This systematic review and meta-analysis examined the use of routine postoperative administration of vitamin D and calcium to reduce the incidence of symptomatic postoperative hypocalcemia after total thyroidectomy. A significant decrease in postoperative hypocalcemia was identified in patients who received routine supplementation of oral calcium or vitamin D, especially with the combined administration of both supplements.
Keywords: Vitamin D, Calcium, Hypocalcemia, Post-thyroidectomy, Prevention
Learning Objectives
Discuss the role of routine early postoperative oral calcium and/or vitamin D supplementation in preventing symptomatic post-thyroidectomy hypocalcemia.
Describe the effect of treatment with a combination of oral calcium and vitamin D supplements on the risk of hypocalcemia in post-thyroidectomy patients and its potential to facilitate early discharge.
Abstract
Background.
Transient hypocalcemia is a frequent complication after total thyroidectomy. Routine postoperative administration of vitamin D and calcium can reduce the incidence of symptomatic postoperative hypocalcemia. We performed a systematic review to assess the effectiveness of this intervention. The primary aim was to evaluate the efficacy of routine postoperative oral calcium and vitamin D supplementation in preventing symptomatic post-thyroidectomy hypocalcemia. The second aim was to draw clear guidelines regarding prophylactic calcium and/or vitamin D therapy for patients after thyroidectomy.
Methods.
We identified randomized controlled trials comparing the administration of vitamin D or its metabolites to calcium or no treatment in adult patients after thyroidectomy. The search was performed in PubMed, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, Google Scholar, and Web of Knowledge databases. Patients with a history of previous neck surgery, calcium supplementation, or renal impairment were excluded.
Results.
Nine studies with 2,285 patients were included: 22 in the vitamin D group, 580 in the calcium group, 792 in the vitamin D and calcium group, and 891 in the no intervention group, with symptomatic hypocalcemia incidences of 4.6%, 14%, 14%, and 20.5%, respectively. Subcomparisons demonstrated that the incidences of postoperative hypocalcemia were 10.1% versus 18.8% for calcium versus no intervention and 6.8% versus 25.9% for vitamin D and calcium versus no intervention. The studies showed a significant range of variability in patients' characteristics.
Conclusions.
A significant decrease in postoperative hypocalcemia was identified in patients who received routine supplementation of oral calcium or vitamin D. The incidence decreased even more with the combined administration of both supplements. Based on this analysis, we recommend oral calcium for all patients following thyroidectomy, with the addition of vitamin D for high-risk individuals.
Implications for Practice:
Excision of the thyroid gland (thyroidectomy) is a common therapy for thyroid diseases. It is a safe procedure if performed by an expert surgeon. Temporary low serum calcium level (transient hypocalcaemia) is the most common complication of this surgery. This complication may occur in 10%–45% of the cases and may result in mild to severe symptoms. Postoperative treatment with calcium and vitamin D may prevent or minimize hypocalcemic symptoms; however, until now, clear guidelines for the optimal use of these supplements were not available. The aim of this review is to compare the outcomes of different supplementation protocols in an attempt to assist surgeons decrease the incidence of hypocalcemia after thyroid surgery.
Introduction
Thyroid surgery is one of the most frequently performed surgical procedures worldwide [1]. In fact, total thyroidectomy is now widely accepted as the gold standard for the management of thyroid carcinoma and benign bilateral thyroid disease due to suspicion of cancer, symptoms of local compression, and a patient's desire for rapid and definitive treatment [2]. Currently, total thyroidectomy is considered a safe procedure when performed by experienced surgeons. The main postoperative complications are recurrent laryngeal nerve palsy and hypocalcemia [1, 3].
By definition, transient hypocalcemia resolves within 6 months after total thyroidectomy; its reported incidence ranges from 0.3% to 49% [4]. Permanent hypocalcemia persists after 6 months, with an incidence ranging from 0% to 13% [4]. Therefore, hypocalcemia is one of the main outcomes for auditing and patient consent [5, 6]. Inpatient admission and close monitoring of postoperative serum calcium level has been proposed to prevent postoperative symptoms related to hypocalcemia [7]. However, this approach has been criticized mainly due to the fact that the lowest concentration of serum calcium is usually not reached until 48–72 hours after thyroidectomy; therefore, it has major implications for safe early discharge planning [8].
