Abstract
Background and Aims
It is widely accepted that hydraulic failure due to xylem embolism is a key factor contributing to drought-induced mortality in trees. In the present study, an attempt is made to disentangle phenotypic plasticity from genetic variation in hydraulic traits across the entire distribution area of a tree species to detect adaptation to local environments.
Methods
A series of traits related to hydraulics (vulnerability to cavitation and hydraulic conductivity in branches), growth performance and leaf mass per area were assessed in eight Pinus canariensis populations growing in two common gardens under contrasting environments. In addition, the neutral genetic variability (FST) and the genetic differentiation of phenotypic variation (QST) were compared in order to identify the evolutionary forces acting on these traits.
Key Results
The variability for hydraulic traits was largely due to phenotypic plasticity. Nevertheless, the vulnerability to cavitation displayed a significant genetic variability (approx. 5 % of the explained variation), and a significant genetic × environment interaction (between 5 and 19 % of the explained variation). The strong correlation between vulnerability to cavitation and survival in the xeric common garden (r = –0·81; P < 0·05) suggests a role for the former in the adaptation to xeric environments. Populations from drier sites and higher temperature seasonality were less vulnerable to cavitation than those growing at mesic sites. No trade-off between xylem safety and efficiency was detected. QST of parameters of the vulnerability curve (0·365 for P50 and the slope of the vulnerability curve and 0·452 for P88) differed substantially from FST (0·091), indicating divergent selection. In contrast, genetic drift alone was found to be sufficient to explain patterns of differentiation for xylem efficiency and growth.
Conclusions
The ability of P. canariensis to inhabit a wide range of ecosystems seemed to be associated with high phenotypic plasticity and some degree of local adaptations of xylem and leaf traits. Resistance to cavitation conferred adaptive potential for this species to adapt successfully to xeric conditions.
Keywords: vulnerability to cavitation, Pinus canariensis, common garden, drought, genetic differentiation, hydraulic conductivity, phenotypic plasticity, fitness, selection, trade-off
INTRODUCTION
The responses of long-lived plant species to changes in environmental conditions are determined by the capacity of individuals to alter their structure and function (i.e. phenotypic plasticity) to novel biotic or abiotic environments, adapt through natural selection or migrate (Nicotra et al., 2010). The whole-plant acclimation to water deficit requires maximizing gas exchange while avoiding hydraulic failure. Above-ground water flow through higher plants in steady state can be described by the following equation (Zimmermann, 1983):
| 1 |
where D is the vapour pressure deficit of the atmosphere; gs is the stomatal conductance; As is the cross-sectional sapwood area; Al is the leaf area; Ks and Kl are the sapwood-specific and leaf-specific hydraulic conductivity, respectively; and ΔΨ is the water potential gradient through the system.
The adjustment of the hydraulic system to deal with climate dryness (greater D) involves: (1) stronger stomatal control to limit water loss; (2) decreasing leaf to sapwood area ratio (Al:As), thus altering the above-ground allocation pattern between water-conducting and transpiring tissues; (3) increasing the efficiency of the conducting elements, i.e. increasing the hydraulic conductivity; and/or (4) decreasing the vulnerability to xylem embolism to limit the risk of hydraulic failure. Furthermore, trees can alter the below-ground hydraulic properties by modifying the depth of the roots, root/leaf area ratio or axial and radial hydraulic traits (Steudle, 1994; Sperry et al., 1998). The combination of these strategies is possible, and different types of hydraulic adjustments have been described (Chaves et al., 2002; Bréda et al., 2006), although with some restrictions. A trade-off between xylem safety (i.e. resistance to embolism) and xylem transport efficiency at the tissue level has been reported (Martínez-Vilalta et al., 2002; Hacke et al., 2006, 2009) as a consequence of mechanical constraints (Pittermann et al., 2006) and protection from air-seeding or from freezing-induced cavitation (Sperry et al., 2008).
Most of our current understanding about the variability and interaction of these hydraulic traits comes from interspecific comparisons (Hacke and Sperry, 2001; Maherali et al., 2004; Jacobsen et al., 2007; Pittermann et al., 2010). However, information about variation within species, and to what extent genotypes are plastic for hydraulic traits, remains scarce (but see Ewers et al., 2000 about the effect of nutrient and water availability in root xylem hydraulics of Pinus taeda). Likewise, analyses of interactions and trade-offs among hydraulic traits and their role in adaptation are based on interspecific rather than intraspecific comparisons.
Genetic differentiation within species is viewed as a key factor to adaptation. A major goal of population genetic analysis is to identify the genetic basis of adaptive phenotypic differentiation and the action of selection on this variation (González-Martínez et al., 2007). Two categories of evolutionary forces determine population differentiation. The first category includes neutral evolutionary processes. The second is related to natural selection under distinct ecological environments (Still et al., 2005). To discern between the influence of both categories, the comparison of differentiation of neutral markers (as reflected in FST; Wright, 1951) and quantitative trait divergence (as reflected in QST; Spitze, 1993) is widely used. Both statistics quantify the proportion of total variation that occurs between populations. Any significant difference between FST and QST (assuming that populations are in drift–migration equilibrium) is held to be evidence for natural selection (Merilä and Crnokrak, 2001). Furthermore, the more QST differs from FST, the stronger is the evidence for local adaptation for a given trait (Merilä and Crnokrak, 2001; Latta and McKay, 2002).
Island ecosystems are natural laboratories for exploring adaptive differentiation (Emerson, 2002). In oceanic islands, volcanic and erosional activities are common, creating extremely diverse habitats that may exert varying selective pressures (Emerson, 2002). The archipelago comprising the Canary Islands is such an example, being the result of the active volcanism during the past 20 million years, with islands differing dramatically in their age (Juan et al., 2000). Isolation, contrasting habitats, complex colonization–extinction processes derived from volcanic activity (Navascués et al., 2006) and human activity in the last millennia (de Nascimento et al., 2009) have contributed to the biology and ecology of the Canary Island flora and fauna.
Pinus canariensis is the only endemic pine of the Canary Islands. Current environmental conditions are very different from those in which this species evolved under a much wetter climate even during the late Holocene (de Nascimento et al., 2009). Nowadays, despite its small distribution area, the species grows across a wide climate envelope: from xeric conditions, with barely 300 mm of rain in south-western slopes, to mixed forest with the monteverde in north-eastern slopes, influenced by the humid trade winds, and from close to sea level to 2400 m altitude (Climent et al., 2002). As is the case for most pines, P. canariensis is outcrossing, and gene flow by seed and pollen is extensive (Navascués et al., 2006; Vaxevanidou et al., 2006; Navascués and Emerson, 2007), particularly in open forests resulting from disturbed pinewoods or at early stages of colonization (López de Heredia et al., 2010). The dispersal ability of P. canariensis may have consequences on the degree of adaptation of the species to local environmental conditions and to promote plasticity. Long-distance gene flow by seed and pollen can promote adaptive evolution in novel environments by increasing genetic variation for fitness (Kremer et al., 2012) and enhancing plastic responses (Alpert and Simms, 2002).
Pines exhibited nearly isohydric behaviour, maintaining rather constant leaf water potential in soils with low water status and/or under high evaporative demand (Martínez-Vilalta et al., 2004). Modifications of Al:As and stomatal control seemed to be the general adjustments of their hydraulic system, whereas anatomical traits or vulnerability to cavitation showed little plasticity in the genus (Maherali and DeLucia, 2000; Martínez-Vilalta and Piñol, 2002; Martínez-Vilalta et al., 2004, 2009), despite the high variability found among conifers (Piñol and Sala, 2000; Maherali et al., 2004; Martinez-Vilalta et al., 2004; Brodribb and Cochard, 2009; Delzon et al., 2010).
In this study, we compared the intraspecific variation and the relative contribution of plasticity and/or genetic adaptation for branch-level hydraulic properties and growth in eight populations of Canary Island pine growing in two common gardens. In addition, we aimed to find evidence of local adaptation in Canary Island pine populations by comparing neutral differentiation (FST) from neutral nuclear genes with phenotypic differentiation (QST) from trait measures in the common gardens. Specifically, we hypothesized that trees would respond to an increase in climate dryness by increasing leaf-specific hydraulic conductivity by means of decreasing the branch leaf:sapwood area ratio. We assumed that water limitation would have been a powerful agent of natural selection, and populations from drier sites, besides adjusted branch Al:As, would be less vulnerable to cavitation and would survive better in the xeric common garden. Conversely, the construction of a safer xylem, and due to the limit plasticity of cavitation resistance found in pines, would result in lower growth in the mesic common garden, reflecting a potential trade-off between these traits.
