soluble plasma proteins (e.g., haptoglobin [Hp], hemopexin [Hpx], α1-microglobulin, and albumin) (Schaer and Alayash 2012). Cell-based receptors bind the Hb, or heme complexes of these proteins, and protect against systemic Hb and heme toxicity, until heme is degraded to metabolites by the heme oxygenases. The most extensively studied pathways are those involving the plasma Hb scavenger Hp and the monocyte/macrophage Hb–Hp scavenger receptor CD163 (Kristiansen et al. 2001; Schaer et al. 2006b). Additionally, the plasma heme-binding protein Hpx and its interaction with the LDL receptor-related protein 1 (LRP-1) appear to be critical when heme is released from Hb and other proteins (Nielsen et al. 2010). Hp remains the most important plasma Hb-binding protein, whereas the role of Hpx is secondary and generally required only when heme is released from Hb following oxidation. Figure 4 depicts the process of Hb/heme clearance and iron recycling within the plasma, macrophages, and erythroid cellular compartments.
Figure 4.
Physiologic hemoglobin scavengers and detoxification systems. Hb can be released from red blood cells (RBC) into the plasma and extracellular environment during hemolysis or tissue injury. In the extracellular compartment, Hb reacts with peroxides (H2O2 and lipid hydroperoxides) and promotes oxidative tissue damage. In the plasma or extracellular space, Hb is sequestered in the Hb–Hp complex. Complex formation prevents Hb-induced hypertensive and oxidative reactions. The Hb–Hp complex is subsequently endocytosed by the macrophage Hb scavenger receptor CD163. Within the macrophage, heme is released from globin and degraded by HO-1 into bilirubin and carbon monoxide (CO). The released iron induces ferritin synthesis. Iron can either be exported for iron recycling or it can be stored in a ferritin complex. Backup systems such as hemopexin (Hpx) and the Hpx–heme complex receptor (LDL receptor-related protein, LRP) pathway can bind and detoxify free heme that is released from oxidized Hb.
Haptoglobin
The primary Hb scavenger in plasma is Hp (Levy et al. 2010). Each Hp β chain binds one Hb αβ dimer in a 1:1 stoichiometry. In mammalian species, the primary phenotype of Hp is 1-1, meaning that the protein contains two identical β chains and two α1 chains assembled as βα1-α1β (90 kDa). The disulfide bond between the α1 chains occurs at only one cysteine residue. In humans, certain evolutionary changes have driven the expression of two additional phenotypes, Hp 2-1 and Hp 2-2, in which the α2 chain has two exposed cysteine residues available for disulfide bonding (Wicher and Fries 2010). Hp 2-1 contains an α2 chain, two α1 chains, and three β chains (135 kDa). Hp 2-2 contains only α2 chains and β chains that can form a multitude of self-polymerizing proteins with a molecular mass ranging from ∼135 kDa to 800 kDa. Additional mutations exist in humans, but remain rare and isolated. The formation of Hb–Hp creates a stable and irreversible complex that circulates in the plasma until it is cleared by CD163-expressing peripheral blood monocytes or tissue-resident macrophages (Kristiansen et al. 2001).
Hemopexin
This protein is primarily expressed in the liver and, like Hp, is classified as a class I acute phase protein induced by proinflammatory cytokines (i.e., IL-1 and IL-6) (Tolosano et al. 1999, 2010). The interaction of free heme with Hpx is reported to be the strongest plasma heme–protein interaction with a Kd < 10−13 m (Tolosano et al. 2010). Following inflammation, Hpx is synthesized in the liver, generating mean plasma concentrations of ∼10 μm (0.6 mg/mL) (Tolosano et al. 1999, 2010). In sickle cell anemia and thalassemia, Hpx is typically depleted in the plasma. Once heme-Hpx binding occurs, interaction and uptake by the macrophage-associated LRP-1 receptor removes the heme from the circulation (Nielsen et al. 2010).
