Abstract
Objective:
To determine whether severe recurrent headache is a risk factor for neurovascular events in children who received radiation for brain tumors.
Methods:
This is a retrospective cohort study of children with brain tumors who received cranial irradiation at a large tertiary care center, aged 0–21 years at diagnosis, with initial treatment between January 1, 1993 and December 31, 2002, and 2 or more follow-up visits. Patients were considered to have severe recurrent headache if this appeared as a complaint on 2 or more visits. Headaches attributed to tumor progression, shunt malfunction, or infection, or appearing at the end of life, were excluded. Medical records were reviewed for events of stroke or TIA.
Results:
Of 265 subjects followed for a median of 6.0 years (interquartile range 1.7–9.2 years), stroke or TIA occurred in 7/37 (19%) with severe headaches compared to 6/228 (3%) without these symptoms (hazard ratio 5.3, 95% confidence interval 1.8–15.9, p = 0.003). Adjusting for multiple variables did not remove the significance of this risk. Median time to first neurovascular event for the entire cohort was 4.9 years (interquartile range 1.7–5.5 years).
Conclusions:
Severe recurrent headache appears to be a risk factor or predictor for subsequent cerebral ischemia in pediatric brain tumor survivors treated with radiation. This finding has clinical implications for both monitoring survivors and targeting a specific population for primary stroke prevention.
Late effects of cranial irradiation for brain tumors in the pediatric population include TIA and stroke.1–5 The mechanisms of these delayed neurovascular events include radiation-induced chronic inflammation and oxidative stress, leading to accelerated atherosclerosis via intimal fibrosis, foam cell accumulation, and thrombosis formation.6 Little data exist regarding factors that might predict later occurrence of neurovascular events.
Based on the increased risk for delayed neurovascular events, the Children's Oncology Group Long-Term Follow-Up Guidelines recommend neurologic surveillance in cancer survivors who received ≥18 Gy of cranial radiation.7 The determination of when and how frequently to use neuroimaging (including vascular imaging), however, depends on the patient's report of symptoms and the clinician's judgment that imaging is “clinically indicated.” Since the patient may only report a neurologic deficit after ischemic damage has occurred, ideal surveillance would identify those patients at greatest risk for neurovascular events to target primary stroke prevention.
A recent study by our group confirmed excess risk for neurovascular events in a cohort of brain tumor survivors seen over 10 years at a major pediatric neuro-oncology tertiary care center, with an incidence of 548/100,000 person-years, compared to 2–8/100,000 in the general pediatric population.8 These data also demonstrated an eightfold increased risk for cranial radiation. As headache is a known symptom of vasculopathies such as moyamoya disease,9 we hypothesized that patients with more severe vascular damage from radiation, and therefore greater risk for late neurovascular events, were more likely to complain of severe headaches during their follow-up visits.
METHODS
Study population.
The Division of Oncology Tumor Registry reflects all children with a diagnosis of a CNS tumor presenting to the Neuro-Oncology division of The Children's Hospital of Philadelphia, a large tertiary care center. Patients aged 0–21 years at brain tumor diagnosis with initial treatment between January 1, 1993 and December 31, 2002, and who had 2 or more follow-up visits in the Division of Neuro-Oncology, were identified from the database. Patients were excluded if they did not receive treatment for the brain tumor, if they experienced intraoperative or perioperative stroke, or if they had a disease known to increase the risk for neurovascular events, such as neurofibromatosis-1. For the current analysis, we included only those patients who received cranial radiation therapy (RT) as part of their treatment.
Each medical record was reviewed for demographics, date of brain tumor diagnosis, tumor pathology, tumor location, dates and location of tumor-directed surgery, dates of radiation along with radiation dose and fields (focal, whole brain, or craniospinal), and whether chemotherapy was given. Additionally, dates of imaging studies, occurrence of stroke or TIA symptoms, date of stroke or TIA occurrence, presence or absence of brain tumor relapse and subsequent therapy, presence or absence of severe recurrent headache, and date of death were identified from the records. Brain MRI reports for each patient were reviewed for the presence of infarction, and magnetic resonance angiography reports, when available, for each patient were reviewed for the presence of vasculopathy.
