Abstract
The coordination of pelvic visceral activity with appropriate elimination behaviors is a complex task that requires reciprocal communication between the brain and pelvic viscera. Barrington’s nucleus in the pons is central to a circuit involved in this function. Barrington’s nucleus neurons diverge to project to pelvic visceral motoneurons and brain norepinephrine neurons that modulate behavior. This circuit is adaptively designed to coordinate the descending limb of the micturition reflex with a central limb that initiates arousal and shifts the focus of attention to facilitate elimination behavior. Although it serves an adaptive function, this same circuitry that links the bladder and brain allows for pathological processes at one end of the circuit to be expressed at the other. Here we show how urologic disorders can have cognitive and behavioral consequences by affecting components of this circuit. In the opposing direction, psychosocial stressors can produce voiding dysfunctions and bladder pathology through this circuit. The stress-related neuropeptide, corticotropin-releasing factor, which is prominent in Barrington’s nucleus neurons, is a potential mediator of these effects. Together, the studies reviewed highlight the potential for co-morbid bladder and neurobehavioral symptoms and suggest how this information can guide new cognitive and pharmacotherapies for urologic disorders.
The clinical consideration of pelvic visceral disorders is often exclusively directed towards specific viscera. However, the activity of these viscera must be coordinated with each other and they do not function ideally independently of the brain. The coexistence of multiple pelvic visceral symptoms and their frequent co-morbidity with psychiatric symptoms highlights the interrelationships between the brain and pelvic viscera1–8. Underlying these interrelationships are reciprocal endocrine and neural lines of communication. In the case of the bladder, reciprocal communication allows for bladder status to be communicated to brain nuclei that serve to increase arousal, redirect attention and modify behavior so it is best coordinated with visceral functions. In this way elimination behaviors can be coordinated with bladder emptying. Conversely, the brain is poised to directly modify visceral activity so that it is best suited to adaptively respond to a changing environment, for example, in response to stress or when urination is used to mark territory or as a part of a sexual repertoire9. These same reciprocal lines of communication that are adaptive can provide routes through which visceral pathology can affect brain activity and conversely, changes in brain activity can impact on the viscera. Despite the physiological importance of communication between the brain and bladder and the role this can play in urological disorders, our understanding of the underlying mechanisms for this is sparse.
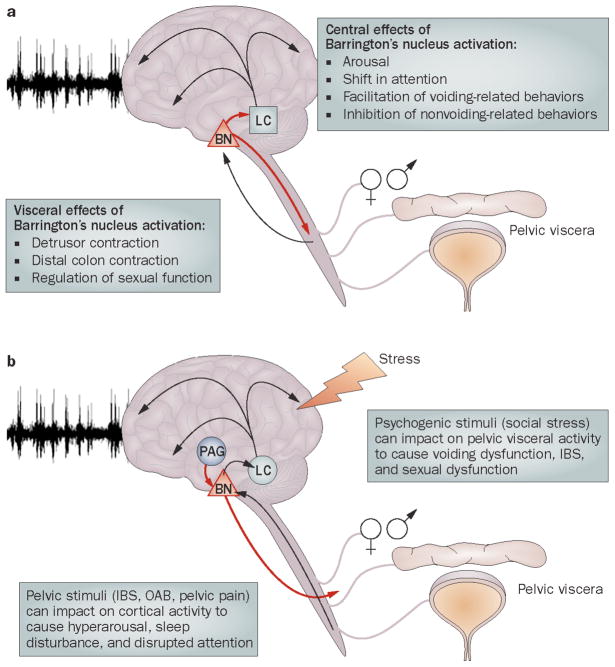
One circuit that underlies communication between the brain and pelvic viscera is centered about Barrington’s nucleus, the pontine micturition center. Initially, considered as the switch that initiates the descending limb of the micturition reflex, Barrington’s nucleus neurons that project to bladder motoneurons were found to diverge to innervate the locus coeruleus (LC), the major norepinephrine-containing brain nucleus10. LC neurons, in turn, collateralize extensively throughout the forebrain to densely innervate the cortex and through this projection system LC activation increases arousal and shifts the mode of attention11–14. This structural organization positions Barrington’s neurons to co-regulate the parasympathetic tone to the bladder and norepinephrine tone to the cortex, providing a mechanism for coordinating elimination behaviors with bladder voiding.
A notable characteristic of Barrington’s nucleus neurons is their high expression of the stress-related neuropeptide, corticotropin-releasing factor (CRF)15–17. CRF was first characterized as the hypothalamic neurohormone released in response to stressors to initiate the endocrine limb of the stress response18. CRF also acts as a brain neurotransmitter that is released from neurons in limbic brain regions to mediate behavioral responses to stress19. The high CRF expression by Barrington’s nucleus neurons has been speculated to play a role in regulation of bladder or colonic motility as a component of the stress response20. Given its prominence in this pathway that can coordinate central and pelvic visceral functions, clarifying the actions of CRF could pave the way for the development of novel pharmacological means for treating pelvic visceral disorders.
Here we review convergent anatomical and physiological evidence for the Barrington’s nucleus network that links the brain and bladder and discuss its role in coordinating a behavioral limb of the micturition reflex with the descending limb. Recent findings demonstrating that partial bladder outlet obstruction adversely affects cortical activity and in turn that psychosocial stress induces voiding dysfunction and bladder pathology through this same circuitry are presented. Evidence for a role of Barrington’s nucleus CRF in pelvic visceral disorders and as a target for treating these disorders is discussed.
Barrington’s Nucleus Circuit
Descending limb of the micturition reflex
Early studies in cats and rats identified a micturition center in the dorsal pons by demonstrating that lesions here abolished micturition and conversely, electrical stimulation of the same region elicited bladder contractions and voiding21–24. Anatomical tracing studies more precisely localized the area critical to micturition as an oval cluster of neurons just ventral to the fourth ventricle25,26. Anterograde tracers injected into Barrington’s nucleus labeled axon terminals in the preganglionic parasympathetic column of the lumbosacral spinal cord in the vicinity of neurons that innervate the bladder27. Consistent with this, Barrington’s nucleus neurons were retrogradely labeled from the lumbosacral spinal cord15,17. Chemical stimulation of Barrington’s nucleus, which has more regional precision compared to electrical stimulation, elicited bladder contraction and provided confirming physiological identification of Barrington’s nucleus as a micturition center22,23. Barrington’s nucleus neurons become activated as bladder pressure increases and they receive bladder afferent information both directly from the spinal cord as well as from the periaqueductal gray region28–30. The input from the periaqueductal gray is particularly relevant to the effects of social stress on Barrington’s nucleus regulation of micturition (see below).