Monocyte/Macrophage CD163
In the circulation and tissues, the Hb–Hp complex is cleared by the Hb scavenger receptor CD163, a 130-kDa scavenger receptor tethered to peripheral blood monocytes/macrophages and tissue-resident macrophages (Kristiansen et al. 2001; Schaer et al. 2006b, 2007). CD163 is the primary receptor responsible for removing free Hb (primarily as Hb–Hp) from the circulation. The binding of the Hb–Hp complex to the scavenger receptor cysteine-rich domain three of CD163 and its subsequent uncoupling following uptake are calcium-dependent. CD163 is cleaved from monocyte and macrophage cell surfaces by a TNF-α-converting enzyme/A Disintigrin and Metalloproteinase 17 mechanism under conditions of inflammation (Etzerodt et al. 2010). The inflammation-linked cleavage and plasma levels of CD163 have been shown to be elevated during general tissue inflammation; the highest concentrations were found in patients with macrophage-activation syndromes (Schaer et al. 2005).
Endocytosis of the Hb–Hp complex results in cellular delivery of heme and up-regulation of HO-1. This provides a link to enhanced macrophage HO-1 clearance of heme (Schaer et al. 2006a; Vallelian et al. 2010). The end result of this process is the up-regulation of the synthesis of heme metabolites (i.e., bilirubin, CO, and ferritin) that may function as antioxidants (bilirubin and ferritin) and signaling molecules (CO) (Otterbein et al. 2003).
Hemoxygenase
The loss of heme oxygenase (HO) function, or HO-1 deficiency, in humans was first reported in 1999 (Yachie et al. 1999). The disease was described in a 6-yr-old boy, who suffered from severe growth retardation, increased RBC fragility, chronic hemolysis with paradoxically elevated Hp levels, elevated ferritin levels, iron deposition in renal and hepatic tissues, and marked kidney injury (Yachie et al. 1999). This very rare condition provided important insight into the functional role and importance of this class of enzymes. HO is the primary enzyme of heme catabolism. Two predominant isoforms are involved in heme metabolism, HO-1 and HO-2, and both enzymatically degrade heme to biliverdin, bilirubin, iron, and CO (Abraham and Kappas 2008). HO-2 is constitutively expressed; however, HO-1 is induced by various stimuli including heme, lipopolysaccharide, hypoxia, and heavy metals (Abraham and Kappas 2008). However, heme is the most potent physiologic inducer of HO-1 (Balla et al. 1993). Although it appears evident that HOs are essential to keep intracellular heme homeostasis within a physiologic (e.g., nontoxic range), many effects that are associated with under- or overexpression of these enzymes are poorly understood. For example, the mechanisms and pathways of cellular heme toxicity (e.g., in cases of inappropriately low HO-1 expression or excessive heme load) have not yet been systematically explored. Cellular heme toxicity is mostly considered to be caused by nonspecific oxidative reactions that may damage cellular proteins and lipid components. However, it is likely that more complex pharmacologic effects of the protoporphyrin exist and could disturb essential metabolic pathways. Likewise, it is uncertain to what extent the protection against heme toxicity by HO is dependent on biologic activities of its metabolic end products (CO, iron, and bilirubin) in addition to the primary catabolic heme “removal” activity (Otterbein et al. 2003).