Standard protocol approvals, registrations, and patient consents.
Institutional review board approval of the protocol was obtained prior to study initiation.
Radiation dose.
Radiation field was categorized in one of 3 ways, in order to estimate the dose to the circle of Willis (COW): whole-brain radiation (COW dose equals whole-brain dose), focal radiation to areas not including the COW (dose to COW equals zero), and focal radiation to areas including the COW (dose to COW equals focal dose).
Assessment of severe recurrent headache.
Patients were considered to have severe recurrent headache if this appeared as a complaint on 2 or more follow-up visits to neuro-oncology. Headaches attributed by the clinician at the time of the visit or clearly linked to tumor progression, shunt malfunction, or infection, or appearing at the end of life were excluded. If the clinician labeled the complaint as “migraine,” these were considered to be severe headaches for the purposes of this study.
Assessment of neurovascular events.
Brain MRI of all patients with stroke symptoms were reviewed to confirm the presence of an ischemic lesion. The event of acute arterial ischemic stroke (AIS) was defined as a new acute clinical deficit corresponding to a vascular territory confirmed by evidence of ischemia on diffusion-weighted imaging (DWI) or clear evidence of hyperintensity on T2-weighted imaging corresponding to the appropriate vascular distribution if DWI was not performed. If imaging was uninterpretable due to postsurgical or postradiation changes, stroke was defined as a new acute clinical deficit corresponding to a vascular territory, lasting >24 hours. TIA was defined as a new acute clinical deficit corresponding to a vascular territory lasting <24 hours without evidence of ischemia on MRI. Possible neurovascular events of TIA and AIS were assessed individually by 3 investigators to reach consensus.
Statistical analysis.