Co-regulation of multiple pelvic visceral functions
The initial anatomical and physiological studies described above identified the role of Barrington’s nucleus as a switch in the micturition reflex. However, more complex functions for Barrington’s nucleus were revealed through the use of pseudorabies virus transsynaptic tracing to identify neurons that are synaptically linked to viscera. These studies indicated that Barrington’s nucleus neurons were synaptically linked to (and thereby able to influence) the distal colon and genitals, as well as the bladder31–34. Of particular clinical relevance, the majority of Barrington’s nucleus neurons are synaptically linked to both the bladder and distal colon allowing for co-regulation of both viscera31 (Fig. 1). In contrast, few Barrington’s nucleus neurons are transsynaptically linked to the stomach, suggesting that brain regulation of the distal colon is more likely to be coordinated with the bladder (another pelvic viscera) rather than other gastrointestinal-related viscera31. The finding that the same Barrington’s nucleus neurons are linked to both bladder and colon may seem at odds with the idea that these viscera should function in a reciprocal manner rather than be activated simultaneously. However, this is consistent with embryologic development as the bladder and rectum both arise from the same hindgut that undergoes a coronal partition by the insertion of the urorectal septum by 6 weeks of fetal development35. Unlike the bladder, distal colon motility is also regulated by multiple factors such as propulsive forces from proximal levels of the gastrointestinal tract that are under vagal control and these factors likely exert a greater influence over distal colonic motility compared to the spinal parasympathetic tone regulated by Barrington’s nucleus. Nonetheless, co-activation of both bladder and colon may occur under certain conditions, such as stress. Importantly, many Barrington’s nucleus neurons are activated by both bladder and colonic distention of non-noxious magnitudes, indicating that bladder and colonic information converge onto common Barrington’s nucleus neurons28. This provides a means through which multiple pelvic visceral symptoms can coexist, i.e., irritable bowel with irritable bladder1,4,5,36.
Figure 1.
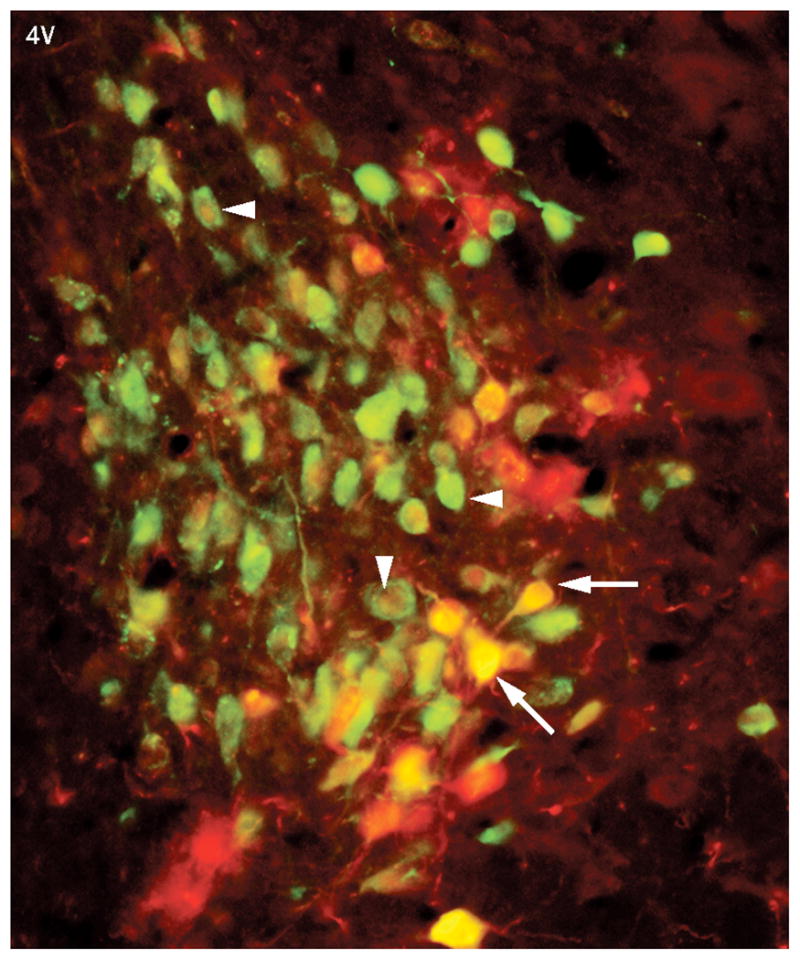
Barrington’s nucleus neurons are transsynaptically-linked to both the bladder and colon. Fluorescent photomicrograph of a rat brain section at the level of Barrington’s nucleus labeled with pseudorabies virus expressing green fluorescent protein that was injected into the bladder (green) and pseudorabies virus expressing β-galactosidase (red) that was injected into the distal colon. Many Barrington’s nucleus neurons are labeled with both viruses. These appear as either yellow or orange (arrows) or cells the have a red nucleus and green cytoplasm (arrowhead). Reproduced from reference 31 with permission from John Wiley and Sons, LTD).
Central limb of the micturition reflex
The perspective of Barrington’s nucleus was changed by the finding that many Barrington’s nucleus neurons that project to the spinal cord also project to the major norepinephrine-containg brain nucleus, the locus coeruleus (LC)10. This finding gave Barrington’s nucleus neurons a more global function in the regulation of micturition. The LC is a cluster of norepinephrine-containing neurons that serves as the major source of norepinephrine in the brain11,12,37. These cells have massive collateralized projections that innervate the entire neuraxis, and particularly the forebrain12. The LC-norepinephrine system initiates and maintains arousal in response to stimuli and facilitates shifts between scanning and focused modes of attention13,14,38. LC neurons are activated by multimodal sensory stimuli and importantly, by visceral stimuli such as non-noxious levels of bladder and colon distention39–43. LC activation by these pelvic visceral stimuli is temporally correlated to cortical electroencephalographic (EEG) indices of arousal (e.g., desynchronization and a shift from high amplitude, low frequency activity to high frequency activity)42,43. This provides the means by which increases in bladder or colon pressure can initiate arousal and shift the focus of attention such that behaviors unrelated to these stimuli will be interrupted and behaviors compatible with the stimuli will be facilitated. Thus, Barrington’s nucleus neurons are central to coordinating the descending limb of the micturition reflex with a central limb that facilitates a switch from on-going non-voiding related behavior to voiding behaviors. As the same neurons are excited by increases in colonic intraluminal pressure, this circuit likely facilitates behaviors related to defecation as well42. Barrington’s nucleus with its divergent projections to spinal cord and the LC is poised to integrate ongoing signals from multiple pelvic viscera and to coordinate activity of each of these viscera with activity in forebrain regions that underlies behavior (Fig. 2A).
Figure 2.