SCAVENGER PROTEIN-BASED THERAPEUTICS FOR HEMOLYTIC CONDITIONS
Several studies have explored the protective activities of Hp and Hpx against Hb/heme-driven disease processes in vitro and in vivo, and novel therapeutics based on these proteins have been proposed. Hp can prevent heme release from met-Hb (Fe3+) and, in the presence of H2O2, attenuate the peroxidation of globin chain amino acids and the oxidative modification of external molecules (i.e., other proteins and lipids) (Miller et al. 1997; Buehler et al. 2009; Pimenova et al. 2010). These protective functions appear to be related to the ability of Hp to firmly stabilize the structure of the bound Hb αβ dimers, and to a less understood mechanism whereby Hp shields peroxidative reactions within the Hb–Hp complex from external substrates. The Hb–Hp complex can still react with many ligands such as O2, NO, H2O2, and nitrite (Boretti et al. 2009; Buehler et al. 2009; Roche et al. 2012). It is unknown whether these reactions have any biological function. Some hypotheses suggest that the H2O2 reaction may attenuate oxidative stress in inflamed tissues, whereas the enhanced nitrite-reductase activity of the complex may help to control the adverse vascular effects of unopposed NO consumption within the circulation. Another essential function of Hp in vivo is the capture of the relatively small Hb dimers within the circulation by a protein complex too large for renal clearance and extravasation (Boretti et al. 2009; Baek et al. 2012). This intravascular sequestration limits toxic Hb exposure in the kidneys and other sensitive tissues, and may also prevent access of the NO scavenger Hb to the vascular wall (Fig. 5). Proof of concept studies of the therapeutic potential of Hp have been conducted in animal models of Hb infusion and blood transfusion (Boretti et al. 2009; Baek et al. 2012). Application of human plasma-derived Hp in these models attenuated the Hb-mediated acute hypertensive response, oxidative renal damage, and vascular injury. Human plasma-derived Hp is approved as a drug for the treatment of hemolytic conditions in Japan (haptoglobin injection, Benesis, Osaka, Japan). Unfortunately, this product is not available outside of Japan and there are insufficient data available to estimate the clinical efficacy of Hp, but there are reports of therapeutic benefits (Imaizumi et al. 1994). Other Hp products are expected to be developed by European and U.S.-based plasma fractionation companies, and further preclinical and clinical studies will likely be conducted in the near future to further explore the efficacy and safety of Hp for the treatment of diverse hemolytic conditions.
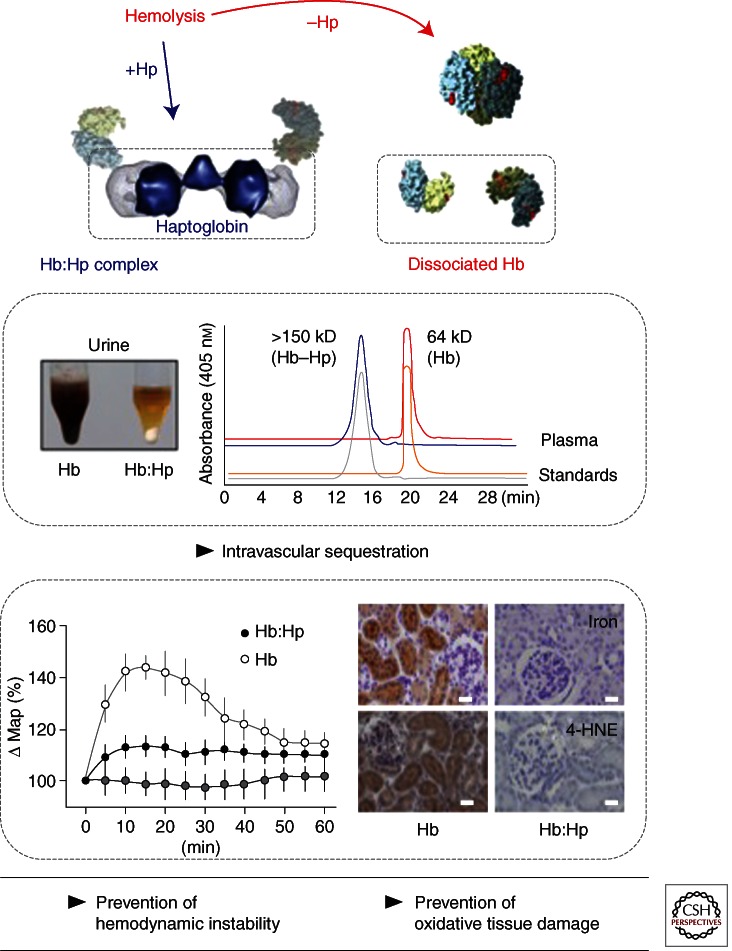
Figure 5.