Transient post-thyroidectomy hypocalcemia is self-limiting, but it could potentially be life-threatening [9]. Hypocalcemia symptoms are uncommon unless serum calcium level is below 8.0 mg/dL (2.0 mmol/L) [10]. Symptoms depend on the degree and rapidity of hypocalcemia onset, ranging from mild paresthesia and tingling to more severe cramps, tetany, seizures, laryngospasm, congestive heart failure, and arrhythmias due to prolonged QT intervals [8, 11]. Hungry bone syndrome could also occur after total thyroidectomy for Graves' disease as the patient recovers from the disease's thyrotoxic effects on bone metabolism [12].
To manage postoperative hypocalcemia, most practitioners obtain serial serum calcium measurements and respond appropriately to low levels [13]. Oral supplementation is started with elemental calcium with or without calcitriol for immediate management of postsurgical hypoparathyroidism [12]. For patients whose symptoms persist despite oral replacement therapy, intravenous replacement and closer monitoring are indicated [8]. Moreover, some centers routinely supplement all patients to facilitate early discharge [14]. Parathyroid autotransplantation also has been used to prevent hypocalcemia. Wells et al. first reported its successful use in patients with multigland hyperplasia, but it has also been used for delayed transplantation with variable success rates [12].
Several studies suggested that low postoperative parathyroid hormone (PTH) levels can predict hypocalcemia; it can be used to tailor supplement treatment options and prevent transient hypocalcemia [8, 15–17]. Despite the different methods to evaluate and predict postoperative hypocalcemia, there is no consensus on the role of routine calcium and/or vitamin D following thyroid surgery. The objectives of this meta-analysis are to evaluate the potential role of routine calcium or vitamin D supplementation for the prevention of post-thyroidectomy hypocalcemia and to draw therapy guidelines that may prevent this common complication.
Methods
The study population included adults who underwent thyroidectomy as presented in the international literature. Interventions included post-thyroidectomy treatment with vitamin D, calcium, or both. The following types of interventions were considered: vitamin D and/or calcium orally, vitamin D and/or calcium intravenously, and vitamin D and/ or calcium in any combination. The control group was compared with one type of treatment or no intervention. Outcomes were the effects of treatment on the incidence of hypocalcemia.
Studies included only randomized controlled trials. There was no time limitation. The review was limited to English language studies of adult patients (>18 years old). Studies that were designed to have a crossover management, were conducted on animals, included a pediatric population, or were unpublished/dissertation data were excluded. Studies that included patients with renal failure or previous neck surgery were also excluded, as the latter group of patients is at an increased risk for hypocalcemia with multiple cofactors involved (and for the same reason was excluded by the majority of randomized controlled trials).
An extensive literature search was conducted. Several electronic databases (including PubMed, Cochrane, Cumulative Index to Nursing and Allied Health Literature, Google Scholar, Web of Knowledge, and Snowball) were searched to locate studies that examined prevention of hypocalcemia after thyroidectomy. References were explored to identify other articles. Titles and abstracts were searched. Articles meeting inclusion criteria were retrieved and reviewed by the first and the second authors.
Key words and MeSH terms included the following: (post-thyroidectomy) OR (after thyroidectomy) AND (hypocalcemia OR low calcium) AND (prevention OR prophylactic or role) AND (calcium or vitamin D, OR calcifirole, OR caltrat, OR 1, 25-dihydroxyvitamin D, OR calcitriol, OR 25-hydroxyvitamin D, OR calcidiol, OR calcifediol, OR calcitroic acid, OR cathelicidin, OR cholecalciferol 7-dehydrocholesterol, OR 22-dihydroergocalciferol, OR dihydrotachysterol, OR 1, 25-dihydroxycholecalciferol, OR ergocalciferol, OR ergosterol, OR lumisterol, OR paricalcitol, OR tacalcitol).