MATERIALS AND METHODS
Plant material and common garden experiments
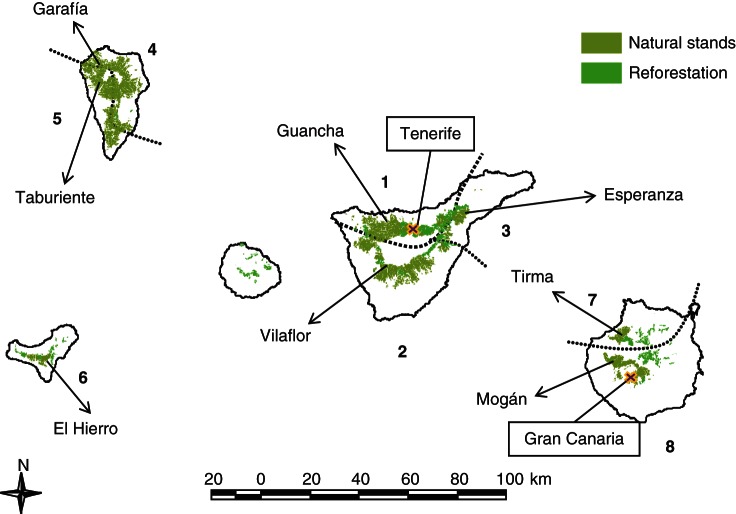
Trees of P. canariensis from eight populations, representing the eight ecological regions of the Canary Island pine (Climent et al., 2004), growing in two common garden experiments were selected for this study (Fig. 1). To establish the common gardens, cones were collected from 25 trees spaced at least 100 m apart. Cones were oven-dried to extract seeds, and, within each population, seeds were pooled across parent trees. Both common gardens were within the range of potential pine forest but they differed significantly in water availability, exposure and soil type. The most humid and productive common garden was located in the north of Tenerife at 1575 m on the windward slope of the Teide volcano and is under the direct influence of the humid trade winds (approx. 800 mm of annual precipitation). The common garden in the south of Gran Canaria combines an arid environment, approx. 300 mm of annual precipitation and periodic gusts of the extreme dry Saharan wind, with a very compact and stony soil (Table 1; for more details see López et al., 2007). Survival, height and basal diameter were measured during the first 6 years after the establishment of the common gardens (for more detail, see López et al., 2007). For the objectives of the present study, we used the height and basal diameter of the same trees used for hydraulics.
Fig. 1.
Location of the sampled population of Pinus canariensis and common gardens in the Canary Islands. Dotted lines are the limits of the ecological regions numbered) described in Climent et al. (2004).
Table 1.
Ecological regions and climatic characterization of the studied Pinus canariensis populations and the common garden experiments
| Population | Elevation | Pa (mm) | T (°C) | Tr (°C) | Dp (months) | ETo (mm d−1) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| sp | sum | aut | win | |||||||
| Ecological region | ||||||||||
| 1 | Guancha | 700 | 939·9 | 12·7 | 14·4 | 3·6 | 3·56 | 4·41 | 2·34 | 2·39 |
| 2 | Vilaflor | 1900 | 505 | 13·2 | 22·2 | 5·36 | 4·15 | 4·77 | 2·73 | 2·84 |
| 3 | Esperanza | 1100 | 629·7 | 14·7 | 17·4 | 4·79 | 3·74 | 4·47 | 2·41 | 2·50 |
| 4 | Garafía | 1500 | 1015·1 | 16·5 | 19·1 | 5·12 | 3·61 | 4·34 | 2·45 | 2·46 |
| 5 | Taburiente | 1000 | 719·9 | 14 | 18 | 5·12 | 3·8 | 4·44 | 2·61 | 2·64 |
| 6 | El Hierro | 1000 | 450·1 | 16·4 | 15·7 | 6·66 | 4·2 | 4·80 | 2·76 | 2·90 |
| 7 | Tirma | 850 | 379·6 | 18 | 20·6 | 6·83 | 4·37 | 4·84 | 2·85 | 2·99 |
| 8 | Mogán | 900 | 334·7 | 17·6 | 21·9 | 7·52 | 4·37 | 4·84 | 3·00 | 3·00 |
| Common gardens | ||||||||||
| 1 | Realejos (Tenerife) | 1575 | 795 | 14·3 | 21·1 | 4·07 | 3·70 | 4·46 | 2·39 | 2·47 |
| 8 | Tirajana (Gran Canaria) | 1259 | 320 | 17·8 | 20·3 | 7·68 | 4·36 | 4·90 | 3·00 | 3·01 |
Pa, annual precipitation; T, mean annual temperature; Tr, annual temperature range; Dp, drought period; ETo, evapotranspiration calculated with the Penman–Monteith equation (sp, spring; sum, summer; aut, autumn; win, winter).
Vulnerability curves
One branch exposed to the sun, longer than 40 cm and with a maximum diameter of 1 cm, was sampled from 8–14 trees per population in each common garden. Trees were 11 years old and sampled branches corresponded to the previous year's growth unit in the mesic site and to the last 2–3 years' growth units in the dry site. Needles were removed and branches were wrapped in a black plastic bag with moist paper towels, to prevent dehydration, and sent to the laboratory in Clermont-Ferrand, France, where they were kept in a cold chamber at 4 °C. Prior to measurement, bark was removed and sample ends were cut in water with a razor blade so as to have a length of exactly 28 cm. The bottom and top diameters of each sample were measured with a caliper. Xylem cavitation was assessed with the Cavitron technique (Cochard et al., 2005). The principle of the technique is to use centrifugal force to increase the water tension in the stem segment while measuring the decrease of its hydraulic conductance. Maximal conductance of each sample (kmax) was determined at a xylem pressure of –0·1 MPa, measuring the flux of a degassed ionic solution (10 mm KCl and 1 mm CaCl2 in deionized water). Xylem pressure was then lowered stepwise by increasing the rotational velocity, and sample conductance (k) was determined again. In each step, k was measured three times, and the average was used to compute the percentage loss of conductance as PLC = 100 × (1 – k/kmax). The procedure was repeated until the PLC reached approx. 90 %. The observed curve was fitted to a logistic function (Pammenter and Van de Willigen, 1998):
| 2 |
where P50 is the pressure inducing 50 % loss of xylem conductance and s is the slope of the vulnerability curve at this point. In addition, xylem pressure at 12 % loss of conductance (P12), an estimate of the xylem water potential at which embolism begins, and xylem pressure at 88% loss of conductance (P88), a proxy of the xylem water potential at full embolism (Domec and Gartner, 2001), were calculated as:
| 3 |
| 4 |
Hydraulic efficiency
Shoot specific conductivity (Ks) was assessed by dividing kmax by sample basal wood area and multiplying by shoot length. Leaf specific conductivity (Kl), a measure of the hydraulic capacity of the shoot to supply water to leaves, was calculated as the ratio of shoot specific conductivity to leaf area (Tyree and Zimmermann, 2002). The projected area of 12 needles removed from the shoots used to construct vulnerability curves was obtained with a scanner and analysed with the program WinFOLIA (Regent Instruments). Then they were dried at 60 °C for 3 d to determine leaf dry mass and leaf mass per area (LMA). The rest of the needles were dried as previously described and total leaf area was calculated dividing the total needle mass by LMA.
Quantitative genetic differentiation
The quantitative variability within a given population was estimated using the coefficient of variation for the phenotypic value (CV). The CVs were obtained from population means and within-common garden standard deviations.
To determine the proportion of total variation that occurs between populations, the statistic QST was calculated for all quantitative traits partitioning the total additive genetic variance into the among-population (σB) and the within-population (σw) components:
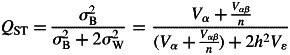 |
5 |
where h2 is the narrow-sense heritability and n the number of common gardens. Since there are not any published values of heritability for P. canariensis, a value of 0·2 was assumed, taking into account the narrow-sense heritability found in other pines (see González-Martínez et al., 2002 for a similar procedure). A simulation procedure was conducted to evaluate the influence of heritability values (in the range 0·2–0·8) on the QST value. The variance components: variance of the population (Vα), variance of the interaction population × common garden (Vαβ) and residual variance (Vɛ) were estimated using the residual maximum likelihood option (REML) of the VARCOMP procedure in SAS 9.1 (SAS/STAT Software, SAS Institute) following the model:
| 6 |
where Yij is the phenotypic value of the ith population at the common garden j; μ is the overall mean, αi is the effect of the ith population, βj is the effect of the jth common garden; αβij is the interaction between the ith population and the jth common garden and ɛ is the experimental error.