Schematic depicting the circuitry centered around Barrington’s nucleus that links the brain and pelvic viscera. A. Signals from pelvic viscera are conveyed to Barrington’s nucleus either directly or indirectly. Barrington’s nucleus modulates visceral activity via spinal efferents and cortical activity via its projections to the locus coeruleus. B. Through the same circuitry signals related to pathology in the pelvic viscera can be relayed to the cortex via Barrington’s nucleus projections to the LC. Psychogenic stimuli, such as social stress can potentially impact on the pelvic viscera through projections from the periaqueductal gray or other afferents to Barrington’s nucleus. Abbreviations: IBS (irritable bowel syndrome), LC (locus coeruleus) and OAB (Overactive bladder).
Corticotropin-releasing factor
Corticotropin-releasing factor serves as a neurohormone and brain neurotransmitter to orchestrate the stress response. CRF release from hypothalamic neurons that terminate in the median eminence initiate the cascade of adrenocorticotropin and corticosteroid secretion that are the hallmark of stress18. CRF in neurons outside of the hypothalamus, particularly in limbic regions that are involved in the expression of emotion serves as a neurotransmitter to mediate emotional, cognitive and behavioral aspects of the stress response19,44. The LC is a target for CRF neurotransmission and in response to stress, CRF is released into the LC from limbic regions to excite LC neurons subsequently resulting in forebrain EEG activation and arousal45–48. This is thought to be an emotional arousal component of the stress response.
CRF is also prominently expressed in Barrington’s nucleus neurons and CRF axons from Barrington’s nucleus terminate within the preganglionic parasympathetic nucleus that gives rise to pelvic innervation, as well as within the LC, indicating that CRF is an important neurotransmitter in this circuit10,17,49 (Fig. 3). The finding of CRF in a circuit regulating micturition is of interest from an evolutionary and phylogenetic standpoint because CRF functioned as an osmoregulatory peptide in early species and CRF analogues are diuretic hormones in insects50,51. As in rat and cat, CRF is prominent in human brain in the region corresponding to Barrington’s nucleus and PET imaging studies have associated this region with micturition52,53.
Figure 3.
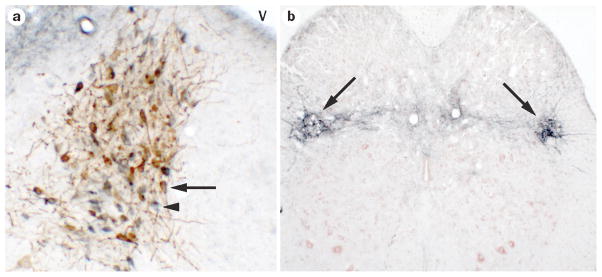
CRF is a major neurotransmitter in Barrington’s nucleus neurons. A. Brightfield photomicrograph of a rat brain section at the level of Barrington’s nucleus showing CRF-immunoreactive neurons (blue) and neurons that are retrogradely labeled with the tracer fluorogold from the lumbosacral spinal cord (brown). Note that most neurons have the hybrid blue/brown color indicating that they are CRF neurons that project to the spinal cord (example indicated by arrow). The arrowhead points to a neuron that is labeled for CRF only. Dorsal is at the top and medial is to the right. V indicates the fourth ventricle. B. Section at the level of the lumbosacral spinal cord showing dense CRF immunoreactive terminal fields (blue) in the region of the preganglionic parasympathetic neurons (arrows). The section is counterstained with Neutral Red. Dorsal is at the top.
The CRF-expressing neurons of Barrington’s nucleus respond to both bladder and colon distention28. In Barrington’s nucleus projections to the LC, CRF is excitatory, activating LC neurons in response to pelvic visceral stimuli and in turn, eliciting cortical arousal42,54. Thus, these CRF neurons are an important step in signaling pelvic visceral information to the cortex thereby initiating behavioral responses to these stimuli. Within spinal projections, Barrington’s nucleus CRF has been speculated to have excitatory effects on colonic motoneurons, perhaps contributing to stress-related increases in colonic motility and symptoms of irritiable bowel syndrome20,55. In vivo cystometry studies examining the effect of CRF or CRF antagonists administered systemically or intrathecally suggest both excitatory and inhibitory effects of CRF on micturition56–58. These discrepant results may be due to the use of different strains and sexes. Additionally, the state of arousal, which is difficult to standardize between laboratories, is an important determinant of urodynamic endpoints and of the effect of CRF58. An important consideration in interpreting the role of CRF in urodynamics from cystometry studies is that cystometry endpoints represent the net effect of afferent and efferent neural activity and activity in bladder urothelium. CRF is present multiple sites that could influence these endpoints, including the urothelium and dorsal horn of the spinal cord, as well as in spinal projections of Barrington’s nucleus neurons that regulate the micturition reflex59–61. The specific role of CRF in Barrington’s nucleus spinal projections has been directly studied using recordings of bladder pressure during localized chemical stimulation of Barrington’s nucleus neurons. These studies support an inhibitory influence of CRF in this spinal pathway because blocking CRF effects at the level of the spinal cord increased the Barrington’s nucleus stimulated contractions22. The inhibitory influence of CRF in these projections may be through a direct action on preganglionic parasympathetic neurons or by inhibition of excitatory amino acid neurotransmitters from Barrington’s nucleus that excite preganglionic parasympathetic neurons22.
An inhibitory influence of CRF on the bladder may seem counterintuitive as urination is often considered a response to fear, which is often equated with stress. However, urination is inhibited in response to certain stressors. Particularly, urinary retention is a prominent response to social stress in rodents where it is likely to be adaptive (see below). Taken with the ability of CRF to engage the LC-cortical pathway and initiate arousal in response to pelvic visceral stimuli, the inhibitory influence in spinal cord may help to maintain continence by allowing arousal to occur prior to bladder contraction (Fig. 4). If bladder contraction and cortical arousal occurred simultaneously, there would be insufficient time to interrupt ongoing behavior and mobilize motor systems for voiding behaviors. By creating a lag between arousal and the visceral response, continence can be maintained. Although speculative at this point, this putative role for CRF in continence should be well researched, given the prevalence of disorders of continence and their cost to the individual and society.
Figure 4.