Only randomized controlled trials were included; they were evaluated for concealment of randomization, blinding, and intention-to-treat analysis. Any study that did not have a reference standard or control group was excluded. Study quality was assessed using the Strength of Recommendation Taxonomy (SORT) grading system [26]; the level of evidence was therefore rated as 1 or 2 only. Assessment was not blinded. Studies were weighted based on their level of evidence as well as the number of patients involved.
Statistical Analyses
For each prophylactic method, separated analyses had been conducted using the difference between the change in the incidence of hypocalcemia for each type of treatment and the change in the control to determine the best treatment. Comparison groups included vitamin D versus no intervention, calcium versus no intervention, calcium versus calcium + vitamin D, and calcium + vitamin D versus no intervention. Final conclusions on the use of calcium or/and vitamin D as a prophylactic therapy for post-thyroidectomy hypocalcemia were based on the analysis of these studies. Mantel-Haenszel methods and raw counts from each study were used in the meta-analysis.
The I2 test and associated p values were used to assess the heterogeneity of the studies. The software used for analysis was R (R Foundation for Statistical Computing, Vienna, Austria). The package that was used within the software to perform meta-analysis (including the figures and funnel plot to assess bias and heterogeneity) was Meta 2.1–1 (Guido Schwarzer, Freiburg, Germany). Two independent reviewers evaluated the studies' eligibility, assessed the quality, and assessed the extracted data, aiming for achieving a high level of correlation in quality and validity of the findings. Disagreements were resolved by consensus.
Results
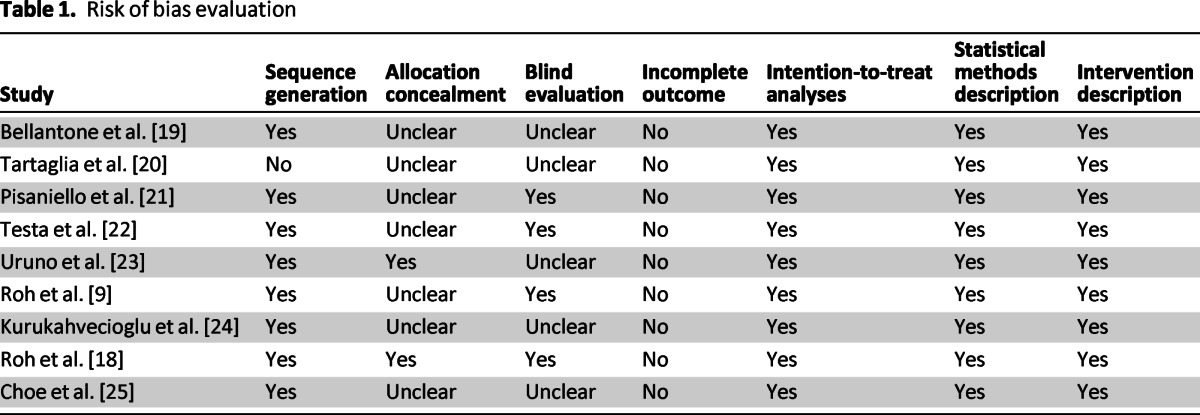
The search identified 1,180 studies focusing on post-thyroidectomy hypocalcemia. A total of 132 studies remained after eliminating those that were duplicated. Of these 132 studies, 88 studies were excluded because they did not investigate post-thyroidectomy hypocalcemia. Another 13 articles were excluded because they had indirect measurement of our inclusion outcomes. Twenty-one articles were excluded because they were not randomized controlled trials and 1 article was excluded because it had crossover management. Thus, nine studies met the inclusion criteria and were chosen for analysis (Fig. 1). Two of the included studies were conducted by the same author: Roh et al. in 2006 and 2009 [9, 18]. Risks of biases were evaluated for all studies (Table 1).
Figure 1.