To assess the significance of random effects, mixed models with the factors described above were fitted using REML, and likelihood ratio tests were performed. Common garden was always treated as a fixed effect. Population was treated as random. Analyses were performed on individual-tree data. Residuals were examined for normality. Wald tests and F-statistics were used to evaluate the significance of fixed effects.
Survival was analysed with a linear logistic model (GLZ). A binomial distribution of the data was assumed and a logit function was used as the link function. The factors included in the model were as described before: common garden, population and the interaction common garden × population.
We estimated the plasticity of each population for each hydraulic and field trait with a log response ratio, L = ln( GC) – ln(
GC) – ln( TF) (Hedges et al., 1999), where
TF) (Hedges et al., 1999), where 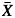 GC represents the population mean in Gran Canaria, and
GC represents the population mean in Gran Canaria, and 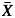 TF the population mean in Tenerife. Approximate 95 % confidence intervals for the individual log response ratios were calculated as L – 1·96 √v ≤ L ≤ L + 1·96 1·96 √v where √v is the variance of L computed as:
TF the population mean in Tenerife. Approximate 95 % confidence intervals for the individual log response ratios were calculated as L – 1·96 √v ≤ L ≤ L + 1·96 1·96 √v where √v is the variance of L computed as:
| 7 |
where n and SD are the sample size and standard deviation and mean, respectively in Gran Canaria (GC) and Tenerife (TF). Populations were considered to be plastic for a specific trait when 95 % confidence intervals of L for that specific trait did not overlap zero. Differences between populations in plasticity for a trait were tested by Duncan's multiple-range test if the interaction population × common garden in Equation 6 was significant. The overall plasticity for each trait was computed as the weighted mean of the log response ratio and its statistical significance was evaluated with the GLM of Equation 6:
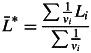 |
8 |
where Li is the log response ratio of the ith population and vi is the variance of Li.
Correlations between traits were evaluated by calculating Pearson's coefficient on the population Best Linear Unbiased Estimator (BLUE). In addition, Spearman's correlation coefficients were determined between the climatic conditions at origin, the BLUEs of hydraulic and growth traits of each population and their plasticity.
Molecular genetic analysis
In order to estimate neutral variation between populations, amplicons from three nuclear genes were sequenced using previously published primer pairs transferred from Pinus pinaster (Grivet et al., 2011), the water stress-inducible protein lp3-3 gene, and from Pinus taeda (Brown et al., 2004), CCoAOMT (cell wall reinforcement) and cad (end of the monolignol biosynthetic pathway) (Table 2). Genomic DNA was extracted from needles from 21–24 trees collected in each of the eight populations using the Invitek kit (Invisorb® Spin Plant Mini Kit). Primer information and amplification conditions are described in Supplementary Data Table S1. The PCR product was directly sequenced using the Secugen S.L. sequencing service (Madrid, Spain).
Table 2.
Description of the three nuclear genes: GeneBank accession number, putative functional category, total number of haploid sequences (n) and coding/non-coding length screened in bp
| Gene ID | GenBank accession no. | Functional category | n | Length screened (bp) |
||
|---|---|---|---|---|---|---|
| Total | Coding region | Non-coding region | ||||
| cad | JX088746–JX088937 | Lignin biosynthesis (end of the monolignol biosynthetic pathway) | 384 | 412 | 301 | 111 |
| lp3-3 | JX089129–JX089315 | Drought stress (belong to the ASR gene family) | 374 | 404 | 237 | 167 |
| CCoAOMT | JX088938–JX089128 | Lignin biosynthesis (cell wall reinforcement) | 382 | 511 | 264 | 247 |
Multiple sequence alignments were obtained using CLUSTALW on BIOEDIT software (http://www.mbio.ncsu.edu/BioEdit/page2.html). Because the data for these three loci were obtained from diploid individuals, they often include multiple heterozygous positions. The program PHASE (Stephens et al., 2001) was used to estimate two haplotypes for each individual at each locus. Hence, 42–48 haplotype sequences per population were scored.
Standard molecular diversity statistics were computed for each gene and population with DnaSP 5·1 (Librado and Rozas, 2009). Departure from neutrality for each locus was evaluated with Tajima's D (Tajima, 1989), Fu's D and F (Fu and Li, 1993) and Fu's FS (Fu, 1997) obtained with DnaSP 5·1. In addition, we used a recently developed statistical test to detect positive selection (MFDM) that has proven analytically and empirically free from the confounding effect of varying population size, including bottlenecks and recent expansions (Li, 2011), which is a realistic scenario for P. canariensis (Navascués et al., 2006). The test uses the maximum frequency of derived mutations within the sample to detect the presence of an unbalanced tree at a locus, which implies that a nearby locus may have experienced positive selection. A matrix of pairwise FST values was computed with Arlequin 3.11 (Excoffier et al., 2005) considering the substitution model for each locus estimated with jModeltest 0.1.1 (Posada, 2008).
Comparison of FST and QST
Confidence intervals and distribution for QST estimations were assessed with a parametric bootstrap procedure (1000 samples) outlined in Whitlock (2008) with replacement at the individual level within a population. In addition, confidence intervals for FST were determined by bootstrapping over loci. For each bootstrap replicate, the mean FST value was calculated from the neutral loci sampled, and from that the predicted χ2 distribution of FST was determined from the Lewontin–Krakauer approach. QST was considered to be statistically different from FST when the 95 % confidence intervals of QST did not overlap the 95 % confidence intervals of FST (Sahli et al., 2008).
RESULTS
Quantitative genetic variability between and within populations
Embolism began at a similar water potential in all populations in each common garden (P12 = –1·69 ± 0·12 MPa in Tenerife and P12 = –2·80 ± 0·13 MPa in Gran Canaria) but progressed differently, and slope, P50 and P88 differed among populations (Table 3). Although the maximum difference in P50 between populations was approx. 1·4 MPa in both common gardens, the population ranking differed (Table 4). In the mesic common garden, populations from La Palma and the leeward slopes of Tenerife and Gran Canaria were less vulnerable to cavitation, whereas in the xeric common garden, populations from La Palma were among the most vulnerable (Fig. 2; Table 4). No significant population effect on traits related to hydraulic efficiency or growth was observed (Table 3). However, plants from sites with a longer drought period showed higher survival rates and produced thicker needles in both common gardens (Table 4). This genetic differentiation for both survival and LMA was more pronounced in the xeric common garden where survival rates of populations from Gran Canaria were double the survival rates of populations from the north of Tenerife, and the LMA differed in 46·6 g m−2 (Table 4).
Table 3.