Wilcoxon rank-sum tests were conducted to compare nonparametrically distributed variables. Cox proportional hazard models were used to estimate the hazard ratios (HRs) of neurovascular event (TIA or AIS) between those patients who complained of severe recurrent headache after receiving cranial RT to those who did not complain of severe recurrent headache. HRs were adjusted for age at diagnosis, treatment with chemotherapy, and inclusion of the COW in the field of radiation. Standard asymptotic inference methods for Cox regression based on the partial likelihood were used to construct 95% confidence intervals (CIs) and to calculate 2-sided significance tests. Statistical tests were performed using SPSS 17.0 (SPSS Inc., Chicago, IL) and STATA SE 10.0 (Stata Corp., College Station, TX).
RESULTS
Of 431 subjects in the epidemiologic study,10 265 received cranial RT and were included in the headache analysis. Table 1 demonstrates that patients with severe recurrent headaches were comparable to those without these symptoms with several exceptions. Age at diagnosis was significantly older in those patients with severe recurrent headaches. Radiation dose in patients with headaches was lower than in the patients without headaches, although there was no significant difference in radiation dose to the COW.
Table 1.
Group characteristics of patients with severe recurrent headache and those without headache
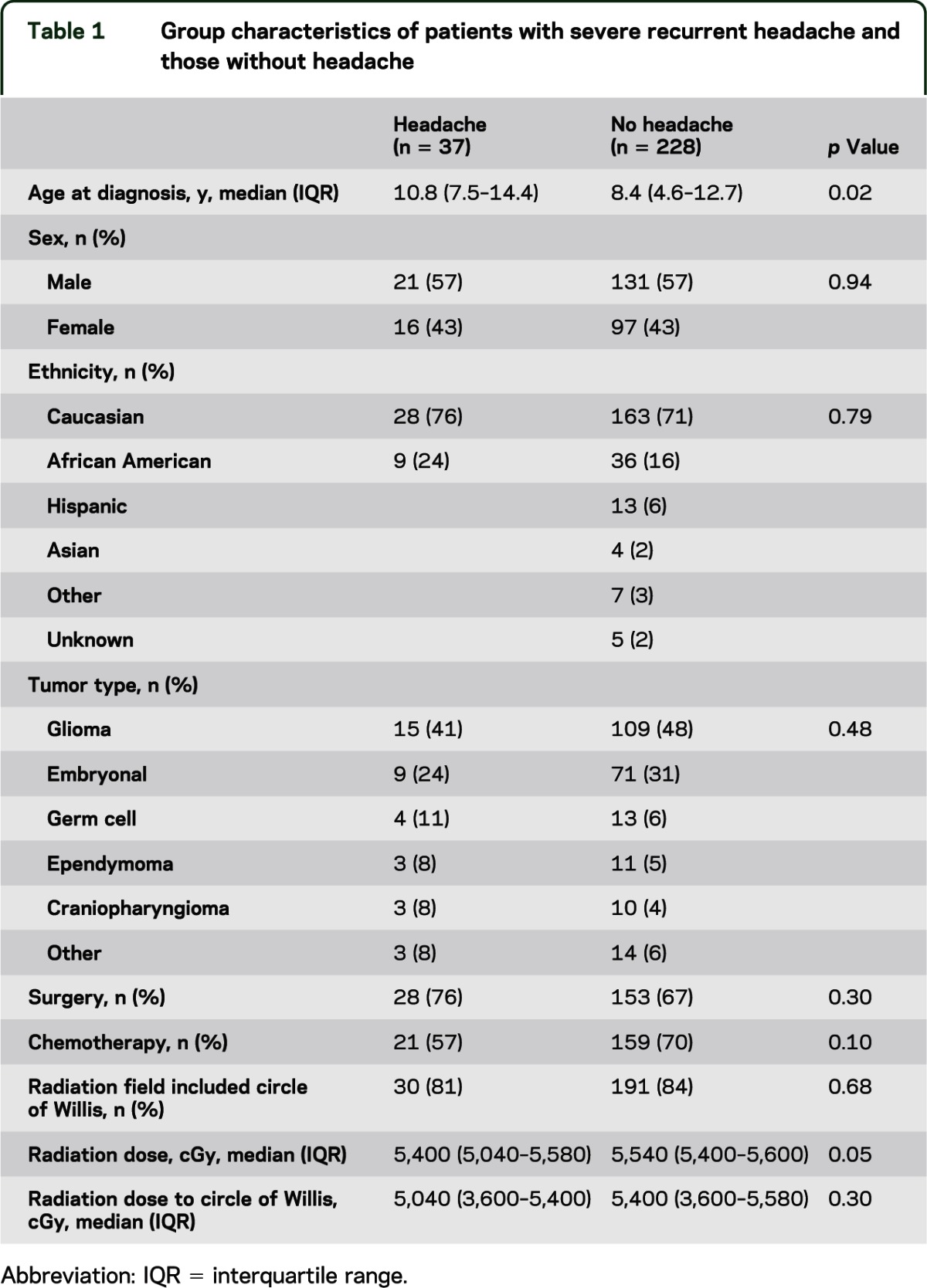
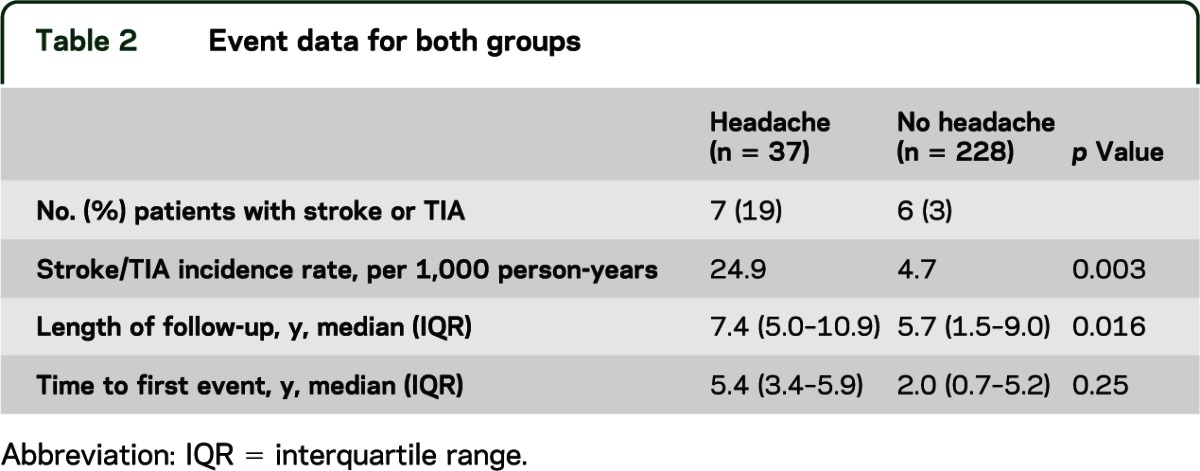
Table 2 shows neurovascular event data for the 2 groups. In 37 patients with severe recurrent headache, there were 7 patients with 11 events of TIA or AIS. In 228 patients without headache, there were 6 patients with 8 events of TIA or AIS. The median follow-up for the entire cohort was 6.0 years (interquartile range 1.7–9.2 years), while median time to first neurovascular event for the entire cohort was 4.9 years (interquartile range 1.7–5.5 years). Length of follow-up was significantly longer in those patients with headache.