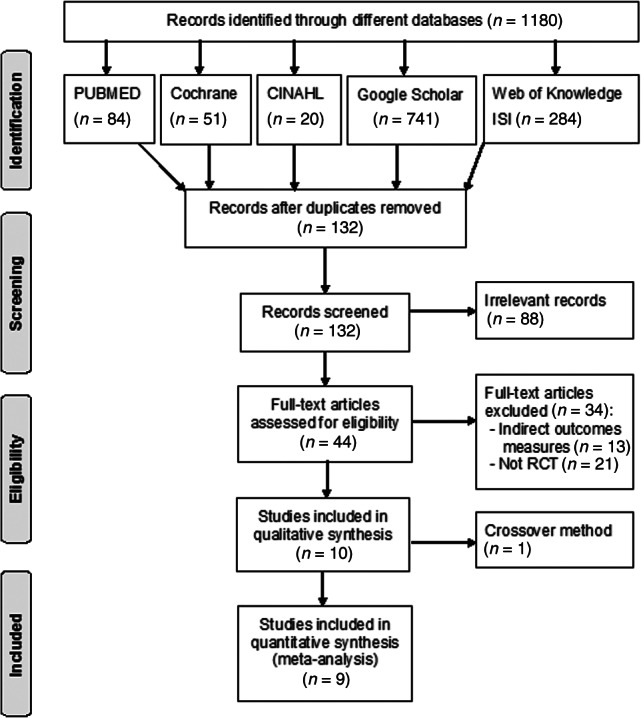
Flow of information through the different phases of the systematic review.
Abbreviations: CINAHL, Cumulative Index to Nursing and Allied Health Literature; RCT, randomized controlled trial.
Table 1.
Risk of bias evaluation
Summary of Study Findings
Bellantone et al. showed that routine supplementation therapy with oral calcium or vitamin D effectively prevents symptomatic hypocalcemia after total thyroidectomy and may allow for a safe early discharge [19]. Tartaglia et al. demonstrated that oral administration of calcitriol and calcium salts after total thyroidectomy significantly decreases the risk of severe postoperative hypocalcemia [20].
Pisaniello et al. concluded that early and combined oral administration of both calcium and vitamin D seemed to have major efficacy in preventing and treating postoperative hypocalcemia, demonstrating mean serum calcium levels higher than those of patients who received only oral calcium administration [21]. Testa et al. observed that the administration of calcitriol plus hydrochlorothiazide was able to prevent transient post-thyroidectomy hypocalcemia and reduce hospital stay [22].
Uruno et al. mentioned that a prophylactic infusion of calcium solution after total thyroidectomy may be useful in reducing the development of symptomatic hypocalcemia and reducing a patient's risk of discomfort and anxiety due to hypocalcemia [23]. In 2006, Roh et al. showed that routine administration of a supplement containing oral calcium and vitamin D is effective in reducing the incidence and severity of hypocalcemia after total thyroidectomy [9]. In 2009, Roh et al. also showed that central lymph dissection significantly increased the rate of postoperative hypocalcemia compared with total thyroidectomy alone; this could be prevented by routine postoperative supplementation with oral calcium and vitamin D [18].
Kurukahvecioglu et al. showed that routine postoperative calcium and vitamin D supplementation therapy may be useful for the prevention of symptomatic hypocalcemia after total thyroidectomy and may allow for a safe and early discharge from the hospital [24]. Finally, Choe et al. confirmed that routine oral calcium and vitamin D supplements were beneficial after total thyroidectomy with central neck lymph node dissection, with no difference between cholecalciferol and calcitriol. If taken after the onset of hypocalcemia, however, calcitriol along with calcium carbonate seem to be more effective than cholecalciferol with calcium carbonate [25].
Overall Results of Meta-Analysis
Among the nine studies included, six were SORT class II and three were SORT class I. The combined studies recruited 2,285 patients, and the overall incidence of post-thyroidectomy hypocalcemia was 26.1% (Table 2). There was a significant range of variability in patient characteristics. A total of 394 males and 1,891 females were included, and the mean age was 50 ± 8 years. Among these patients, 1,183 had thyroid cancer and 904 patients had benign disease. Central lymph node dissection was performed for 845 patients (Table 3).
