Abstract
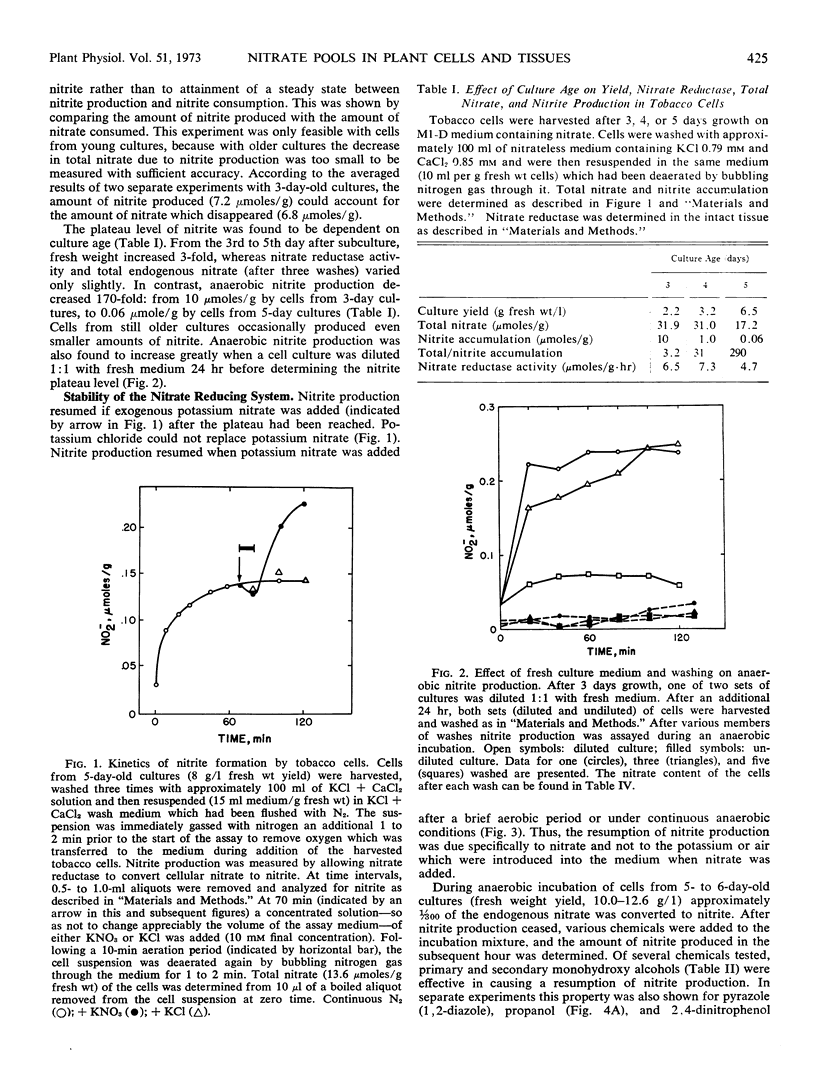
Tobacco (Nicotiana tabacum L. cv. Xanthi) XD cells containing nitrate and nitrate reductase stopped producing nitrite after approximately 1 hour when incubated under anaerobic conditions. The cessation of nitrite production was not due to an inactivation of the nitrate reducing system. This was shown by the ability of the cells to resume anaerobic nitrite production at a rate similar to the initial rate of nitrite production upon exposure to nitrate, monohydroxy alcohols or pyrazole. Cessation of nitrite production also could not be attributed to leakage of nitrate from the cells. Although some nitrate did leak from the cells, most of the nitrate was still in the cells by the time anaerobic nitrite production ceased. We infer the existence of a small metabolic pool and a large storage pool of nitrate, such that nitrite production ceases when the metabolic pool is depleted of nitrate. The metabolic pool of nitrate in tobacco cells decreased 170-fold as the culture aged from 3 to 5 days. However, total cellular nitrate during this period remained relatively constant.