Table 2.
Event data for both groups
The figure demonstrates event-free survival over time for the 2 groups. AIS or TIA occurred in 19% (7/37) of subjects with severe headaches compared to 3% (6/228) without these symptoms (unadjusted HR 5.3; 95% CI 1.8–15.9; p = 0.003). Severe headache remained significantly associated with subsequent AIS or TIA (HR 4.7; 95% CI 1.52–14.5, p = 0.007) after adjustments for other potential confounders, none of which independently impacted risk in this population, including age at diagnosis (HR = 1.08 per year; 95% CI 0.9–1.23; p = 0.27), inclusion of COW in radiation field (HR = 2.7; 95% CI 0.3–19.8; p = 0.37), or treatment with chemotherapy (HR = 2.6; 95% CI 0.7–9.7; p = 0.15).
Figure. Kaplan-Meier stroke/TIA-free survival estimates.
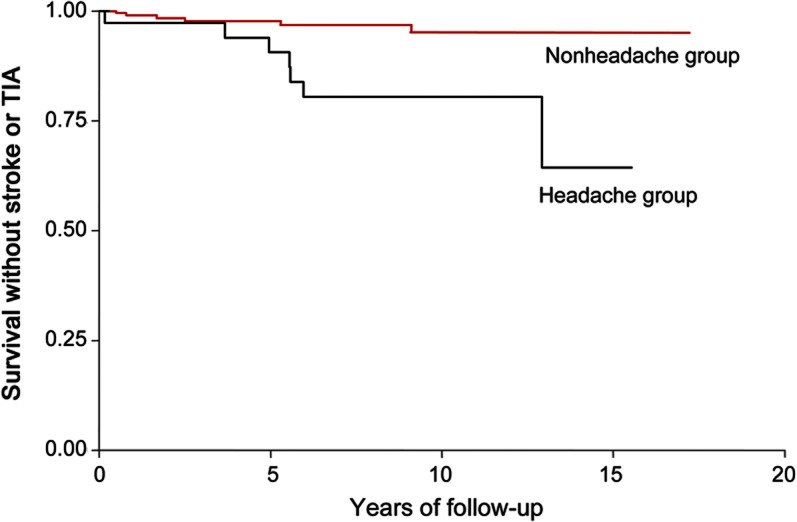
Event-free survival over time in patients with severe recurrent headache and those without headache.
DISCUSSION
Stroke as a delayed complication of radiation-induced vascular changes in the brain was first proposed in the literature more than 30 years ago, but there have been few large-scale studies to address this issue.11 Our recent epidemiologic study confirmed that there is an increased risk of stroke for brain tumor survivors who received cranial RT.10 In this same large cohort, we found that severe recurrent headache is associated with an increased risk of neurovascular events and therefore may be a warning sign of ongoing neurovascular changes. Headache is a common symptom in other vasculopathies, including moyamoya disease, arterial dissection, reversible cerebral vasoconstriction syndrome, and vasculitis, and may be due to chronic mild ischemia9 or referred pain due to arterial injury.12 These mechanisms may also contribute to headache associated with radiation-induced vasculopathy. We cannot conclusively demonstrate that patients with severe recurrent headache are more likely to have strokes or TIAs on the basis of vasculopathy. Due to the retrospective nature of the study, in which vascular imaging was not uniformly obtained and is not done as standard of care in tumor surveillance imaging in this cohort, this question could not be analyzed for this study. Future studies should address the role of large- and small-vessel vasculopathies in the pathogenesis of stroke and TIA after cranial RT, and how these changes clinically manifest.