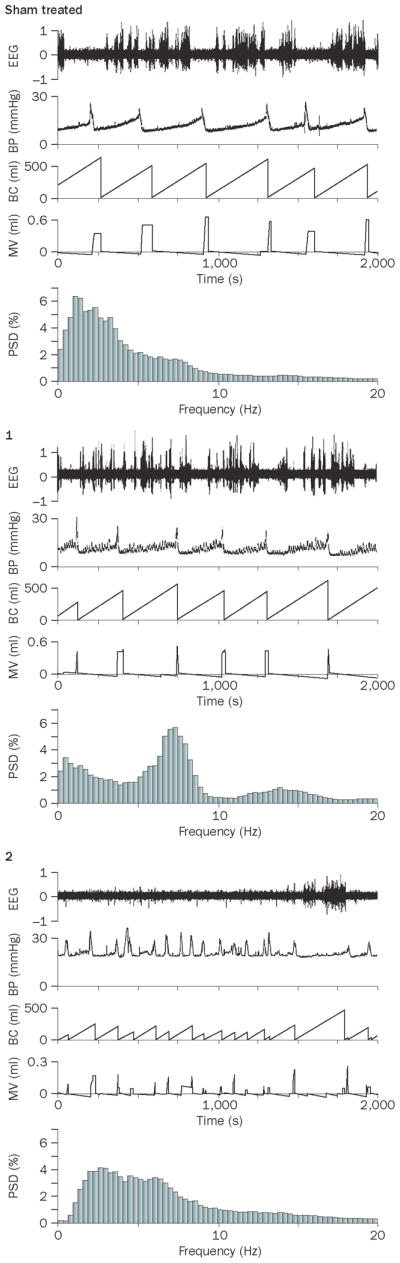
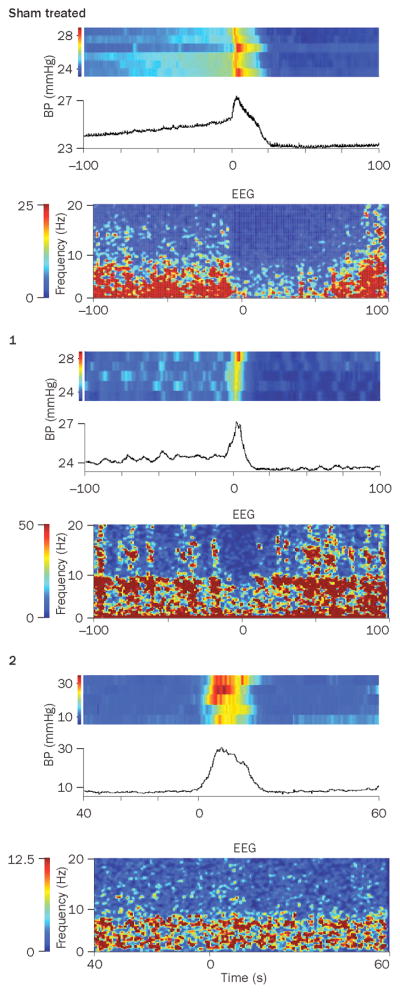
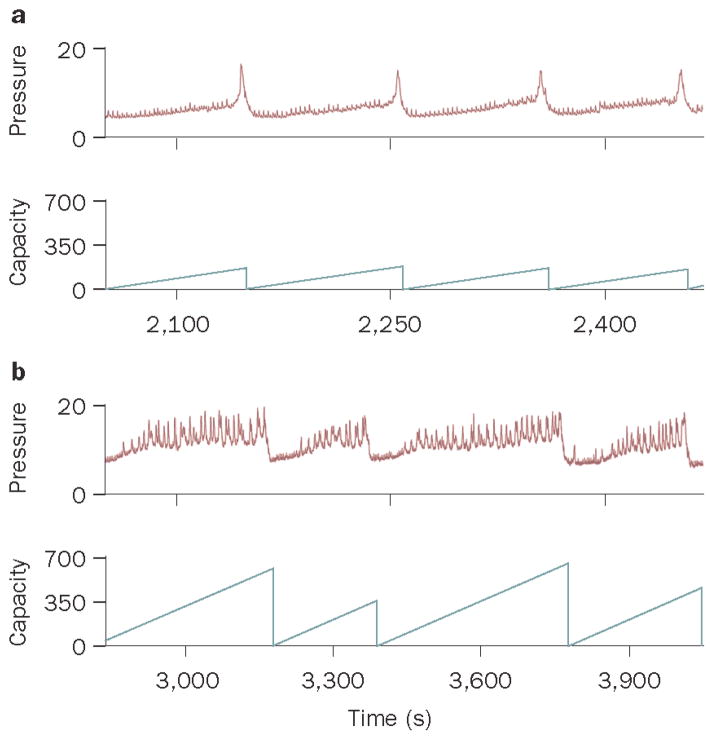
Effect of partial bladder obstruction on the relationship between bladder pressure and cortical EEG. A. Simultaneous EEG and cystometry recordings from sham and obstructed rats. From top to bottom, the traces show raw EEG, bladder pressure (BP, mm Hg), bladder capacity (BC, ml), micturition volume (MV, ml), and EEG power spectrum (PSD). An obstructed rat (1) has a similar urodynamic pattern as the sham rat with the exception of spontaneous non-voiding contractions but has a distinct EEG pattern characterized by a peak at 7.5 Hz (theta oscillation). Obstructed rat 2 does not exhibit non-voiding contractions, but shows increased micturition frequency. In this case the EEG is characterized by lower amplitude and a shift towards higher frequencies. B1 and B2 show peri-event spectrograms generated from the same subjects as in A, indicating how power in different EEG frequency bands co-varies with bladder pressure during individual micturition cycles. In B1, the traces represent mean bladder pressure over 4–5 micturition cycles and centered at the micturition threshold (time=0). The heat map above each trace represents bladder pressure for each micturition cycle. For the sham rat, a uniform increase in bladder pressure up to micturition threshold can be seen. In contrast, for Obstructed (1) non-voiding contractions are indicated in the heat map as sporadic episodes (lighter blue blocks interspersed within darker blue) that occur up to the point at which micturition threshold is reached. Obstructed (2) does not exhibit non-voiding contractions. B2 shows heat maps that indicate the mean relative power in different EEG frequency bands (0–20 Hz, ordinate) and these are time-locked with B1, such that time=0 indicates the point of the micturition threshold. Note how the different patterns of bladder activity produced by obstruction impact on the relationship between bladder pressure and cortical EEG activity. In sham rats a decrease in power in all frequencies (i.e., desynchronization) precedes the micturition threshold and is maintained. Obstructed 1 with non-voiding contractions has greater power in higher frequencies (7–10 Hz and 14–15 Hz) that fluctuate like the contractions. In Obstructed 2 that has frequent micturition cycles and no non-voiding contractions, cortical EEG is desynchronized throughout the session and increases in bladder pressure up to the micturition threshold are without effect. Reproduced with permission from reference 63.
Central Symptoms of Bladder Pathology
Partial bladder outlet obstruction
The findings discussed above highlight the importance of the Barrington’s nucleus circuit in coordinating behavior with visceral activity. However, the same reciprocal lines of communication can act as a conduit to convey signals from pathologic events occurring in viscera to the brain, thereby providing a structural basis for neurobehavioral sequelae of pelvic visceral disorders (Fig. 2B). This was recently investigated in the rat model of partial bladder outlet obstruction (PBOO), an animal model that has been used to examine the consequences of outlet obstruction on bladder function and structure62. An initial consequence of PBOO on the Barrington’s nucleus circuit was the loss of responses of Barrington’s nucleus and LC neurons to increases in bladder pressure to micturition threshold63. This is relevant to the ongoing visceral pathology as this represents a loss of central regulation of bladder function. Analogous to spinal cord injury, the loss of central control could contribute to the development of local spinal regulation of the micturition reflex that is thought to underlie bladder hyperactivity33. Thus, consequences of PBOO on Barrington’s nucleus neuronal activity may feed-forward to further contribute to the bladder dysfunction. Importantly, this can account for the finding that some individuals can have chronic retention and enlarged bladder, yet no sensation or urge to urinate.