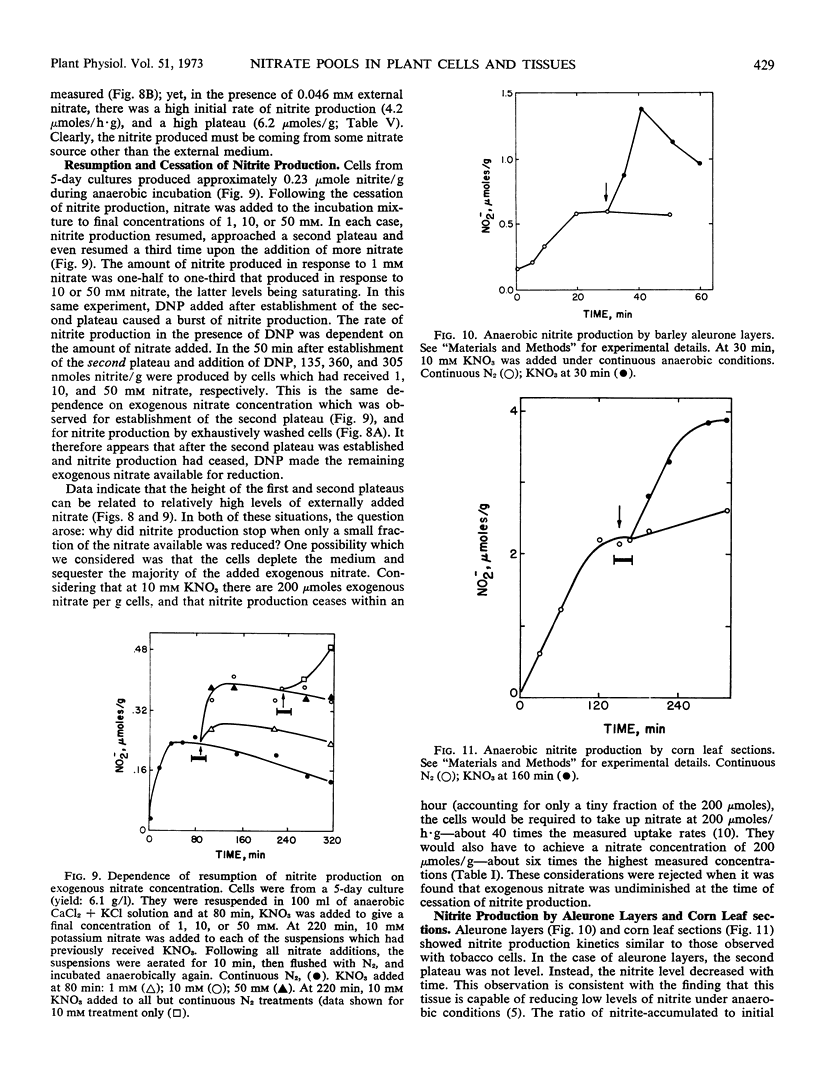
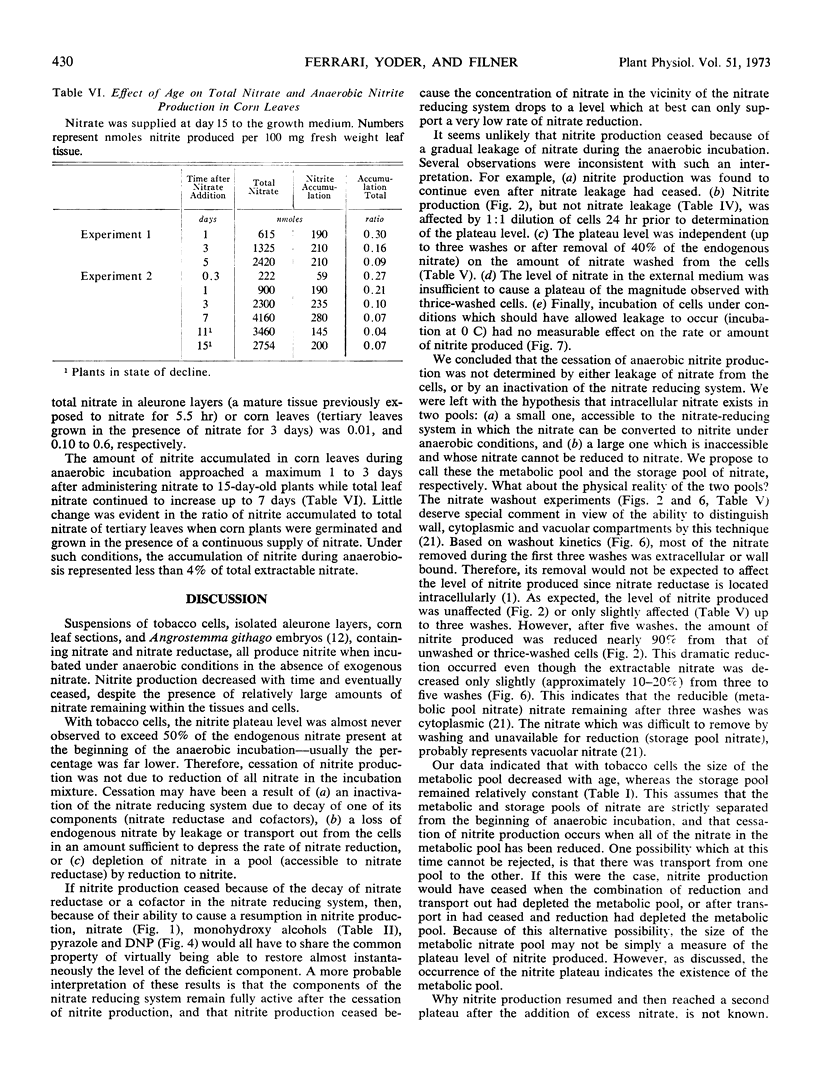
Anaerobic nitrite production by barley (Hordeum vulgare) aleurone layers and corn (Zea mays) leaf sections also ceased after only a small fraction of endogenous nitrate was reduced and resumed again upon addition of exogenous nitrate. In contrast to that found with tobacco cells, the metabolic pool of nitrate in corn leaf sections remained constant with age, while total endogenous nitrate increased. These results were interpreted to mean that higher plants in general contain metabolic and storage pools of nitrate, the properties of which vary with species and physiological variables.
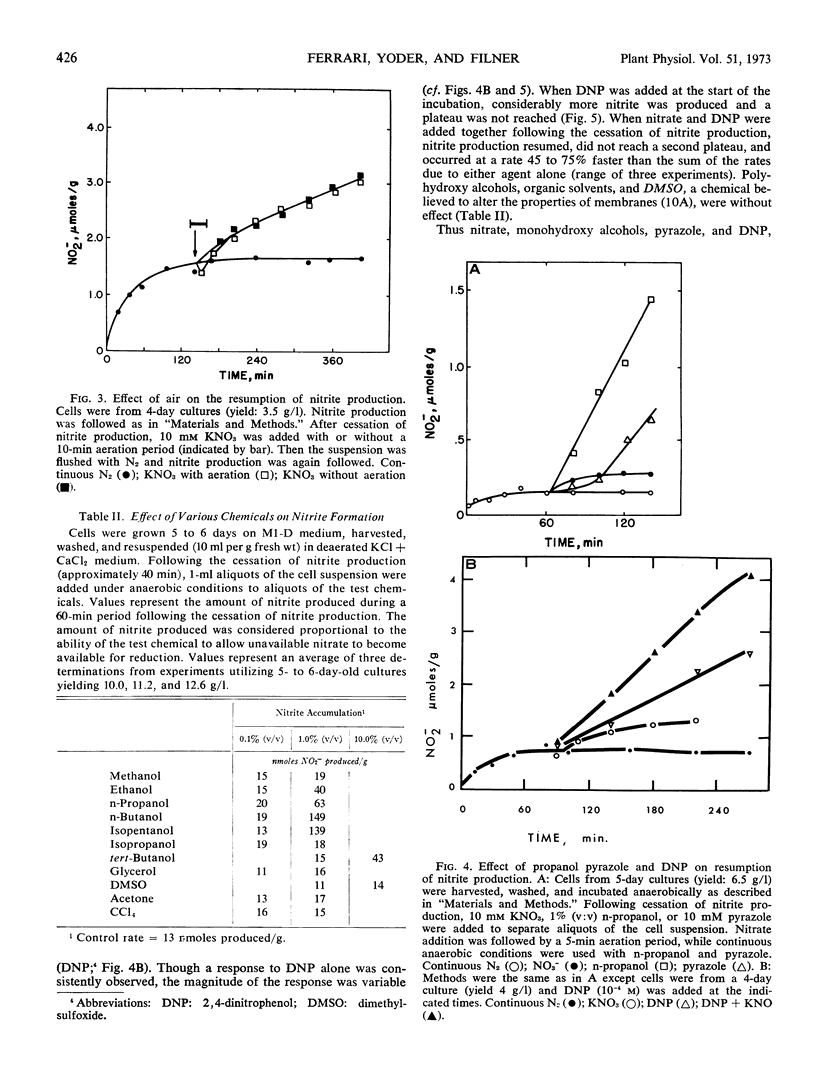
Full text
PDF

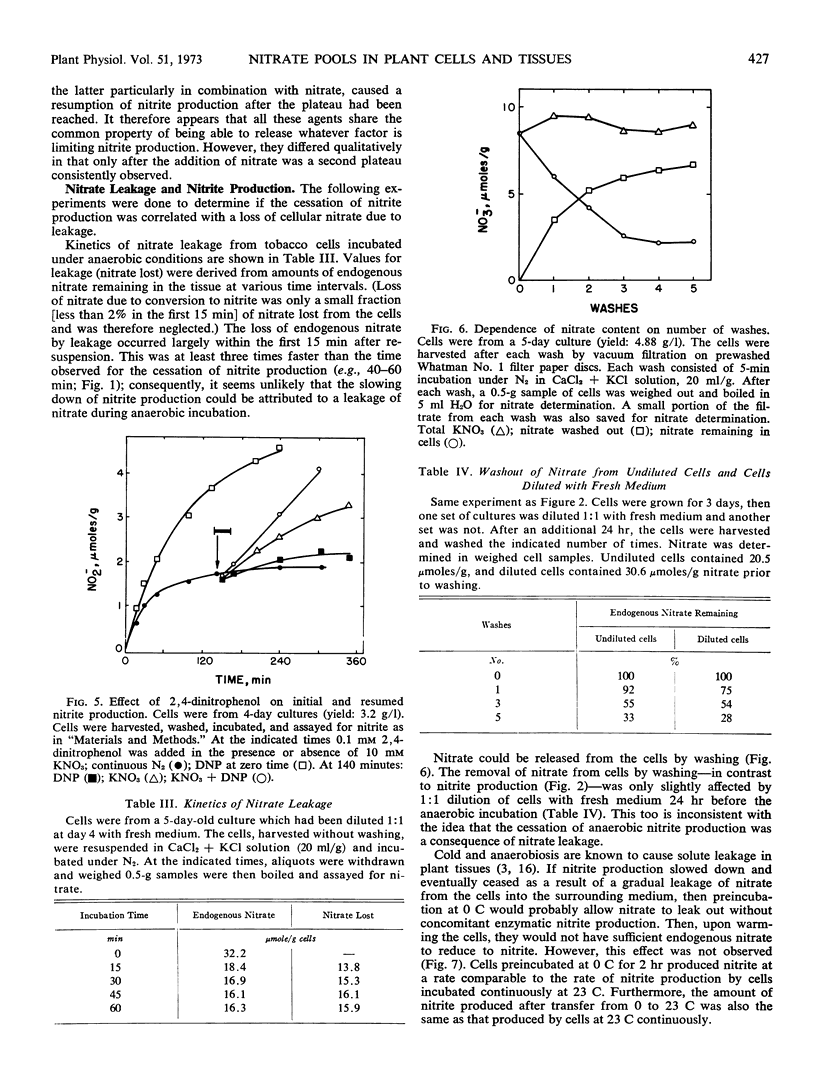

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