Collecting the data by chart review allowed stroke and TIA events to be cross-referenced with imaging. Because the complaint of severe recurrent headache was gathered during retrospective chart review, one limitation of this study is the inability to characterize further the headache symptoms. Most of the visit notes did not include enough details to apply International Headache Society criteria,13 and the complaint of “migraine” or “headache” was therefore ascertained directly from the chart. While differentiating headache type was not possible in our study, attempting to classify accurately the patient's headache would be of great utility in clinical practice, given the existing data regarding ischemic stroke in migraineurs with aura.14 While patients who received cranial RT may experience migraine with aura secondary to a different mechanism than those without any history of radiation exposure, the possibility of hypoperfusion during aura would make identifying migraineurs with aura particularly relevant. Separating brain tumor survivors into subgroups based on headache type may thus have predictive implications with regard to neurovascular events.
The significantly longer duration of follow-up in the headache group may represent an ascertainment bias in this population. It is possible that patients were more likely to continue to follow up in the clinic if they had symptoms to report, while those without headaches or other neurologic symptoms were less likely to follow up. Such a bias may have led to overestimation of the HR for headache as a risk factor for neurovascular events. However, the HR associated with severe recurrent headache is high enough in this study that the potential limitation of a 2- to 3-year difference in follow-up is unlikely to overshadow this risk. A randomly sampled prospective study would be necessary to fully address this issue. Age at diagnosis was significantly older in those with severe headaches than those without headache. While this does not represent the age at which the complaint of headache was registered, it does suggest that the patients complaining of headache may have been older than those without complaints of headache. Older children may be more likely to communicate specific complaints than younger children, thereby overestimating the risk associated with headache. Despite this difference between the 2 groups, the HR of severe headache remained significant when adjusted for age at diagnosis.
It is important to draw a distinction between the neurovascular events experienced by these patients and the syndrome of stroke-like migraine after radiation therapy (SMART). SMART has been described in the literature as a clinical syndrome of neurologic deficits with complete recovery, sometimes lasting weeks to months, with no EEG evidence of seizures, and striking transient MRI changes of posterior cortical gyral enhancement on MRI.15,16 While SMART is characterized by its resolution, both clinically and radiographically, some authors propose that these patients be treated with migraine prophylaxis and that antiplatelet agents be considered.15 As SMART is a rare disorder, it is not known if these patients are at increased risk for vascular events, although it seems plausible given the similarity of these attacks to migraine with aura.
While some authors note that recognition of the SMART syndrome may save patients from invasive procedures such as angiography, our data would argue that severe headaches in patients who received cranial RT should raise suspicion for impending brain ischemia. We, therefore, recommend that patients with severe recurrent headaches after cranial RT for brain tumors receive vigilant monitoring, and that consideration of prompt brain and neurovascular imaging may be warranted.
Although antiplatelet therapy is frequently offered to patients considered at risk for delayed ischemic events, prophylaxis is not considered standard of care in pediatric brain tumor survivors due to the lack of supporting evidence. However, within the subgroup of patients with severe recurrent headaches after cranial RT, both antiplatelet therapy as well as migraine prevention could be targeted in future prospective studies to evaluate their impact on the risk of subsequent neurovascular events.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Phillip B. Storm and Dr. Daniel J. Licht for discussions and input regarding this work.