The haptoglobin paradigm of damage prevention during hemolysis. After hemolysis free Hb can dissociate into αβ dimers with a relatively small molecule size of ∼32 kD (eluting at 19.8 min in the shown plasma size exclusion chromatography [SEC] profile). Susceptible tissues are exposed to toxic Hb. The results are hemoglobinuria, hemodynamic instability with acute hypertension, and oxidative tissue damage. With haptoglobin (Hp) treatment 100% of the free Hb is captured within the large sized (>150 kD) Hb:Hp complex that elutes at 14.7 min in plasma SEC (note that there remains no free Hb in the Hp-treated plasma sample). As a result, free Hb remains compartmentalized within the circulation. Hemoglobinuria, hemodynamic instability, and oxidative tissue damage are prevented. The image in the left middle panel shows urine collections of Hb and Hb:Hp infused guinea pigs. The lower left panel shows blood pressure recordings of Hb, Hb:Hp, or starch (control) infused animals. The kidney sections in the lower right panel were stained for 4-HNE as a marker of oxidative tissue damage (brown color) in Hb-infused (but not in Hb transfused +Hp-treated) animals.
The rationale for the use of Hpx as a therapeutic scavenger protein in hemolytic conditions is based on the idea that free heme could be an ultimate mediator of Hb toxicity. This idea is supported by multiple in vitro observations and by findings in the Hpx-knockout mouse, which is susceptible to renal damage after acute intravascular hemolysis (Tolosano et al. 1999). Severe sepsis can be accompanied by hemolysis and Hpx depletion. In a mouse model of severe sepsis, plasma-derived Hpx was protective against organ damage and death (Larsen et al. 2011). The protective effect in this model may be related to the ability of Hpx to prevent the interaction of free heme with innate immune receptors (e.g., toll-like receptors), interrupting the synergistic proinflammatory effects of heme and the sepsis-inducing pathogen. Currently, there is no Hpx product available for clinical application and, therefore, no safety and/or efficacy data exist for its therapeutic use.
Several functions of Hpx have been described that are not directly related to its heme scavenging activity (Tolosano et al. 2010). These functions involve enzymatic activities as well as direct anti-inflammatory effects. Nonheme-binding effects of Hpx might be responsible for the intriguing observation that, in another mouse model of sepsis, Hpx inhibited neutrophil migration to the focus of infection. In this model, lethality was paradoxically increased in septic wild-type mice treated with the heme-binding protein, and reduced in Hpx-knockout animals (Spiller et al. 2011). It was suggested that many of the reported nonheme-binding functions of Hpx were related to the experimental conditions or to impurities and degradation products in some experimental Hpx preparations (Mauk et al. 2011); careful examination of such effects may be critical in the preclinical evaluation of Hpx-based therapeutics. Also, no comparative experimental data currently exist to determine which of the plasma-scavenging proteins within the Hb/heme-binding cascade might be the most protective against hemoglobin-driven pathologies.
ACKNOWLEDGMENTS
This work is supported by the Swiss National Science Foundation (grants 310030/120658 and 31003A/138500), the University of Zurich Research Priority Program “Integrative Human Physiology,” the Swiss Federal Commission for Technology and Innovation (CTI), and FDA Internal Funding.