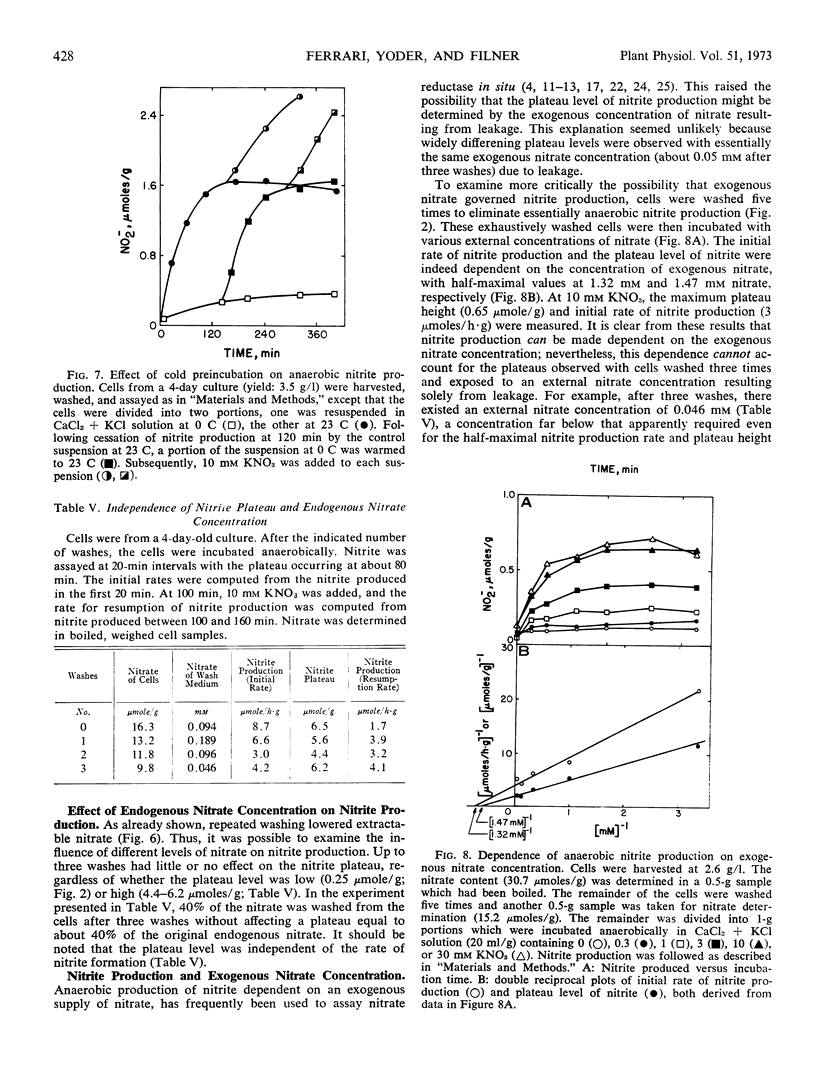
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen M. N., Carns H. R., Slyter D. J. Stimulation of Solute Loss from Radicles of Gossypium hirsutum L. by Chilling, Anaerobiosis, and Low pH. Plant Physiol. 1970 Jul;46(1):53–56. doi: 10.1104/pp.46.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Varner J. E. Control of nitrate reductase activity in barley aleurone layers. Proc Natl Acad Sci U S A. 1970 Mar;65(3):729–736. doi: 10.1073/pnas.65.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Varner J. E. Intact tissue assay for nitrite reductase in barley aleurone layers. Plant Physiol. 1971 Jun;47(6):790–794. doi: 10.1104/pp.47.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- Filner P. Semi-conservative replication of DNA in a higher plant cell. Exp Cell Res. 1965 Aug;39(1):33–39. doi: 