Percentage of the explained variation and significance values due to common garden, population and the interaction between common garden and population from the GLM and the GLZ for survival (Equation 6); coefficient of variation between (CVbp) and within (CVwp) populations, population differentiation for quantitative traits (QST) and log response ratio of the trait (L°*) for eight Pinus canariensis populations growing in two common gardens
| Trait | Com Gard | Pop | Com Gard × Pop | CVbp | CVwp | QST | 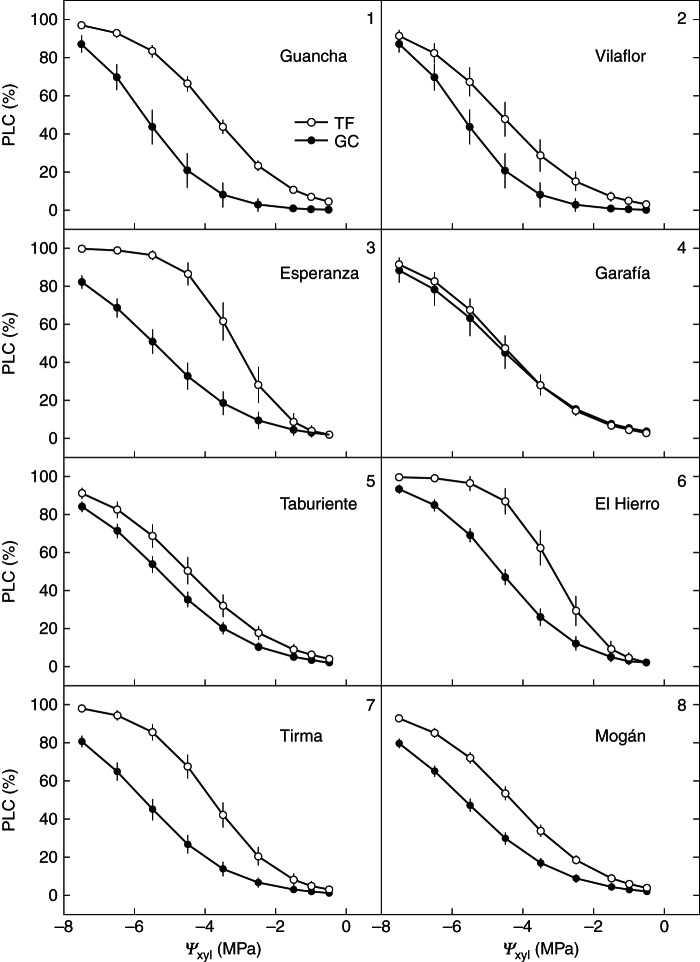 |
|---|---|---|---|---|---|---|---|
| Hydraulic efficiency | |||||||
| Ks | 15·33*** | 1·45 | 0 | 10·06 | 76·09 | 0·076 (0·10) | –0·42 (0·10) |
| Kl | 10·53*** | 3·58 | 1·28 | 20·72 | 79·11 | 0·089 (0·11) | 0·40 (0·16) |
| Vulnerability to cavitation | |||||||
| P12 | 34·35*** | 0 | 4·96 | 6·60 | 46·13 | 0·093 (0·08) | 0·48 (0·15) |
| P50 | 53·11*** | 5·48*** | 5·50* | 10·21 | 17·37 | 0·365 (0·09) | 0·31 (0·12) |
| P88 | 42·75*** | 9·00*** | 8·39** | 11·41 | 16·22 | 0·452 (0·08) | 0·25 (0·13) |
| Slope | 7·83*** | 5·80*** | 19·31** | 17·80 | 31·66 | 0·365 (0·09) | –0·16 (0·15) |
| Biomass allocation | |||||||
| Al:As | 40·26*** | 0·35 | 1·26 | 3·71 | 47·99 | 0·062 (0·09) | –0·61 (0·15) |
| Stem morphology | |||||||
| H | 94·12*** | 0·11 | 0 | 2·88 | 21·13 | 0·044 (0·09) | –0·92 (0·15) |
| Db | 59·96*** | 2·35 | 0 | 9·43 | 37·79 | 0·135 (0·09) | –0·72 (0·12) |
| Needle morphology | |||||||
| LMA | 73·16*** | 4·31* | 4·34* | 9·85 | 25·61 | 0·157 (0·09) | 0·35 (0·10) |
| Surv | 17·87*** | 4·80*** | 2·21 | ||||
The standard deviation is given in parentheses·
*P < 0·05; **P < 0·01; ***P < 0·001·
Ks, specific hydraulic conductivity; Kl, leaf specific hydraulic conductivity; P12, P50, P88, pressure causing 12, 50 and 88 % loss of conductance, respectively; slope, slope at the inflection point of the vulnerability curve; Al:As, branch leaf-to-sapwood area ratio; H, height 6 years after planting; Db, basal diameter 6 years after planting; LMA: leaf mass per area; Surv, survival.
Table 4.
Mean values (± s.e.) of survival, hydraulic efficiency and safety traits, field performance and leaf mass per area of eight populations of Pinus canariensis growing in two common gardens of contrasted environmental conditions (Tenerife and Gran Canaria)
| Hydraulic efficiency |
Vulnerability to cavitation |
Biomass allocation | Field performance |
Needle morphology | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common garden | Ecol. region | Ks (kg m−1 s−1 MPa−1) | Kl × 104 (kg m−1 s−1 MPa−1) | P12 (MPa) | P50 (MPa) | P88 (MPa) | Slope | Al:As (m2 cm−2) | Surv (%) | H (cm) | Db (cm) | LMA (g m−2) |
| Tenerife | 1 | 32·8 (5·2) | 3·22 (0·69) | –1·54 (0·21) | –3·77 (0·16) | –6·01 (0·29) | 23·4 (1·6) | 0·11 (0·02) | 64·3 (9·0) | 168 (13) | 4·9 (0·4) | 142 (6·7) |
| 2 | 45·1 (5·5) | 3·86 (0·62) | –1·84 (0·35) | –4·61 (0·42) | –7·38 (0·67) | 20·4 (2·9) | 0·14 (0·02) | 82·0 (7·1) | 127 (10) | 4·3 (0·2) | 158 (7·3) | |
| 3 | 39·2 (10·2) | 3·51 (0·47) | –1·64 (0·32) | –3·16 (0·33) | –4·69 (0·40) | 34·9 (3·2) | 0·11 (0·02) | 64·3 (9·2) | 149 (9) | 4·9 (0·4) | 148 (6·0) | |
| 4 | 73·2 (11·9) | 6·47 (0·15) | –2·00 (0·16) | –4·61 (0·29) | –7·21 (0·54) | 20·7 (2·4) | 0·12 (0·02) | 96·7 (3·1) | 140 (11) | 4·6 (0·2) | 143·4 (6·0) | |
| 5 | 83·2 (18·9) | 6·12 (0·21) | –1·60 (0·36) | –4·47 (0·33) | –7·33 (0·47) | 19·2 (2·9) | 0·13 (0·01) | 81·9 (7·2) | 139 (10) | 4·7 (0·3) | 149·8 (5·9) | |
| 6 | 62·8 (11·4) | 4·82 (0·75) | –1·52 (0·27) | –3·13 (0·32) | –4·73 (0·45) | 34·6 (4·3) | 0·13 (0·01) | 85·9 (7·1) | 151 (15) | 4·1 (0·3) | 139·8 (5·7) | |
| 7 | 62·4 (12·6) | 5·89 (1·37) | –1·70 (0·38) | –3·80 (0·26) | –5·90 (0·30) | 26·2 (2·8) | 0·11 (0·01) | 99·9 (1·0) | 144 (10) | 5·0 (0·3) | 163·1 (7·0) | |
| 8 | 78·6 (18·6) | 6·52 (1·22) | –1·68 (0·19) | –4·32 (0·15) | –6·97 (0·26) | 20·2 (1·4) | 0·11 (0·01) | 78·6 (8·5) | 126 (9) | 4·2 (0·3) | 159·7 (6·3) | |
| Gran Canaria | 1 | 32·2 (4·6) | 5·77 (1·77) | –3·75 (0·49) | –5·73 (0·41) | –7·71 (0·46) | 27·1 (2·2) | 0·09 (0·02) | 32·1 (9·8) | 59 (5) | 0·7 (0·1) | 181·5 (7·2) |
| 2 | 39·0 (8·5) | 4·26 (0·94) | –3·70 (0·21) | –6·05 (0·24) | –8·40 (0·29) | 21·5 (0·9) | 0·11 (0·02) | 55·3 (9·3) | 51 (4) | 0·8 (0·2) | 228·1 (7·6) | |
| 3 | 37·1 (7·4) | 9·31 (2·84) | –2·62 (0·53) | –5·44 (0·39) | –8·26 (0·40) | 18·8 (2·0) | 0·09 (0·02) | 42·9 (9·3) | 57(3) | 0·8 (0·1) | 210·6 (7·0) | |
| 4 | 18·9 (6·8) | 3·13 (0·51) | –1·86 (0·17) | –4·76 (0·48) | –7·67 (0·43) | 18·5 (2·2) | 0·06 (0·02) | 50·7 (9·8) | 49 (3) | 0·7 (0·2) | 210·5 (6·9) | |
| 5 | 37·9 (5·0) | 10·10 (1·70) | –2·49 (0·29) | –5·29 (0·22) | –8·08 (0·30) | 18·9 (1·3) | 0·05 (0·01) | 64·3 (9·2) | 59 (3) | 1·1 (0·1) | 228·1 (7·5) | |
| 6 | 33·8 (4·5) | 9·11 (2·15) | –2·17 (0·34) | –4·62 (0·20) | –7·07 (0·26) | 22·9 (2·0) | 0·05 (0·01) | 28·6 (9·4) | 48 (3) | 0·6 (0·1) | 227·1 (7·5) | |
| 7 | 38·2 (6·8) | 12·33 (3·78) | –3·17 (0·42) | –5·74 (0·26) | –8·30 (0·22) | 20·3 (1·6) | 0·05 (0·01) | 64·3 (9·2) | 57(3) | 0·8 (0·2) | 235·1 (8·3) | |
| 8 | 43·5 (6·3) | 8·64 (1·38) | –2·62 (0·32) | –5·65 (0·18) | –8·68 (0·29) | 18·4 (1·5) | 0·06 (0·01) | 71·4 (9·1) | 75 (2) | 1·3 (0·1) | 234·2 (8·1) | |
Ks, specific hydraulic conductivity; Kl, leaf specific hydraulic conductivity; P12, P50, P88, pressure causing 12, 50 and 88 % loss of conductance, respectively; slope, slope at the inflection point of the vulnerability curve; Al:As, branch leaf-to-sapwood area ratio; Surv, survival 6 years after planting; H, height 6 years after planting; Db, basal diameter 6 years after planting; LMA, leaf mass per area.