Table 2.
Randomized controlled trials included in meta-analysis
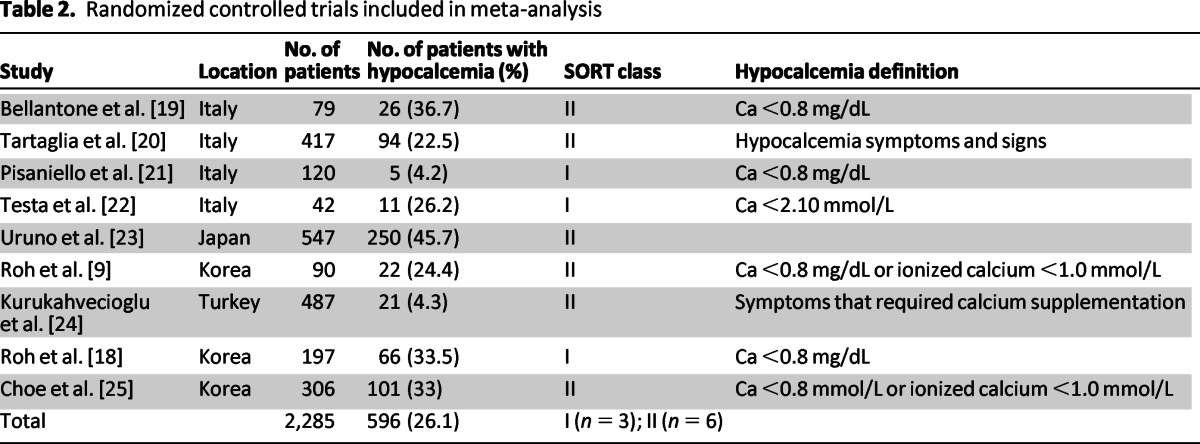
Table 3.
Demographic data
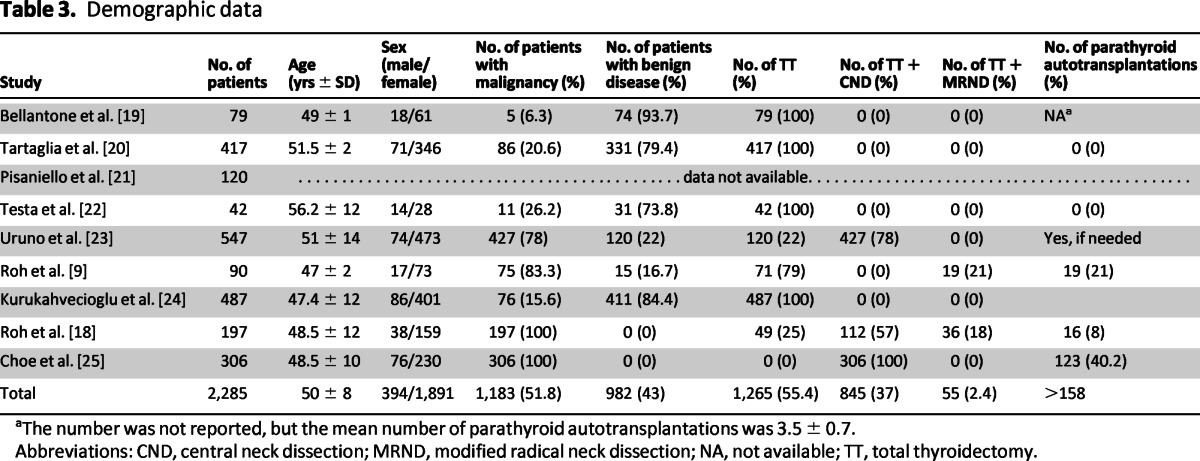
aThe number was not reported, but the mean number of parathyroid autotransplantations was 3.5 ± 0.7.