Footnotes
Editors: David Weatherall, Alan N. Schechter, and David G. Nathan
Additional Perspectives on Hemoglobin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
- Abraham NG, Kappas A 2008. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127 [DOI] [PubMed] [Google Scholar]
- Alayash AI 2004. Oxygen therapeutics: Can we tame haemoglobin? Nat Rev Drug Discov 3: 152–159 [DOI] [PubMed] [Google Scholar]
- Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW 2012. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122: 1444–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AL, Wiley EB, Alayash AI 2002. Comparison of effects of two hemoglobin-based O(2) carriers on intestinal integrity and microvascular leakage. Am J Physiol Heart Circ Physiol 283: H1292–H1301 [DOI] [PubMed] [Google Scholar]
- Baldwin AL, Wiley EB, Summers AG, Alayash AI 2003. Sodium selenite reduces hemoglobin-induced venular leakage in the rat mesentery. Am J Physiol Heart Circ Physiol 284: H81–H91 [DOI] [PubMed] [Google Scholar]
- Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM 1993. Endothelial-cell heme uptake from heme proteins: Induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci 90: 9285–9289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM 2012. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology 116: 637–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr PJ, Bailie KE 2011. Transfusion thresholds in FOCUS. N Engl J Med 365: 2532–2533 [DOI] [PubMed] [Google Scholar]
- Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM 2003. Transgenic sickle mice have vascular inflammation. Blood 101: 3953–3959 [DOI] [PubMed] [Google Scholar]
- Berra L, Coppadoro A, Yu B, Lei C, Spagnolli E, Steinbicker AU, Bloch KD, Lin T, Sammy FY, Warren HS, et al. 2012. Transfusion of stored blood does not alter reactive hyperemia in healthy volunteers. Anesthesiology 117: 56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boretti FS, Buehler PW, D’Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, et al. 2009. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 119: 2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutaud O, Moore KP, Reeder BJ, Harry D, Howie AJ, Wang S, Carney CK, Masterson TS, Amin T, Wright DW, et al. 2010. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci 107: 2699–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO 2009. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol 174: 1097–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JJ, Johns M, Kampfer T, Nguyen AT, Game L, Schaer DJ, Mason JC, Haskard DO 2012. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res 110: 20–33 [DOI] [PubMed] [Google Scholar]
- Buehler PW, D’Agnillo F, Hoffman V, Alayash AI 2007. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther 323: 49–60 [DOI] [PubMed] [Google Scholar]
- Buehler PW, Abraham B, Vallelian F, Linnemayr C, Pereira CP, Cipollo JF, Jia Y, Mikolajczyk M, Boretti FS, Schoedon G, et al. 2009. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood 113: 2578–2586 [DOI] [PubMed] [Google Scholar]
- Buehler PW, D’Agnillo F, Schaer DJ 2010. Hemoglobin-based oxygen carriers: From mechanisms of toxicity and clearance to rational drug design. Trends Mol Med 16: 447–457 [DOI] [PubMed] [Google Scholar]
- Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE 2010. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 116: 687–692 [DOI] [PubMed] [Google Scholar]
- Butt OI, Buehler PW, D’Agnillo F 2010. Differential induction of renal heme oxygenase and ferritin in ascorbate and nonascorbate producing species transfused with modified cell-free hemoglobin. Antioxid Redox Signal 12: 199–208 [DOI] [PubMed] [Google Scholar]
- Cunnington AJ, de Souza JB, Walther M, Riley EM 2011. Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med 18: 120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agnillo F, Alayash AI 2001. Redox cycling of diaspirin cross-linked hemoglobin induces G2/M arrest and apoptosis in cultured endothelial cells. Blood 98: 3315–3323 [DOI] [PubMed] [Google Scholar]
- Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD 1998. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol 16: 672–676 [DOI] [PubMed] [Google Scholar]
- Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, et al. 2011. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 124: 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzerodt A, Maniecki MB, Moller K, Moller HJ, Moestrup SK 2010. Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol 88: 1201–1205 [DOI] [PubMed] [Google Scholar]
- Farber HW, Loscalzo J 2004. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665 [DOI] [PubMed] [Google Scholar]
- Ferreira A, Marguti I, Bechmann I, Jeney V, Chora A, Palha NR, Rebelo S, Henri A, Beuzard Y, Soares MP 2011. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 145: 398–409 [DOI] [PubMed] [Google Scholar]