Sample size was n = 144 for all traits except for survival, n = 448.
Fig. 2.
Xylem vulnerability curves for shoots of Pinus canariensis from populations of the eight ecological regions described for the species (numbered in the graphs) planted in two common gardens (TF, Tenerife; GC, Gran Canaria). Error bars represent the s.e. Ψxyl, xylem water potential; PLC, percentage loss of conductance.
Genetic variability in phenotypic plasticity
All traits were strongly influenced by common garden (Table 3). As expected, in Tenerife, more plants survived and grew taller, branches were more vulnerable to cavitation (higher values of P12, P50 and P88) and branch Al:As was higher. Conversely, higher values of Kl were found in the xeric common garden, mostly due to the low leaf area of trees growing in this location, which only retained leaves formed in the current year. All populations exhibited significant plasticity (the confidence interval around L did not overlap zero) for height, diameter and LMA, and were equally plastic (the interaction common garden × population in Equation 6 was not significant). The only differences in plasticity between populations were for parameters of the vulnerability curve (Fig. 3). Noticeably, both populations from La Palma were the least plastic for P50 [no plasticity of population 4 and L(P50) = 0·17 of population 5].
Fig. 3.
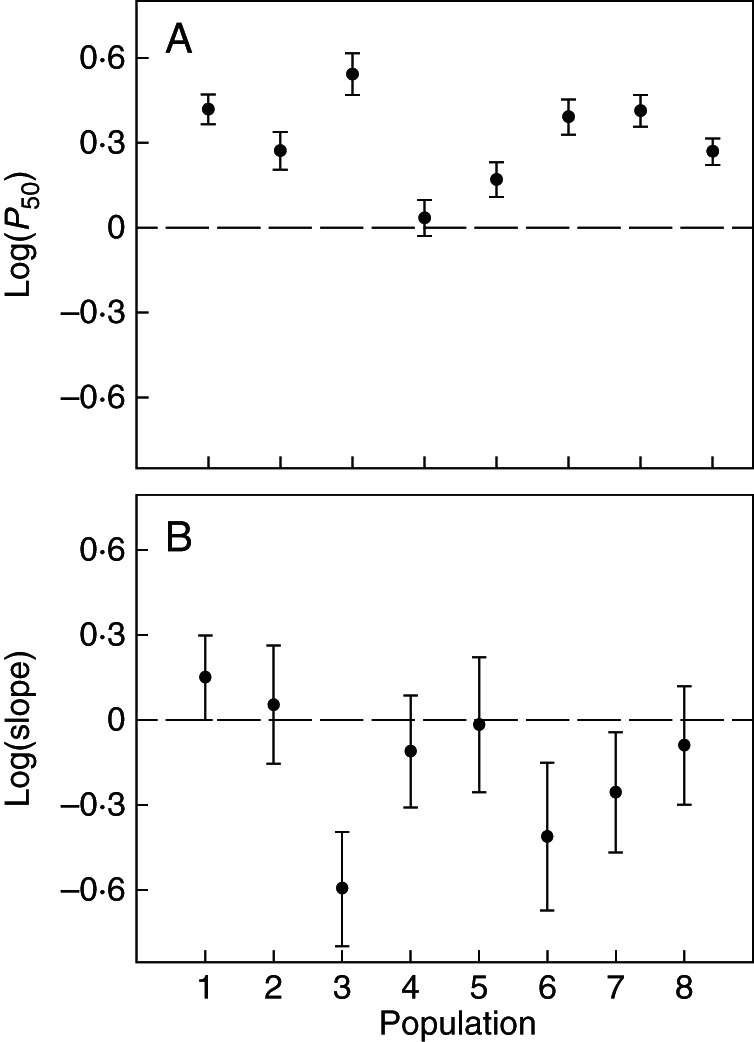
(A) Log response ratio ± 95 % confidence interval of the pressure causing 50 % loss of conductance (P50) and (B) the slope at the inflection point of the vulnerability curve of eight populations of Pinus canariensis growing in two common garden experiments. If the 95 % confidence intervals are above (below) zero, this indicates a significant increase (decrease) in the trait in Tenerife (mesic common garden); if the confidence intervals cross the line, then there is no significant effect of the common garden.
Relationships among field performance, hydraulic traits and climate conditions of origin
The parameters of the vulnerability curve were positively correlated with each other (r between slope and P50 = 0·43). There was also a positive correlation between variables measuring hydraulic efficiency (r = 0·52). No relationship was found between both sets of traits, indicating the absence of a trade-off between hydraulic efficiency and safety.
Parameters of the vulnerability curve were related to field performance and climate of origin of the populations only in the xeric common garden. Survival was strongly correlated with population mean values of P88 (r = –0·81) and slope (r = –0·71) (Fig. 4). P88 was negatively related to the temperature range (r = –0·76) and positively to the mean annual precipitation, if we excluded the population from El Hierro (r = 0·96) (Fig. 5). Populations occurring in forests with a longer drought period showed higher LMA (r = 0·76). No relationship was found between plasticity and fitness traits.
Fig. 4.
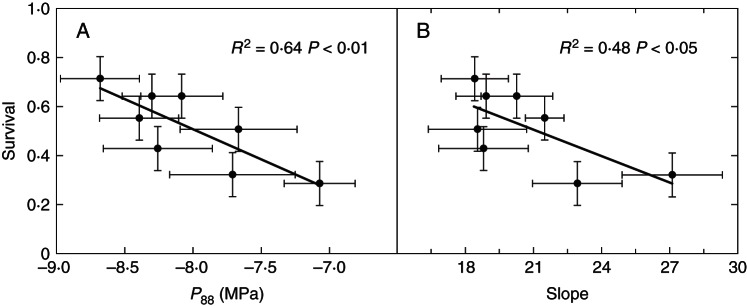
Correlations between mean values of survival of Pinus canariensis populations growing at the xeric common garden in Gran Canaria and two parameters obtained from the vulnerability curves: (A) the pressure causing 88 % loss of conductance (P88) and (B) the slope at the inflection point.
Fig. 5.
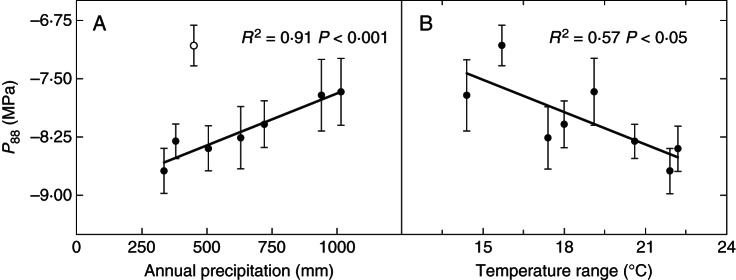
Relationship of (A) mean annual precipitation and (B) temperature range with the pressure causing 88 % loss of conductance in the xeric common garden in Gran Canaria (P88). The population from El Hierro (open circle) is excluded in (A).
Molecular genetic analysis
The partial sequences of the three nuclear genes (GeneBank accession nos: cad, JX088746–JX088937; CCoAOMT, JX088938–JX089128; and lp3-3, JX089129–JX089315) covered coding and non-coding regions (Supplementary Data Table S2). The number of segregating sites was similar for all three genes (10–11) when the whole range of the species was considered but was variable for each population, from a low of two in El Hierro to a high of eight in Esperanza for cad. The number of haplotypes was variable between populations and genes (Supplementary Data Table S2). While haplotypes were shared across all populations, private haplotypes were scored in almost all the populations for the three candidate genes. Neither standard neutrality tests (Tajima's D, Fu's D and F, and Fu's FS) nor the MFDM test generated significant values for any of the genes when all ecological regions were considered or when they were analysed alone (Supplementary Data Table S2).
Neutral vs. adaptive differentiation
FST differentiation was low for neutral genes. The overall estimate of genetic differentiation for cad FST was 0·091 (0·053, P < 0·01), for CCoAOMT 0·066 (0·062, P < 0·01) and for lp3-3 0·061 (0·048, P < 0·01). QST values ranged between 0·044 (0·09; P < 0·001) for height and 0·452 (0·08; P < 0·001) for P88 (Table 3). For height and hydraulic efficiency traits, QST and FST values did not differ significantly. Conversely, for parameters of the vulnerability curve, the average QST value was significantly higher than FST (Fig. 6), suggesting that populations displayed more differentiation than would be expected by drift alone. The differences between quantitative QST and neutral FST were still significant when heritability values in the range 0·2–0·8 were assumed (Fig. 6).
Fig. 6.