GLOSSARY
- AIS
arterial ischemic stroke
- CI
confidence interval
- COW
circle of Willis
- DWI
diffusion-weighted imaging
- HR
hazard ratio
- RT
radiation therapy
- SMART
stroke-like migraine after radiation therapy
Footnotes
Editorial, page 1448
AUTHOR CONTRIBUTIONS
Sarah Kranick: study concept and design, acquisition of data, statistical analysis, analysis and interpretation, drafting/revision of manuscript. Cynthia Campen: study concept and design, acquisition of data, revision of manuscript. Scott Kasner: study concept and design, statistical analysis, interpretation, revision of manuscript. Sudha Kessler: statistical analysis, interpretation, revision of manuscript. Robert Zimmerman: acquisition of data, analysis and interpretation, revision of manuscript. Robert Lustig: acquisition of data, analysis and interpretation, revision of manuscript. Peter Phillips: acquisition of data, analysis and interpretation, revision of manuscript. Lauren Beslow: acquisition of data, analysis and interpretation, revision of manuscript. Rebecca Ichord: acquisition of data, analysis and interpretation, revision of manuscript. Michael Fisher: study concept and design, acquisition of data, analysis and interpretation, revision of manuscript, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
S. Kranick and C. Campen report no disclosures. S. Kasner has served on scientific advisory boards for BrainsGate (DSMB), Medtronic (DSMB), and PhotoThera (trial steering committee); serves as a consultant for AstraZeneca and Daiichi Sankyo and on endpoint adjudication committees for Novartis, Pfizer Inc, and Merck/PAREXEL International; receives/has received research support from WL Gore, Acorda Pharma, and the NIH; and has reviewed cases related to medico-legal proceedings. S. Kessler is supported by a grant from NIH-K12-NS049453. R. Zimmerman, R. Lustig, and P. Phillips report no disclosures. L. Beslow received grant funding from NIH-K12-NS049453, NIH-T32-NS007413, and The L. Morton Morley Funds of The Philadelphia Foundation. R. Ichord received consulting fees and reimbursement for travel as a member of the clinical event committee for the IDE trial of the Berlin Heart EXCOR pediatric ventricular assist device. M. Fisher reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2006;24:5277–5282 [DOI] [PubMed] [Google Scholar]
- 2.Omura M, Aida N, Sekido K, Kakehi M, Matsubara S. Large intracranial vessel occlusive vasculopathy after radiation therapy in children: clinical features and usefulness of magnetic resonance imaging. Int J Radiat Oncol Biol Phys 1997;38:241–249 [DOI] [PubMed] [Google Scholar]
- 3.Bowers DC, Mulne AF, Reisch JS, et al. Nonperioperative strokes in children with central nervous system tumors. Cancer 2002;94:1094–1101 [PubMed] [Google Scholar]
- 4.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol 1999;45:393–396 [DOI] [PubMed] [Google Scholar]
- 5.Ullrich NJ, Robertson R, Kinnamon DD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology 2007;68:932–938 [DOI] [PubMed] [Google Scholar]
- 6.Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol 2005;44:13–22 [DOI] [PubMed] [Google Scholar]
- 7.Children's Oncology Group Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, Version 3.0. Arcardia, CA: Children's Oncology Group; 2008 [Google Scholar]
- 8.Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke 2012;43:3035–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seol HJ, Wang KC, Kim SK, Hwang YS, Kim KJ, Cho BK. Headache in pediatric moyamoya disease: review of 204 consecutive cases. J Neurosurg 2005;103:439–442 [DOI] [PubMed] [Google Scholar]
- 10.Painter MJ, Chutorian AM, Hilal SK. Cerebrovasculopathy following irradiation in childhood. Neurology 1975;25:189–194 [DOI] [PubMed] [Google Scholar]
- 11.Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry 2006;77:1021–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24:9–160 [DOI] [PubMed] [Google Scholar]
- 13.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartleson JD, Krecke KN, O'Neill BP, Brown PD. Reversible, strokelike migraine attacks in patients with previous radiation therapy. Neuro Oncol 2003;5:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partap S, Walker M, Longstreth WT, Jr, Spence AM. Prolonged but reversible migraine-like episodes long after cranial irradiation. Neurology 2006;66:1105–1107 [DOI] [PubMed] [Google Scholar]
- 16.Pruitt A, Dalmau J, Detre J, Alavi A, Rosenfeld MR. Episodic neurologic dysfunction with migraine and reversible imaging findings after radiation. Neurology 2006;67:676–678 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