Abbreviations: CND, central neck dissection; MRND, modified radical neck dissection; NA, not available; TT, total thyroidectomy.
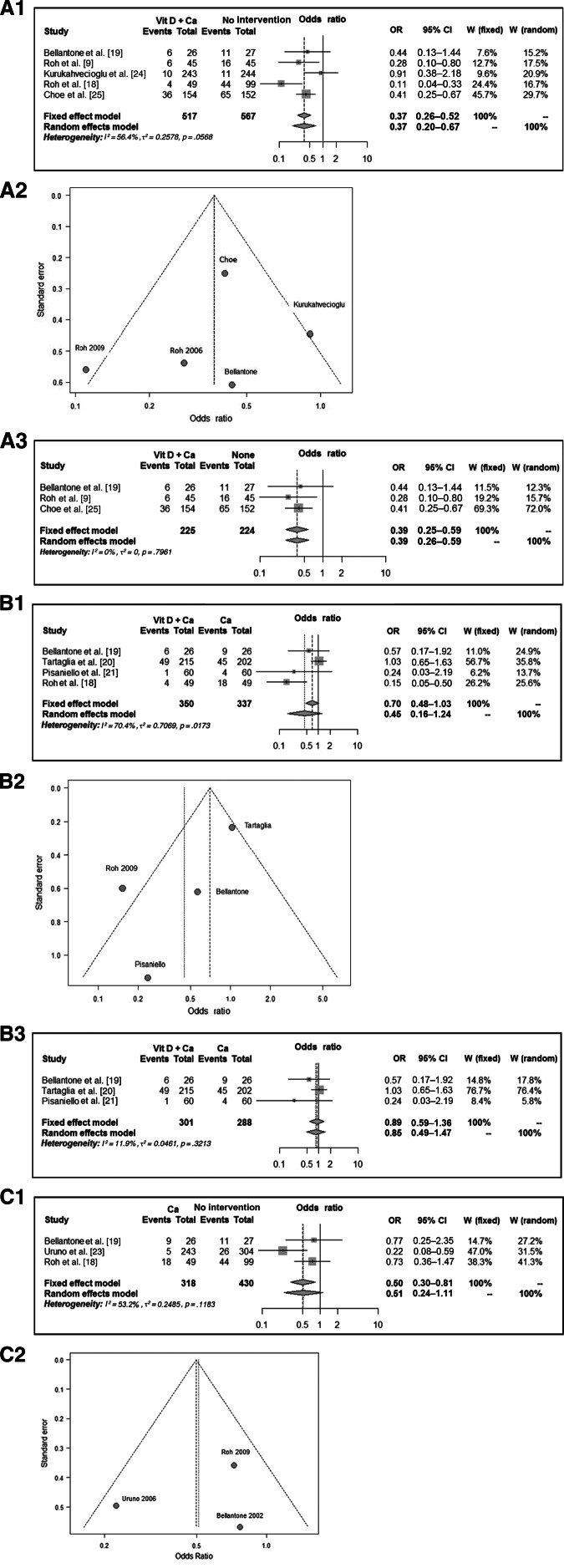
Four different groups were identified: vitamin D treatment (n = 22), calcium treatment (n = 580), vitamin D and calcium treatment (n = 792), and no intervention (n = 891). Accordingly, the rates of hypocalcemia amongst the different groups were 4.6%, 14%, 14%, and 20.5%, respectively. Thus, there were four comparisons: vitamin D and calcium versus no intervention, calcium versus vitamin D and calcium, calcium versus no intervention, and vitamin D versus no intervention. The incidences of postoperative hypocalcemia were 6.8% versus 25.9%, 22.6% versus 17.1%, 10.1% versus 18.8%, and 5% versus 50%, respectively (Table 4, Fig. 2). The meta-analysis showed a significant decrease in the rate of symptomatic postoperative hypocalcemia between the groups treated with vitamin D or its metabolites along with calcium compared with no prophylaxis or calcium alone. There was a high heterogeneity between the studies for vitamin D and calcium versus no intervention, as well as for calcium versus calcium and vitamin D. However, there was no significant heterogeneity for calcium versus no intervention. Two separate analyses were performed for the first and second comparisons, with and without the studies with high heterogeneity. The results were the same, but the confidence interval was narrower in the second analysis (Fig. 2).