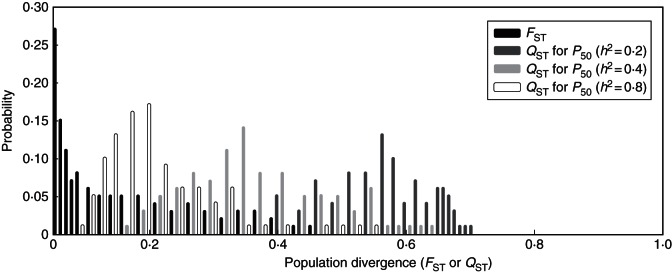
Frequency distributions of the cad gene (FST) and quantitative trait for P50 (QST) differentiation based on 1000 bootstrap samples. Estimated QST considering different values of narrow-sense heritability (h2).
DISCUSSION
Genetic variation of vulnerability to cavitation and phenotypic plasticity of hydraulic traits
Canary Island pine populations from a wide range of ecological conditions showed evidence for climate-driven divergence for branch vulnerability to cavitation under xeric conditions. According to our expectations, and with the exception of the population from El Hierro, populations did differ consistently in their vulnerability to water stress-induced cavitation in the xeric common garden: the dry populations were the least vulnerable and the mesic populations the most susceptible (Fig. 5). The construction of a safer xylem in drier habitats has been commonly found in interspecific comparisons (Pockman and Sperry, 2000; Choat et al., 2007), but this is the first time it has been assessed in conifers at the intraspecific level. Until now, studies evaluating populations of several pines had reported little or no difference between populations for this trait, even considering a wide range of climates (Maherali and DeLucia, 2000; Martínez-Vilalta et al., 2009; Lamy et al., 2011). However, the high intrapopulation variability for vulnerability to cavitation in P. canariensis (CVwp = 17 %) compared with other pines (Martínez-Vilalta et al., 2009; Lamy et al., 2011) suggested the evolvability, i.e. ability to respond to selection, of this trait in this species (Houle, 1992) as reflected by the comparisons between neutral and quantitative differentiation (Fig. 6).
Hydraulic efficiency, biomass allocation and, in particular, growth were especiallly sensitive to changes in environmental conditions (higher values of the log response ratio; Table 3) and, interestingly, all populations performed similarly in both common gardens, as reflected by the non-significant population effect and negligible population × common garden interaction (Table 3). Most of the variability of these traits resided within rather than between populations (Table 3). The general response to overcome low water availability was producing stiffer needles to reduce transpiration (Cernusak et al., 2011) and reducing branch Al:As by means of a dramatic reduction of leaf area, resulting in higher Kl to guarantee water supply to leaves despite the reduction of Ks (Sperry and Pockman, 1993). Substituting the relative differences between common gardens in Equation 1, a 35 % decreased in Ks was offset by a 71 % decrease in Al:As without any adjustment in ΔΨ. In contrast, plasticity for vulnerability to cavitation was genotype dependent, contrary to our initial hypothesis of a limitation in plasticity of P50. Only vulnerability curves of populations from La Palma almost coincided (Fig. 2), more in accordance with the lack of plasticity previously reported for pines (Martínez-Vilalta et al., 2004). However, populations from the windward slopes of the main islands were highly plastic; P50 increased almost 50 % in the mesic common garden, where the development of unnecessary drought-tolerant tissues could compromise competitive ability (Pockman and Sperry, 2000). Higher cavitation resistance has been linked to increased wood density to sustain the compressive forces generated by lower negative pressures (Hacke et al., 2001). Construction of denser wood may correspond to slower growth rates (Enquist et al., 1999), further decreasing fitness of cavitation-resistant genotypes when water is readily available. Nevertheless, we have not found such a trade-off in P. canariensis, at least in above-ground growth, and other traits related to below-ground properties or fructification could be involved.
A possible shortcoming of the present study was that hydraulic properties were compared on 1-year-old branches in the mesic common garden and in 2- to 3-year-old branches in the xeric garden. However, a previous study with Fagus sylvatica did not find any significant age effect in P50 even comparing 1-year-old with 6-year-old stems (Herbette et al., 2010). Moreover, the youngest branches tended to be less vulnerable to cavitation than the largest branches (Cochard, 1992). Thus if there was a bias, we probably would have underestimated the difference in P50 between the two common gardens. Another limitation was that conductance in branches was only a small fraction of total conductance. Other features such as below-ground properties can affect transpiration; for example, root Ks may have been higher in xeric populations, or may be more plastic in populations that are less plastic in P50. Genetic differences in biomass allocation to roots in P. canariensis seedlings subjected to water deficit in hydroponic culture have been observed previously (López et al., 2009).
Vulnerability to cavitation and fitness traits
We used survival and growth as the best available fitness proxies, and the results were conclusive for the adaptive value of decreasing vulnerability to cavitation in dry conditions. We found a strong correlation between survival in the xeric common garden in Gran Canaria, and both the xylem water potential at full embolism (P88) and the slope of the vulnerability curve (Fig. 4). The strong correlation between P50 and annual precipitation for conifers and evergreen angiosperms had already suggested the adaptive significance of decreasing vulnerability to cavitation as a mechanism of drought tolerance at the interspecific level (Brodribb and Hill, 1999; Maherali et al., 2004), and our results also pointed in this direction at the intraspecific level when trees were growing in xeric conditions. Additionally, the resistance of the hydraulic system appeared to be the key factor for survival and posterior gas exchange recovery from drought (Brodribb and Cochard, 2009), and to achieve higher midday stomatal conductance to water vapour in soils with low water retention or under water deficit conditions (Holste et al., 2006; Beikircher and Mayr, 2009), and thus to maintain a favourable carbon balance.
Although an overall trade-off among hydraulic conductivity and growth with P50 was found when pooling data of the two common gardens, this trade-off was not so evident within sites, and trees which constructed a safer xylem were not necessarily those exhibiting lower growth or less efficiency in terms of water transport. This result, when trees grew in the same environment, was consistent with a growing body of evidence suggesting a lack of a trade-off between xylem safety and efficiency in conifers (Willson et al., 2008; Martínez-Vilalta et al., 2009; Peguero-Pina et al., 2011; but see Cochard, 1992; Kavanagh et al., 1999; Piñol and Sala, 2000; Domec and Gartner, 2002, 2003). The exact mechanism by which xylem vulnerability to cavitation acclimates to soil water deficit remains to be explained, but it should be related to changes in size, permeability or stability of the torus of the pits and wall reinforcement (Hacke and Sperry, 2001; Hacke et al., 2001) rather than to conduit diameter.
Effect of natural selection on population differentiation
Our results indicated that natural selection has shaped the observed genetic differentiation in vulnerability to cavitation and LMA across the natural range of P. canariensis. The comparison of QST values of P50, P88, slope of the vulnerability curve and LMA between populations far exceeded that expected through random drift and gene flow alone, as estimated from FST. These measurements of among-population divergence, i.e. values of QST, are inherently dependent on the heritability of the character, but even with high heritability values the distribution of QST differed significantly from FST (Fig. 6), consistent with divergent selection acting on drought resistance across the geographic range of this species. In fact, population differentiation could have been more pronounced if mortality had affected the most vulnerable genotypes. Slope and P50 could be underestimated then, particularly in populations with the lowest survival rates. The QST value of 0·452 for P88 and 0·365 for P50 and slope is close to the upper range of values reported for drought-related traits in Quercus suber (Ramírez-Valiente et al., 2009) but it differed widely from the low value of differentiation among populations of P. pinaster planted in a mesic environment (Lamy et al., 2011). In this latter study, uniform selection for vulnerability to cavitation in P. pinaster was suggested as a consequence of canalization to buffer against genetic or environmental disturbances. In our case, and despite evidence for extensive gene flow among islands (Navascués et al., 2006) and along elevational transects (Navascués et al., 2008), the broad range of environments and historical perturbations could have exerted strong selection pressures leading to both phenotypically plastic genotypes and local adaptation. The occurrence of shared and private haplotypes in all the populations found for chloroplast microsatellites (Vaxevanidou et al., 2006) and for nuclear genes in the present study is compatible with a scenario of substantial gene flow that, rather than counteracting local adaptation, enhances it, as in other wind-dispersed species (Kremer et al., 2012). Quantitative trait genetic differentiation in cavitation (the present study), vegetative phase change (Climent et al., 2006), biomass allocation and osmotic adjustment (López et al., 2009), and foliar morphology and anatomy (López et al., 2010) strengthen the idea of divergent selection.