10.1016/0014-4827(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway of cultured tobacco cells. II. Properties of a variant cell line. Biochim Biophys Acta. 1970 Jul 21;215(1):152–165. doi: 10.1016/0304-4165(70)90398-3. [DOI] [PubMed] [Google Scholar]
- JACOB S. W., BISCHEL M., HERSCHLER R. J. DIMETHYL SULFOXIDE: EFFECTS ON THE PERMEABILITY OF BIOLOGIC MEMBRANES (PRELIMINARY REPORT). Curr Ther Res Clin Exp. 1964 Mar;6:193–198. [PubMed] [Google Scholar]
- Jaworski E. G. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1274–1279. doi: 10.1016/s0006-291x(71)80010-4. [DOI] [PubMed] [Google Scholar]
- Kende H., Hahn H., Kays S. E. Enhancement of Nitrate Reductase Activity by Benzyladenine in Agrostemma githago. Plant Physiol. 1971 Dec;48(6):702–706. doi: 10.1104/pp.48.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of organic acids in corn roots I. Differential labeling of 2 malate pools. Plant Physiol. 1966 Apr;41(4):709–712. doi: 10.1104/pp.41.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel K., Pflüger R. The Release of Potassium and Sodium from Young Excised Roots of Zea mays under Various Efflux Conditions. Plant Physiol. 1972 Jan;49(1):16–19. doi: 10.1104/pp.49.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula P. N. The comparative penetrant-carrier action of dimethyl sulfoxide and ethyl alcohol in vivo. Ann N Y Acad Sci. 1967 Mar 15;141(1):277–278. doi: 10.1111/j.1749-6632.1967.tb34890.x. [DOI] [PubMed] [Google Scholar]
- Streeter J. G., Bosler M. E. Comparison of in vitro and in vivo assays for nitrate reductase in soybean leaves. Plant Physiol. 1972 Mar;49(3):448–450. doi: 10.1104/pp.49.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]