Although LC neurons of PBOO rats were not activated by acute increases in bladder pressure up to micturition threshold, their spontaneous rate of discharge was tonically elevated compared to rats with sham surgery63. Theories of the function of the LC-norepinephrine system in relation to behavior suggest a role in facilitating decisions related to task-directed behavior, i.e., whether to maintain behavior in an ongoing task or to disengage and seek alternative strategies in a dynamic environment13. Tonic LC activation favors disengagement from ongoing behavior and tasks involving focused attention and promotes scanning of the environment for alternate strategies. With this perspective, the transient tonic excitation of LC neurons elicited as bladder pressure rises towards micturition threshold in control rats is speculated to be a central limb of the micturition reflex that serves to increase arousal and facilitate disengagement from ongoing behavior and a shift to elimination-related behaviors. The persistent elevation of LC discharge in subjects with PBOO would be associated with hyperarousal, sleep disorders, altered attention and inability to stay on task, effects that would disrupt normal behavioral function.
Importantly, the effects of PBOO on LC activity and responses to bladder distention are reflected at the level of the cortical EEG as desynchronization and a shift from high amplitude, low frequency activity to low amplitude, high frequency activity, effects indicative of hyperarousal63. A salient feature of the PBOO EEG is a theta frequency oscillation that is particularly prominent in subjects with a urodynamic profile characterized by numerous non-voiding contractions63. Theta oscillations are involved in sensorimotor integration and are hypothesized to coordinate activity in different brain regions in preparation for motor responses to sensory input65. Their persistence in overactive bladder may reflect constant or disordered processing of bladder signals. That they are present even when selective responses to the major micturition event are attenuated, may suggest a loss of ability to grade or discriminate between different magnitudes of bladder pressure changes. Importantly, an effect on sensorimotor processing could affect cortical processing of non-bladder related stimuli and adversely impact on functions requiring focused attention. Tonic LC activation is necessary for PBOO effects on cortical EEG activity because selective chemical lesion of forebrain LC projections prevents these effects without altering urodynamics63. The potential role of CRF in PBOO-induced changes in LC activity is a compelling area of research as it could provide a target for therapeutic intervention.
Simultaneous EEG and cystometry recordings are beginning to reveal how bladder and cortical activity co-vary (Fig. 4). These recordings show how even in rat, arousal (as desynchronization) increases with bladder pressure and precedes the voiding event. In subjects with PBOO the relationship between bladder pressure and cortical activity is markedly altered, underscoring the potential disruption of sensory processing.
Clinical translation
The PBOO model exemplifies how pathology that originates in the bladder can have pronounced adverse central consequences through the Barrington’s nucleus circuit. In the clinic, central sequelae of bladder disorders may go unrecognized as urologists focus on bladder complaints, and psychiatrists will not typically link neurobehavioral symptoms to the bladder. Nonetheless, there is evidence for anxiety and sleep disorders, which are associated with tonic LC activation, in men with benign prostatic hypertrophy and in males and females exhibiting other bladder disorders66,67. Notably, attention deficit disorder, which would be produced by excessive activation of the LC-norepinephrine system, is highly associated with enuresis in children68. Cognitive impairments associated with urge incontinence have been reported that may be explained by the Barrington’s nucleus circuit69. Notably, overactive bladder is prevalent in the elderly, a population that is also vulnerable to neurobehavioral deficits and sleep disturbances70. This new model raises the possibility that improvement of bladder symptoms may also improve cognition. Importantly, evidence for a causal role of the LC-NE system suggests that fine-tuning the activity of this system may be a useful therapeutic approach for central sequelae of overactive bladder.
The link between Barrington’s nucleus neurons and other pelvic viscera implies that pathology arising from pelvic viscera other than the bladder could have similar central consequences as those reported for bladder. For example, this circuit may underlie the well-documented co-morbidity of psychiatric and colonic symptoms that characterize irritable bowel disorder2,3,36.
Social stress-induced bladder dysfunction
The Barrington’s nucleus circuitry that allows bladder pathology to adversely affect cortical activity provides a structure through which psychogenic stimuli can affect the pelvic viscera to result in voiding dysfunctions. Regulating pelvic visceral activity in response to sexual signals, predators and social hierarchy is an adaptive brain function in animals that has been conserved through evolution. Importantly, social stress or competition in male rodents causes urinary retention that is likely to be adaptive9,71–73. However, if the urinary retention persists, this results in bladder enlargement and in some cases death from nephritis ensues71. In a rodent model of social stress (the resident-intruder model), subordinate rats develop a urodynamic profile that resembles that produced by PBOO, with increased intermicturition interval, bladder capacity and micturition volume and numerous non-micturition contractions (Fig. 5)74. The urodynamic profile associated with social stress is distinct from that of PBOO by its lack of elevated micturition pressure, implying that it results from a loss of central drive to the detrusor as opposed to increased tone to the sphincter. Like PBOO, social stress increases bladder mass and the magnitude of this effect is negatively correlated to the latency to become subordinate, suggesting that subjects that are prone to claim defeat in a social conflict are more vulnerable to bladder dysfunction. Urinary retention may be a visceral component of a defensive response that is mediated by the periaqueductal gray, a brain structure that organizes defensive behaviors and is a major input to Barrington’s nucleus75,76.
Figure 5.
Social stress alters bladder urodynamics. A and B show representative cystometry recordings of bladder pressure and bladder capacity of a control rat (A) and a rat that was exposed to the resident-intruder stress for 7 consecutive days (B). Note that the bladder of the stressed rat shows numerous non-micturition contractions, longer intermicturition intervals and greater bladder capacity. (Reproduced from ref 74, Wood et al., Am J Physiol Regul Integr Comp Physiol, 2009 with permission of the American Physiological Society).
In line with increased bladder mass, social stress upregulates some of the same transcription factors that are upregulated by PBOO and are thought to be important in structural remodeling of the bladder during PBOO, including NFAT and MEF277. This is a striking example of how a social signal can induce visceral restructuring that has important biological, as well as clinical implications.
Given the role of CRF in stress and its inhibitory influence in Barrington’s nucleus projections to bladder motoneurons, this was speculated to underlie social stress-induced voiding dysfunction. Consistent with this, social stress upregulates CRF protein and mRNA specifically in Barrington’s nucleus neurons, an effect that can account for the urinary retention74. Notably, repeated restraint stress does not produce urinary dysfunction, nor does this stressor increase CRF protein or mRNA in Barrington’s nucleus neurons, indicating that the visceral dysfunction may be specific to social stress and further implicating CRF in Barrington’s nucleus in this dysfunction. That the effects on bladder may be specific to social stress is not surprising given that different stressors engage distinct circuits in brain and can have very different effects on transcription in the same neurons78,79. Interesting, social stress would be expected to engage brain regions involved in defensive responses such as the periaqueductal gray region, which has prominent projections to Barrington’s nucleus76,80.