A second line of evidence that natural selection has driven genetic diversification in vulnerability to cavitation was the significant correlation between survival and parameters of the vulnerability curve. Moreover, increased resistance to drought and water use efficiency appear to have evolved in populations subjected to greater temperature seasonality and lower precipitation as in populations of Eucalyptus globulus (Dutkowski and Potts, 2012), Pinus halepensis (Voltas et al., 2008) and Cordia alliodora (Choat et al., 2007). In contrast, the QST of xylem efficiency and growth is consistent with genetic drift alone arising from founder effects.
Conclusions
Phenotypic variability for branch hydraulic traits in P. canariensis was largely the result of phenotypic plasticity. Acclimation of the hydraulic system to xeric conditions implied modifications of Al:As and changes in the vulnerability to cavitation. We inferred that divergent selection must have acted in the past on xylem vulnerability to cavitation more evidently than in other traits sensitive to water deficit such as growth or hydraulic efficiency. Our results strongly support the adaptive role of cavitation resistance in xeric environments.
SUPPLEMENTARY DATA
Supplemenatry data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: primers and amplification conditions for the three candidate genes in the study. Table S2: diversity parameters for the three candidate genes.
ACKNOWLEDGEMENTS
We are grateful to the Canary Islands Government, the Cabildos of Tenerife and Gran Canaria and the National Park of Caldera de Taburiente for long-standing support in the study of Canary Island pine. We thank all people involved in the plantation and measurements of the common gardens and to Christian Bodet and Pierre Conchon for their assistance with the Cavitron. R.L. was supported by a González Esparcia fellowship during her stay in Clermont-Ferrand. This work was supported by the Spanish Ministry of Science in the Project AGL2009-10606 (VULCAN).
LITERATURE CITED
- Alpert P, Simms EL. The relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust? Evolutionary Ecology. 2002;16:285–297. [Google Scholar]
- Beikircher B, Mayr S. Intraspecific differences in drought tolerance and acclimation in hydraulics of Ligustrum vulgare and Viburnum lantana. Tree Physiology. 2009;29:765–775. doi: 10.1093/treephys/tpp018. [DOI] [PubMed] [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences, review. Annals of Forest Sciences. 2006;63:625–644. [Google Scholar]
- Brodribb TJ, Cochard H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Hill RS. The importance of xylem constraints in the distribution of conifer species. New Phytologist. 1999;143:365–375. [Google Scholar]
- Brown GR, Gill GP, Kuntz RJ, Langley CH, Neale DB. Nucleotide diversity and linkage disequilibrium in loblolly pine. Proceedings of the National Academy of Sciences, USA. 2004;101:15255–15260. doi: 10.1073/pnas.0404231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusak LA, Hutley LB, Beringer J, Holtum JAM, Turner BL. Photosynthetic physiology of eucalypts along a sub-continental rainfall gradient in northern Australia. Agricultural and Forest Meteorology. 2011;151:1462–1470. [Google Scholar]
- Chaves MM, Pereira JS, Maroco J, et al. How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany. 2002;89:907–916. doi: 10.1093/aob/mcf105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Sack L, Holbrook NM. Diversity of hydraulic traits in nine Cordia species growing in tropical forests with contrasting precipitation. New Phytologist. 2007;175:686–698. doi: 10.1111/j.1469-8137.2007.02137.x. [DOI] [PubMed] [Google Scholar]
- Climent J, Chambel MR, Pérez E, Gil L, Pardos J. Relationship between heartwood radius and early radial growth, tree age, and climate in Pinus canariensis. Canadian Journal of Forest Research. 2002;32:103–111. [Google Scholar]
- Climent J, Tapias R, Pardos JA, Gil L. Fire adaptations in the Canary Islands pine (Pinus canariensis) Plant Ecology. 2004;171:185–196. [Google Scholar]
- Climent J, Chambel MR, López R, Mutke S, Alía R, Gil L. Population divergence for heteroblasty in the Canary Island pine (Pinus canariensis, Paceae) American Journal of Botany. 2006;93:840–848. doi: 10.3732/ajb.93.6.840. [DOI] [PubMed] [Google Scholar]
- Cochard H. Vulnerability of several conifers to air embolism. Tree Physiology. 1992;11:73–83. doi: 10.1093/treephys/11.1.73. [DOI] [PubMed] [Google Scholar]
- Cochard H, Damour G, Bodet C, Tharwat I, Poirier M, Améglio T. Evaluation of a new centrifuge technique for rapid generation of xylem vulnerability curves. Physiologia Plantarum. 2005;124:410–418. [Google Scholar]
- Delzon S, Douthe C, Sala A, Cochard H. Mechanism of water-stress induced cavitation in conifers: bordered pit structure and function support the hypothesis of seal capillary-seeding. Plant, Cell and Environment. 2010;33:2101–2111. doi: 10.1111/j.1365-3040.2010.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domec JC, Gartner BL. Cavitation and water storage in bole segments of mature and young Douglas-fir trees. Trees-Structure and Function. 2001;15:204–214. [Google Scholar]
- Domec JC, Gartner BL. How do water transport and water storage differ in coniferous earlywood and latewood? Journal of Experimental Botany. 2002;53:2369–2379. doi: 10.1093/jxb/erf100. [DOI] [PubMed] [Google Scholar]
- Domec JC, Gartner BL. Relationship between growth rates and xylem hydraulic characteristics in young, mature and old-growth ponderosa pine trees. Plant, Cell and Environment. 2003;26:471–483. [Google Scholar]
- Dutkowski GW, Potts BM. Genetic variation in the susceptibility of Eucalyptus globulus to drought damage. Tree Genetics and Genomes. 2012;8:757–773. [Google Scholar]
- Emerson BC. Evolution on oceanic islands: molecular phylogenetic approaches to understanding pattern and process. Molecular Ecology. 2002;15:104–109. doi: 10.1046/j.1365-294x.2002.01507.x. [DOI] [PubMed] [Google Scholar]
- Enquist BJ, West GB, Charnov EL, Brown JH. Allometric scaling of production and life-history variation in vascular plants. Nature. 1999;401:907–911. [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin 3.01: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Ewers BE, Oren R, Sperry JS. Influence of nutrient versus water supply on hydraulic architecture and water balance in Pinus taeda. Plant, Cell and Environment. 2000;23:1055–1066. [Google Scholar]
- Fu YX. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Li WH. Statistical tests of neutrality of mutations. Genetics. 1993;133:693–709. doi: 10.1093/genetics/133.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Martínez SC, Alía R, Gil L. Population genetic structure in a Mediterranean pine (Pinus pinaster Ait.): a comparison of allozyme markers and quantitative traits. Heredity. 2002;89:199–206. doi: 10.1038/sj.hdy.6800114. [DOI] [PubMed] [Google Scholar]
- González-Martínez SC, Wheeler NC, Ersoz E, Nelson CD, Neale DB. Association genetics in Pinus taeda L. I. Wood property traits. Genetics. 2007;175:399–409. doi: 10.1534/genetics.106.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivet D, Sebastiani F, Alía R, et al. Molecular footprints of local adaptation in two Mediterranean conifers. Molecular Biology and Evolution. 2011;28:101–116. doi: 10.1093/molbev/msq190. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS. Functional and ecological xylem anatomy. Perspectives in Plant Ecology Evolution and Systematics. 2001;4:97–115. [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressures. Oecologia. 2001;126:457–461. doi: 10.1007/s004420100628. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiology. 2006;26:689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Jacobsen AL, Pratt RB. Xylem function of aridland shrubs from California, USA: an ecological and evolutionary analysis. Plant, Cell and Environment. 2009;32:1324–1333. doi: 10.1111/j.1365-3040.2009.02000.x. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- Herbette S, Wortemann R, Awad H, Huc R, Cochard H, Barigah TS. Insights into xylem vulnerability to cavitation in Fagus sylvatica L.: phenotypic and environmental sources of variability. Tree Physiology. 2010;30:1448–1455. doi: 10.1093/treephys/tpq079. [DOI] [PubMed] [Google Scholar]
- Holste EK, Jerke MJ, Matzner SL. Long-term acclimatization of hydraulic properties, xylem conduit size, wall strength and cavitation resistance in Phaseolus vulgaris in response to different environmental effects. Plant, Cell and Environment. 2006;29:836–843. doi: 10.1111/j.1365-3040.2005.01454.x. [DOI] [PubMed] [Google Scholar]
- Houle D. Comparing evolvability and variability of quantitative traits. Genetics. 1992;130:195–204. doi: 10.1093/genetics/130.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen AL, Pratt RB, Ewers FW, Davis SD. Cavitation resistance among twenty-six chaparral species of southern California. Ecological Monographs. 2007;77:99–115. [Google Scholar]