- Finn AV, Nakano M, Polavarapu R, Karmali V, Saeed O, Zhao X, Yazdani S, Otsuka F, Davis T, Habib A, et al. 2012. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol 59: 166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, et al. 2004. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 350: 886–895 [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Kanias T, Kim-Shapiro DB 2012. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest 122: 1205–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC 2006. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med 12: 1417–1422 [DOI] [PubMed] [Google Scholar]
- Heller A, Ragaller M, Schmeck J, Fluth H, Muller M, Albrecht DM, Koch T 1998. Role of NO and endothelin in hemoglobin-induced pulmonary vasoconstriction. Shock 10: 401–406 [DOI] [PubMed] [Google Scholar]
- Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, et al. 2010. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 115: 4284–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, et al. 2011. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 118: 6675–6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi H, Tsunoda K, Ichimiya N, Okamoto T, Namiki A 1994. Repeated large-dose haptoglobin therapy in an extensively burned patient: Case report. J Emerg Med 12: 33–37 [DOI] [PubMed] [Google Scholar]
- Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G 2002. Pro-oxidant and cytotoxic effects of circulating heme. Blood 100: 879–887 [DOI] [PubMed] [Google Scholar]
- Jia Y, Buehler PW, Boykins RA, Venable RM, Alayash AI 2007. Structural basis of peroxide-mediated changes in human hemoglobin: A novel oxidative pathway. J Biol Chem 282: 4894–4907 [DOI] [PubMed] [Google Scholar]
- Kaempfer T, Duerst E, Gehrig P, Roschitzki B, Rutishauser D, Grossmann J, Schoedon G, Vallelian F, Schaer DJ 2011. Extracellular hemoglobin polarizes the macrophage proteome toward Hb-clearance, enhanced antioxidant capacity and suppressed HLA class 2 expression. J Proteome Res 10: 2397–2408 [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, et al. 2003. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 349: 2316–2325 [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK 2001. Identification of the haemoglobin scavenger receptor. Nature 409: 198–201 [DOI] [PubMed] [Google Scholar]
- Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, et al. 2011. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2: 51ra71. [DOI] [PubMed] [Google Scholar]
- Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, Lache O, Nakhoul FM, Asaf R, et al. 2010. Haptoglobin: Basic and clinical aspects. Antioxid Redox Signal 12: 293–304 [DOI] [PubMed] [Google Scholar]
- Lin T, Kwak YH, Sammy F, He P, Thundivalappil S, Sun G, Chao W, Warren HS 2010. Synergistic inflammation is induced by blood degradation products with microbial Toll-like receptor agonists and is blocked by hemopexin. J Infect Dis 202: 624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Zhong Q, Lv FL, Zhou Y, Li JQ, Wang JZ, Yang QW, Yin Q 2012. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation 9: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MR, Smith A, Mauk AG 2011. An alternative view of the proposed alternative activities of hemopexin. Protein Sci 20: 791–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelburg RA, van den Watering LM, Briet E, van der Bom JG 2012. Storage time of red blood cells and mortality of transfusion recipients. Transfus Med Rev 27: 36–43 [DOI] [PubMed] [Google Scholar]
- Miller YI, Altamentova SM, Shaklai N 1997. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: Antioxidant role of haptoglobin. Biochemistry 36: 12189–12198 [DOI] [PubMed] [Google Scholar]
- Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB 2005. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest 115: 3409–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KP, Holt SG, Patel RP, Svistunenko DA, Zackert W, Goodier D, Reeder BJ, Clozel M, Anand R, Cooper CE, et al. 1998. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem 273: 31731–31737 [DOI] [PubMed] [Google Scholar]
- Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, Szentmiklosi J, Mehes G, Csonka T, Smith A, et al. 2010. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol 30: 1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM 2008. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: A meta-analysis. JAMA 299: 2304–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MJ, Moller HJ, Moestrup SK 2010. Hemoglobin and heme scavenger receptors. Antioxid Redox Signal 12: 261–273 [DOI] [PubMed] [Google Scholar]
- Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD 2004. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med 36: 685–697 [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH 2003. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol 24: 449–455 [DOI] [PubMed] [Google Scholar]
- Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, et al. 2007. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med 13: 703–710 [DOI] [PubMed] [Google Scholar]
- Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, et al. 2011. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 365: 44–53 [DOI] [PubMed] [Google Scholar]
- Pimenova T, Pereira CP, Gehrig P, Buehler PW, Schaer DJ, Zenobi R 2010. Quantitative mass spectrometry defines an oxidative hotspot in hemoglobin that is specifically protected by haptoglobin. J Proteome Res 9: 4061–4070 [DOI] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO III, Schechter AN, Gladwin MT 2002. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389 [DOI] [PubMed] [Google Scholar]
- Roche C, Dantsker D, Alayash AI, Friedman JM 2012. Enhanced nitrite reductase activity associated with the haptoglobin complexed hemoglobin dimer: Functional and antioxidative implications. Nitric Oxide 27: 32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal PJ 2011. Lessons from sickle cell disease in the treatment and control of malaria. N Engl J Med 364: 2549–2551 [DOI] [PubMed] [Google Scholar]
- Rother RP, Bell L, Hillmen P, Gladwin MT 2005. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 293: 1653–1662 [DOI] [PubMed] [Google Scholar]
- Schaer DJ, Alayash AI 2012. Clearance and control mechanisms of hemoglobin from cradle to grave. Antioxid Redox Signal 12: 181–184 [DOI] [PubMed] [Google Scholar]
- Schaer DJ, Schleiffenbaum B, Kurrer M, Imhof A, Bachli E, Fehr J, Moller HJ, Moestrup SK, Schaffner A 2005. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol 74: 6–10 [DOI] [PubMed] [Google Scholar]
- Schaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ 2006a. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res 99: 943–950 [DOI] [PubMed] [Google Scholar]
- Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, Schaffner A 2006b. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 107: 373–380 [DOI] [PubMed] [Google Scholar]
- Schaer CA, Vallelian F, Imhof A, Schoedon G, Schaer DJ 2007. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. J Leukoc Biol 82: 106–110 [DOI] [PubMed] [Google Scholar]
- Silverman TA, Weiskopf RB 2009. Hemoglobin-based oxygen carriers: Current status and future directions. Anesthesiology 111: 946–963 [DOI] [PubMed] [Google Scholar]
- Spiller F, Costa C, Souto FO, Vinchi F, Mestriner FL, Laure HJ, Alves-Filho JC, Freitas A, Rosa JC, Ferreira SH, et al. 2011. Inhibition of neutrophil migration by hemopexin leads to increased mortality due to sepsis in mice. Am J Respir Crit Care Med 183: 922–931 [DOI] [PubMed] [Google Scholar]
- Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, Altruda F 1999. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood 94: 3906–3914 [PubMed] [Google Scholar]
- Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V 2010. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal 12: 305–320 [DOI] [PubMed] [Google Scholar]
- Vallelian F, Pimenova T, Pereira CP, Abraham B, Mikolajczyk MG, Schoedon G, Zenobi R, Alayash AI, Buehler PW, Schaer DJ 2008. The reaction of hydrogen peroxide with hemoglobin induces extensive α-globin crosslinking and impairs the interaction of hemoglobin with endogenous scavenger pathways. Free Radic Biol Med 45: 1150–1158 [DOI] [PubMed] [Google Scholar]
- Vallelian F, Schaer CA, Kaempfer T, Gehrig P, Duerst E, Schoedon G, Schaer DJ 2010. Glucocorticoid treatment skews human monocyte differentiation into a hemoglobin-clearance phenotype with enhanced heme-iron recycling and antioxidant capacity. Blood 116: 5347–5356 [DOI] [PubMed] [Google Scholar]
- Wang D, Sun J, Solomon SB, Klein HG, Natanson C 2012. Transfusion of older stored blood and risk of death: A meta-analysis. Transfusion 52: 1184–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher KB, Fries E 2010. Evolutionary aspects of hemoglobin scavengers. Antioxid Redox Signal 12: 249–259 [DOI] [PubMed] [Google Scholar]
- Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S 1999. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103: 129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh K, Bao W, Zhong H 2011. Immunoregulatory effects of stored red blood cells. Hematology Am Soc Hematol Educ Program 2011: 466–469 [DOI] [PubMed] [Google Scholar]
- Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM 2008. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation 117: 1982–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Volpato GP, Chang K, Bloch KD, Zapol WM 2009. Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide. Anesthesiology 110: 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM 2012. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion 52: 1410–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager RA, Gamelin LM 1989. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol 256: F446–F455 [DOI] [PubMed] [Google Scholar]