Clinical translation
The social stress-induced voiding dysfunction in the rodent may model dysfunctional voiding characterized by urinary retention, a problem seen in both children and adults of both sexes and in the elderly81–85. Dysfunctional voiding remains the most common presentation to pediatric urologists86. In children dysfunctional voiding associated with detrusor hypoactivity has been hypothesized to arise from adverse events that are temporally proximal to toilet training87,88. Consistent with this, children with voiding postponement have a less balanced family environment and more psychiatric co-morbidity compared to children with other types of voiding dysfunction89. The impact of childhood social stress on bladder function can extend into adulthood and childhood sexual or physical abuse are associated with urinary disorders characterized by retention in adults90,91. In adults urinary retention has been associated with a history of sexual abuse, depression and social anxiety6,90. As in the rat model, the persistence of retention can have severe structural consequences and proceed to chronic renal insufficiency82. Even in the absence of such severe consequences, the importance of the impact of social stress on urological health is underscored by a recent study of over 1000 women demonstrating statistically significant associations between urinary incontinence and psychosocial problems with feelings of vulnerability 92.
To date the underlying mechanisms of psychogenic voiding disorders or the ability of social stress to adversely affect bladder function have remained unknown, making treatment options difficult. Evidence for an involvement of CRF in the Barrington’s nucleus circuit suggests that manipulation of this peptide may provide a target for the treatment of these disorders.
Summary and Conclusions
The divergent projections of Barrington’s nucleus neurons to the major brain norepinephrine nucleus, LC and the lumbosacral spinal preganglionic neurons that give rise to parasympathetic innervation of the pelvic viscera provide a mechanism for coordinating behavior with pelvic visceral activity. The same structural organization allows signals from dysfunctional viscera to change cortical activity and produce neurobehavioral effects that are co-morbid with visceral pathology. Similarly, psychogenic stimuli, particularly social stress, can produce pelvic visceral dysfunctions by affecting components of the same circuit. With specific regard towards the bladder, this circuit helps to organize voiding behaviors with elimination. At the same time, it provides a means by which bladder disorders can produce hyperarousal, anxiety and inattentiveness and by which social events can produce a dysfunctional bladder. Because the stress-related neuropeptide, CRF, is an important component of this circuit, it may be a novel target for the treatment of urological disorders, particularly those with a psychogenic component. The findings reviewed underscore the importance of considering central components in the etiology of urological disorders, as well as being part of the symptom complex. Elucidating the mechanisms through which the brain and bladder communicate would provide a more holistic and effective approach to the treatment of urinary disorders in patients and offer new avenues for the development novel agents in treating these disorders.
Acknowledgments
The authors acknowledge funding through an NIDDK George O’Brien Center Grant (PO5-DK052620-13) that was essential in facilitating discussions and collaborations between the neuroscientists and urologists involved in this review.
Footnotes
The authors have no competing interests as defined by Nature Publishing Group or other interests that might be perceived to influence the interpretation of the article.
References
- 1.Longstreth GF. Irritable bowel syndrome and chronic pelvic pain. Obstetrical and Gynecological Survey. 1994;49:505–507. doi: 10.1097/00006254-199407000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Lydiard RB, Fossey MD, Marsh W, Ballenger JC. Prevalence of psychiatric disorders in patients with irritable bowel syndrome. Psychosomatics. 1993;34:229–234. doi: 10.1016/S0033-3182(93)71884-8. [DOI] [PubMed] [Google Scholar]
- 3.Lydiard RB, et al. Panic disorder and gastrointestinal symptoms: findings from the NIMH epidemiologic catchment area project. Am J Psych. 1994;151:64–70. doi: 10.1176/ajp.151.1.64. [DOI] [PubMed] [Google Scholar]
- 4.Whorwell PJ, Lupton EW, Erduran D, Wilson K. Bladder smooth muscle dysfunction in patients with irritable bowel syndrome. Gut. 1986;27:1014–1017. doi: 10.1136/gut.27.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1987;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry S, McGrother CW, Turner K. An investigation of the relationship between anxiety and depression and urge incontinence in women: development of a psychological model. Br J Health Psychol. 2006;11:463–482. doi: 10.1348/135910705X60742. [DOI] [PubMed] [Google Scholar]
- 7.Von Gontard A, Hollmann E. Comorbidity of functional urinary incontinence and encopresis: somatic and behavioral associations. J Urol. 2004;171:2644–2647. doi: 10.1097/01.ju.0000113228.80583.83. [DOI] [PubMed] [Google Scholar]
- 8.Baeyens D, et al. Attention deficit/hyperactivity disorder in children with nocturnal enuresis. J Urol. 2004;171:2576–2579. doi: 10.1097/01.ju.0000108665.22072.b2. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 10.Valentino RJ, Chen S, Zhu Y, Aston-Jones G. Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington’s nucleus to the locus coeruleus and spinal cord. Brain Res. 1996;732:1–15. doi: 10.1016/0006-8993(96)00482-9. [DOI] [PubMed] [Google Scholar]
- 11.Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Brain. Academic Press; 1995. pp. 183–213. [Google Scholar]
- 12.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat using dopamine-B-hydroxylase as a marker. J Comp Neurol. 1976;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 13.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 14.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 15.Valentino RJ, Pavcovich LA, Hirata H. Evidence for corticotropin-releasing hormone projections from Barrington’s nucleus to the periaqueductal gray region and dorsal motor nucleus of the vagus in the rat. J Comp Neurol. 1995;363:402–422. doi: 10.1002/cne.903630306. [DOI] [PubMed] [Google Scholar]
- 16.Sakanaka M, Shibasaki T, Lederes K. Corticotropin-releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxide-diaminobenzidene method. J Comp Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- 17.Vincent SR, Satoh K. Corticotropin-releasing factor (CRF) immunoreactivity in the dorsolateral pontine tegmentum: further studies on the micturition reflex system. Brain Res. 1984;308:387–391. doi: 10.1016/0006-8993(84)91085-0. [DOI] [PubMed] [Google Scholar]