- Juan C, Emerson BC, Oromí P, Hewitt GM. Colonization and diversification: towards a phylogeographic synthesis for the Canary Islands. Trends in Ecology and Evolution. 2000;15:104–109. doi: 10.1016/s0169-5347(99)01776-0. [DOI] [PubMed] [Google Scholar]
- Kavanagh KL, Bond BJ, Aitken SN, Gartner BL, Knowe S. Shoot and root vulnerability to xylem cavitation in four populations of Douglas-fir seedlings. Tree Physiology. 1999;19:31–37. doi: 10.1093/treephys/19.1.31. [DOI] [PubMed] [Google Scholar]
- Kremer A, Ronce O, Robledo-Arnuncio JJ, et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecology Letters. 2012;15:378–392. doi: 10.1111/j.1461-0248.2012.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy J-B, Bouffier L, Burlett R, Plomion C, Cochard H, Delzon S. Uniform selection as the primary evolutionary force of cavitation resistance across a species range. PloS ONE. 2011;6 doi: 10.1371/journal.pone.0023476. e23476. http://dx.doi.org/10.1371/journal.pone.0023476 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta RG, McKay JK. Genetic population divergence: markers and traits. Trends in Ecology and Evolution. 2002;17:501–502. [Google Scholar]
- Li H. A new test for detecting recent positive selection that is free from the confounding impacts of demography. Molecular Biology and Evolution. 2011;28:365–375. doi: 10.1093/molbev/msq211. [DOI] [PubMed] [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- López R, Zehavi A, Climent J, Gil L. Contrasting ecotypic differentiation for growth and survival in Pinus canariensis. Australian Journal of Botany. 2007;55:759–769. [Google Scholar]
- López R, Rodríguez-Calcerrada J, Gil L. Physiological and morphological response to water deficit in seedlings of five provenances of Pinus canariensis: potential to detect variation in drought-tolerance. Trees-Structure and Function. 2009;23:509–519. [Google Scholar]
- López R, Climent J, Gil L. Intraspecific variation and plasticity in growth and foliar morphology along a climate gradient in the Canary Island pine. Trees-Structure and Function. 2010;24:343–350. [Google Scholar]
- López de Heredia U, Venturas M, López R, Gil L. High biogeographical and evolutionary value of Canary Island pine populations out of the elevational pine belt: the case of a relict coastal population. Journal of Biogeography. 2010;37:2371–2383. [Google Scholar]
- Maherali H, DeLucia EH. Xylem conductivity and vulnerability to cavitation of ponderosa pine growing in contrasting climates. Tree Physiology. 2000;20:859–867. doi: 10.1093/treephys/20.13.859. [DOI] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- Martínez-Vilalta J, Piñol J. Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. Forest Ecology and Management. 2002;161:247–256. [Google Scholar]
- Martínez-Vilalta J, Prat E, Oliveras I, Piñol J. Xylem hydraulic properties of roots and stems of nine Mediterranean woody species. Oecologia. 2002;133:19–29. doi: 10.1007/s00442-002-1009-2. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Sala A, Piñol J. The hydraulic architecture of Pinaceae – a review. Plant Ecology. 2004;171:3–13. [Google Scholar]
- Martinez-Vilalta J, Cochard H, Mencuccini M, et al. Hydraulic adjustment of Scots pine across Europe. New Phytologist. 2009;184:353–364. doi: 10.1111/j.1469-8137.2009.02954.x. [DOI] [PubMed] [Google Scholar]
- Merilä J, Crnokrak P. Comparison of marker gene and quantitative genetic differentiation among populations. Journal of Evolutionary Biology. 2001;14:892–903. [Google Scholar]
- de Nascimento L, Willis KJ, Fernández-Palacios JM, Criado C, Whittaker RJ. The long-term ecology of the lost forests of La Laguna, Tenerife (Canary Islands) Journal of Biogeography. 2009;36:499–514. [Google Scholar]
- Navascués M, Emerson BC. Restoration of genetic diversity in reforested areas of the endemic Canary Island pine, Pinus canariensis. Forest Ecology and Management. 2007;244:122–128. [Google Scholar]
- Navascués M, Vaxevanidou Z, González-Martínez SC, Climent J, Gil L. Chloroplast microsatellites reveal colonisation and metapopulation dynamics in the Canary Island pine. Molecular Ecology. 2006;15:2691–2698. doi: 10.1111/j.1365-294X.2006.02960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navascués M, Vendramin GG, Emerson BC. The effect of altitude on the pattern of gene flow in the endemic Canary Island pine, Pinus canariensis. Silvae Genetica. 2008;57:357–363. [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. Plant phenotypic plasticity in a changing climate. Trends in Plant Science. 2010;15:684–692. doi: 10.1016/j.tplants.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Vander Willigen C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology. 1998;18:589–593. doi: 10.1093/treephys/18.8-9.589. [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Alquezar-Alquezar JM, Mayr S, Cochard H, Gil-Pelegrin E. Embolism induced by winter drought may be critical for the survival of Pinus sylvestris L. near its southern distribution limit. Annals of Forest Science. 2011;68:565–574. [Google Scholar]
- Piñol J, Sala A. Ecological implications of xylem cavitation for several Pinaceae in the Pacific Northern USA. Functional Ecology. 2000;14:538–545. [Google Scholar]
- Pittermann J, Sperry JS, Wheeler JK, Hacke UG, Sikkema EH. Mechanical reinforcement of tracheids compromises the hydraulic efficiency of conifer xylem. Plant, Cell and Environment. 2006;29:1618–1628. doi: 10.1111/j.1365-3040.2006.01539.x. [DOI] [PubMed] [Google Scholar]
- Pittermann J, Choat B, Jansen S, Stuart SA, Lynn L, Dawson TE. The relationships between xylem safety and hydraulic efficiency in the Cupressaceae: the evolution of pit membrane form and function. Plant Physiology. 2010;153:1919–1931. doi: 10.1104/pp.110.158824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS. Vulnerability to cavitation and the distribution of Sonoran Desert vegetation. American Journal of Botany. 2000;87:1287–1299. [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Ramírez-Valiente JA, Lorenzo Z, Soto A, Valladares F, Gil L, Aranda I. Elucidating the role of genetic drift and natural selection in cork oak differentiation regarding drought tolerance. Molecular Ecology. 2009;18:3803–3815. doi: 10.1111/j.1365-294X.2009.04317.x. [DOI] [PubMed] [Google Scholar]
- Sahli HF, Conner JK, Shaw FH, Howe S, Lale A. Adaptive differentiation of quantitative traits in the globally distributed weed, wild radish (Raphanus raphanistrum) Genetics. 2008;180:945–955. doi: 10.1534/genetics.107.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Pockman WP. Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis. Plant, Cell and Environment. 1993;16:279–288. [Google Scholar]
- Sperry JS, Adler FR, Campbell GS, Comstock JP. Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant, Cell and Environment. 1998;21:347–359. [Google Scholar]
- Sperry JS, Meinzer FC, McCulloh KA. Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant, Cell and Environment. 2008;31:632–645. doi: 10.1111/j.1365-3040.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- Spitze K. Population structure in Daphnia obtusa: quantitative genetic and allozymic variation. Genetics. 1993;135:367–374. doi: 10.1093/genetics/135.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E. Water transport across roots. Plant and Soil. 1994;167:79–90. [Google Scholar]
- Still DW, Aoyama N, Kim DH. Genetic variation in Echinacea angustifolia along a climatic gradient. Annals of Botany. 2005;96:467–477. doi: 10.1093/aob/mci199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Zimmermann MH. Xylem structure and the ascent of sap. Berlin: Springer; 2002. [Google Scholar]
- Vaxevanidou Z, González-Martínez SC, Climent J, Gil L. Tree populations bordering on extinction: a case study in the endemic Canary Island pine. Biological Conservation. 2006;129:451–460. [Google Scholar]
- Voltas J, Chambel MR, Prada MA, Ferrio JP. Climate-related variability in carbon and oxygen stable isotopes among populations of Aleppo pine grown in common-garden tests. Trees-Structure and Function. 2008;22:759–769. [Google Scholar]
- Whitlock MC. Evolutionary inference from QST. Molecular Ecology. 2008;17:1885–1896. doi: 10.1111/j.1365-294X.2008.03712.x. [DOI] [PubMed] [Google Scholar]
- Willson CJ, Manos PS, Jackson RB. Hydraulic traits are influenced by phylogenetic history in the drought-resistant, invasive genus Juniperus (Cupressaceae) American Journal of Botany. 2008;95:299–314. doi: 10.3732/ajb.95.3.299. [DOI] [PubMed] [Google Scholar]
- Wright S. The genetical structure of populations. Annals of Eugenics. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann MH. Xylem structure and the ascent of sap. Berlin: Springer; 1983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.