- 18.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 19.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Revs. 1991;43:425–474. [PubMed] [Google Scholar]
- 20.Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: Pharmacological target for pelvic visceral dysfunctions. Trends Pharmacol Sci. 1999;20:253–266. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- 21.Noto H, Roppolo JR, Steers WD, De Groat WC. Excitatory and inhibitory influences on bladder activity elicited by electrical stimulation in the pontine micturition center in the rat. Brain Res. 1989;492:99–115. doi: 10.1016/0006-8993(89)90893-7. [DOI] [PubMed] [Google Scholar]
- 22.Pavcovich LA, Valentino RJ. Central regulation of micturition in the rat by corticotropin- releasing hormone from Barrington’s nucleus. Neurosci Lett. 1995;196:185–188. doi: 10.1016/0304-3940(95)11873-u. [DOI] [PubMed] [Google Scholar]
- 23.Willette RN, Morrison S, Sapru HN, Reis DJ. Stimulation of opiate receptors in the dorsal pontine tegmentum inhibits reflex contraction of the urinary bladder. J Pharmacol Exp Ther. 1988;244:403–409. [PubMed] [Google Scholar]
- 24.Barrington FJT. The effect of lesion of the hind- abnd mid-brain on micturition in the cat. Quart J Exp Physiol. 1925;15:81–102. [Google Scholar]
- 25.Hida T, Shimazu N. The interrelation between the laterdorsal tegmental area and lumbosacral segments of rats as studied by HRP method. Arch Histol Jap. 1982;45:495–504. doi: 10.1679/aohc.45.495. [DOI] [PubMed] [Google Scholar]
- 26.Loewy AD, Saper CB, Baker RP. Descending projections from the pontine micturition center. Brain Res. 1979;172:533–538. doi: 10.1016/0006-8993(79)90584-5. [DOI] [PubMed] [Google Scholar]
- 27.Blok BFM, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to parasympathetic preganglionic motoneurons of the bladder of the cat. Neurosci Lett. 1997;222:195–198. doi: 10.1016/s0304-3940(97)13384-5. [DOI] [PubMed] [Google Scholar]
- 28.Rouzade-Dominguez ML, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington’s nucleus neurons to pelvic visceral stimuli: a juxtacellular labeling study. Eur J Neurosci. 2003;18:3325–3334. doi: 10.1111/j.1460-9568.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- 29.Ding YQ, et al. Direct projections from lumbosacral spinal cord to Barrington’s nucleus in the rat: a special reference to micturition reflex. J Comp Neurol. 1997;389:149–160. doi: 10.1002/(sici)1096-9861(19971208)389:1<149::aid-cne11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Blok BFM, De Weerd H, Holstege G. Ultrastructural evidence for a paucity of projections from the lumbosacral cord to the pontine micturition center or M-region in the cat: a new concept for the organization of the micturition reflex with the periaqueductal gray as a central relay. J Comp Neurol. 1995;359:300–309. doi: 10.1002/cne.903590208. [DOI] [PubMed] [Google Scholar]
- 31.Rouzade-Dominguez ML, Miselis R, Valentino RJ. Central representation of bladder and colon revealed by dual transsynaptic tracing: substrates for pelvic visceral coordination. Eur J Neurosci. 2003;18:3311–3324. doi: 10.1111/j.1460-9568.2003.03071.x. [DOI] [PubMed] [Google Scholar]
- 32.Marson L. Central nervous system neurons identified after injection of pseudorabies virus into the rat clitoris. Neurosci Lett. 1995;190:41–44. doi: 10.1016/0304-3940(95)11495-i. [DOI] [PubMed] [Google Scholar]
- 33.Marson L, McKenna KE. CNS cell groups involved in the control of the ischiocavernosus and bulbospongiosus muscles; a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1996;374:161–179. doi: 10.1002/(SICI)1096-9861(19961014)374:2<161::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Vizzard MA, Brisson M, de Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res. 2000;299:9–26. doi: 10.1007/s004419900128. [DOI] [PubMed] [Google Scholar]
- 35.Lambert SM, Zderic SA. Textbook of Female Urology and Urogynecology. I. Informa healthcare; Essex, UK: 2010. Embryology of the female urogenital system and clinical applications; pp. 172–184. [Google Scholar]
- 36.Guo YJ, et al. Lower urinary tract symptoms in women with irritable bowel syndrome. Int J Urol. 2010;17:175–181. doi: 10.1111/j.1442-2042.2009.02442.x. [DOI] [PubMed] [Google Scholar]
- 37.Grzanna R, Molliver ME. The locus coeruleus in the rat: an immunohistochemical delineation. Neuroscience. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- 38.Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elam M, Thoren T, Svensson TH. Locus coeruleus neurons and sympathetic nerves: activation by visceral afferents. Brain Res. 1986;375:117–125. doi: 10.1016/0006-8993(86)90964-9. [DOI] [PubMed] [Google Scholar]
- 41.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci US A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lechner S, Curtis A, Brons R, Valentino R. Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res. 1997;756:114–124. doi: 10.1016/s0006-8993(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 43.Page ME, Akaoka H, Aston-Jones G, Valentino RJ. Bladder distention activates locus coeruleus neurons by an excitatory amino acid mechanism. Neuroscience. 1992;51:555–563. doi: 10.1016/0306-4522(92)90295-d. [DOI] [PubMed] [Google Scholar]
- 44.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 45.Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Bockstaele EJ, Colago EEO, Valentino RJ. Corticotropin-releasing factor- containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 47.Page ME, Berridge CW, Foote SL, Valentino RJ. Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci Lett. 1993;164:81–84. doi: 10.1016/0304-3940(93)90862-f. [DOI] [PubMed] [Google Scholar]
- 48.Curtis AL, Bello NT, Connally KR, Valentino RJ. Corticotropin-releasing factor neurons of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. J Neuroendocrinol. 2002;14:667–682. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- 49.Valentino RJ, Kosboth M, Winogren ML, Miselis RR. Transneuronal labeling from the rat distal colon: anatomical evidence for regulation of distal colon function by a pontine corticotropin-releasing factor system. J Comp Neurol. 2000;417:399–414. [PubMed] [Google Scholar]
- 50.Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- 51.Denver R. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann N Y Acad Sci. 2009;1163:1–16. doi: 10.1111/j.1749-6632.2009.04433.x. [DOI] [PubMed] [Google Scholar]
- 52.Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V. Corticotropin-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience. 1999;91:1343–1354. doi: 10.1016/s0306-4522(98)00703-9. [DOI] [PubMed] [Google Scholar]
- 53.Blok BFM, Willemsen ATM, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- 54.Rouzade-Dominguez ML, Curtis AL, Valentino RJ. Role of Barrington’s nucleus in the activation of rat locus coeruleus neurons by colonic distension. Brain Res. 2001;917:206–218. doi: 10.1016/s0006-8993(01)02917-1. [DOI] [PubMed] [Google Scholar]
- 55.Pavcovich LA, Yang M, Miselis RR, Valentino RJ. Novel role for the pontine micturition center, Barrington’s nucleus: evidence for coordination of colonic and forebrain activity. Brain Res. 1998;784:355–361. doi: 10.1016/s0006-8993(97)01178-5. [DOI] [PubMed] [Google Scholar]
- 56.Klausner AP, Steers WD. Corticotropin-releasing factor: a mediator of emotional influences on bladder function. J Urol. 2004;172:2570–2573. doi: 10.1097/01.ju.0000144142.26242.f3. [DOI] [PubMed] [Google Scholar]
- 57.Klausner AP, et al. The role of corticotropin releasing factor and its antagonist, astressin, on micturition in the rat. Auton Neurosci. 2005;123:26–35. doi: 10.1016/j.autneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Kiddoo DA, et al. Impact of the State of Arousal and Stress Neuropeptides on Urodynamic Function in the Freely Moving Rat. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00742.2005. [DOI] [PubMed] [Google Scholar]
- 59.Kawatani M, Suzuki T, de Groat WC. Corticotropin-releasing factor-like immunoreactivity in afferent projections to the sacral spinal cord of the cat. J Auton Nerv Sys. 1996;61:218–226. doi: 10.1016/s0165-1838(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 60.LaBerge J, Malley SE, Girard B, Corrow K, Vizzard MA. Postnatal expression of corticotropin releasing factor (CRF) in rat urinary bladder. Auton Neurosci. 2008;141:83–93. doi: 10.1016/j.autneu.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R692–703. doi: 10.1152/ajpregu.00086.2006. [DOI] [PubMed] [Google Scholar]
- 62.Saito M, Longhurst PA, Tammela TL, Wein AJ, Levin RM. Effects of partial outlet obstruction of the rat urinary bladder on micturition characteristics, DNA synthesis and the contractile response to field stimulation and pharmacological agents. J Urol. 1993;150:1045–1051. doi: 10.1016/s0022-5347(17)35683-5. [DOI] [PubMed] [Google Scholar]
- 63.Rickenbacher E, et al. Impact of overactive bladder on the brain: central sequelae of a visceral pathology. Proc Natl Acad Sci U S A. 2008;105:10589–10594. doi: 10.1073/pnas.0800969105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010;29:63–76. doi: 10.1002/nau.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 66.Kirby RS. The natural history of benign prostatic hyperplasia: what have we learned in the last decade? Urology. 2000;56:3–6. doi: 10.1016/s0090-4295(00)00747-0. [DOI] [PubMed] [Google Scholar]
- 67.Tubaro A. Defining overactive bladder: epidemiology and burden of disease. Urology. 2004;64:2–6. doi: 10.1016/j.urology.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 68.Shreeram S, He JP, Kalaydjian A, Brothers S, Merikangas KR. Prevalence of enuresis and its association with attention-deficit/hyperactivity disorder among U.S. children: results from a nationally representative study. J Am Acad Child Adolesc Psychiatry. 2009;48:35–41. doi: 10.1097/CHI.0b013e318190045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Griffiths D. Clinical studies of cerebral and urinary tract function in elderly people with urinary incontinence. Behav Brain Res. 1998;92:151–155. doi: 10.1016/s0166-4328(97)00187-3. [DOI] [PubMed] [Google Scholar]
- 70.Rovner ES, Wein AJ. Incidence and prevalence of overactive bladder. Curr Urol Rep. 2002;3:434–438. doi: 10.1007/s11934-002-0093-5. [DOI] [PubMed] [Google Scholar]
- 71.Henry JP, Meehan WP, Stephens PM. Role of subordination in nephritis of socially stressed mice. Contr Nephrol. 1982;30:38–42. doi: 10.1159/000406416. [DOI] [PubMed] [Google Scholar]
- 72.Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav. 1999;67:769–775. doi: 10.1016/s0031-9384(99)00131-6. [DOI] [PubMed] [Google Scholar]
- 73.Hoshaw BA, Evans JC, Mueller B, Valentino RJ, Lucki I. Social competition in rats: cell proliferation and behavior. Behav Brain Res. 2006;175:343–351. doi: 10.1016/j.bbr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1671–1678. doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- 76.Blok BFM, Holstege G. Direct projections from the periaqueductal gray to the pontine micturition center (M-region). An anterogreade and retrograde tracing study in the cat. Neurosci Lett. 1994;166:93–96. doi: 10.1016/0304-3940(94)90848-6. [DOI] [PubMed] [Google Scholar]
- 77.Chang A, et al. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol. 2009;297:F1101–1108. doi: 10.1152/ajprenal.90749.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 80.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 81.Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology. 2007;69:436–440. doi: 10.1016/j.urology.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 82.Varlam DE, Dippell J. Non-neurogenic bladder and chronic renal insufficiency in childhood. Pediatr Nephrol. 1995;9:1–5. doi: 10.1007/BF00858952. [DOI] [PubMed] [Google Scholar]
- 83.Fenster H, Patterson B. Urinary retention in sexually abused women. Can J Urol. 1995;2:185–188. [PubMed] [Google Scholar]
- 84.Bilanakis N. Psychogenic urinary retention. Gen Hosp Psychiatry. 2006;28:259–261. doi: 10.1016/j.genhosppsych.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Groutz A, Blaivas JG. Non-neurogenic female voiding dysfunction. Curr Opin Urol. 2002;12:311–316. doi: 10.1097/00042307-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 86.Feldman AS, Bauer SB. Diagnosis and management of dysfunctional voiding. Curr Opin Pediatr. 2006;18:139–147. doi: 10.1097/01.mop.0000193289.64151.49. [DOI] [PubMed] [Google Scholar]
- 87.Bauer SB. Special considerations of the overactive bladder in children. Urology. 2002;60:43–48. doi: 10.1016/s0090-4295(02)01793-4. discussion 49. [DOI] [PubMed] [Google Scholar]
- 88.Ellsworth PI, Merguerian PA, Copening ME. Sexual abuse: another causative factor in dysfunctional voiding. J Urol. 1995;153:773–776. [PubMed] [Google Scholar]
- 89.Lettgen B, et al. Urge incontinence and voiding postponement in children: somatic and psychosocial factors. Acta Paediatr. 2002;91:978–984. doi: 10.1080/080352502760272696. discussion 895–976. [DOI] [PubMed] [Google Scholar]
- 90.Davila GW, Bernier F, Franco J, Kopka SL. Bladder dysfunction in sexual abuse survivors. J Urol. 2003;170:476–479. doi: 10.1097/01.ju.0000070439.49457.d9. [DOI] [PubMed] [Google Scholar]
- 91.Romans S, Belaise C, Martin J, Morris E, Raffi A. Childhood abuse and later medical disorders in women. An epidemiological study. Psychother Psychosom. 2002;71:141–150. doi: 10.1159/000056281. [DOI] [PubMed] [Google Scholar]
- 92.Franzen K, Johansson JE, Andersson G, Pettersson N, Nilsson K. Urinary incontinence in women is not exclusively a medical problem: a population-based study on urinary incontinence and general living conditions. Scand J Urol Nephrol. 2009;43:226–232. doi: 10.1080/00365590902808566. [DOI] [PubMed] [Google Scholar]