Table 4.
Hypocalcaemia incidence
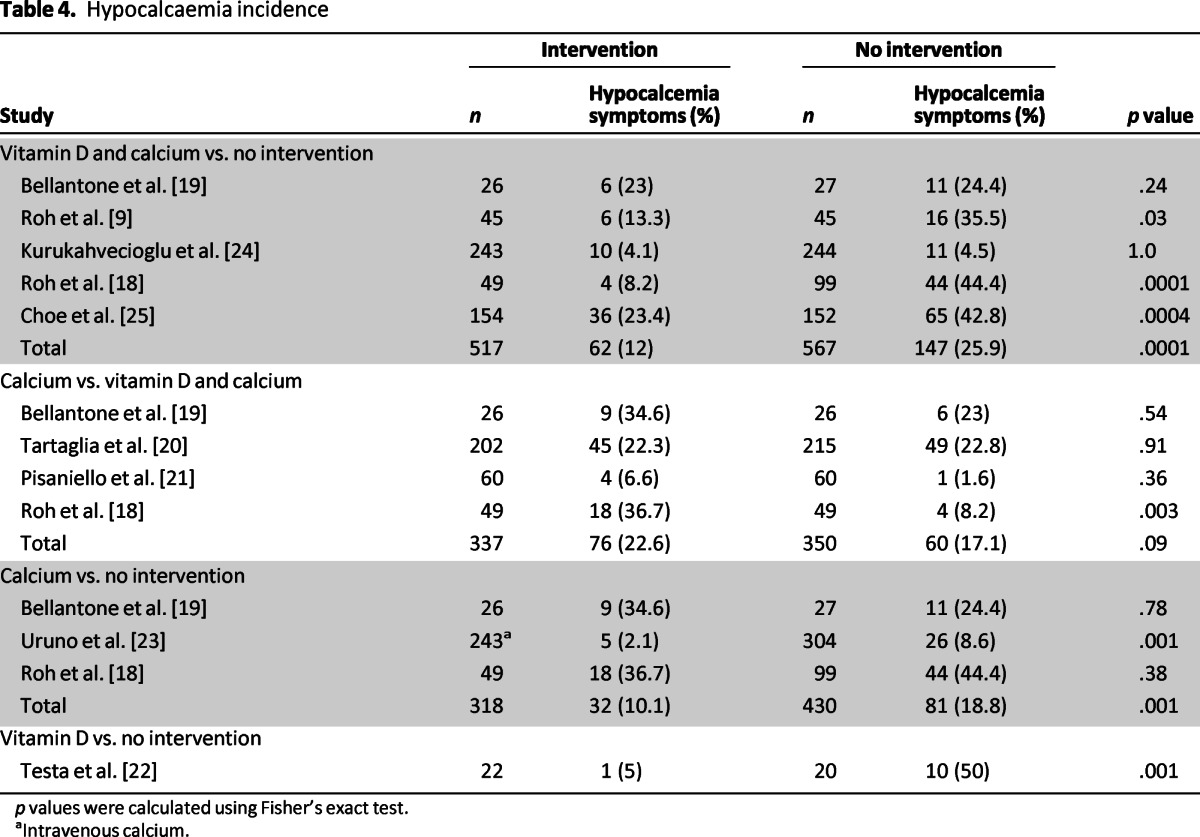
p values were calculated using Fisher's exact test.
aIntravenous calcium.
Figure 2.
(A1): Vitamin D and calcium versus no intervention. (A2): Funnel plot for heterogeneity. (A3): Vitamin D and calcium versus no intervention, excluding the studies with high heterogeneity. (B1): Vitamin D and calcium versus calcium. (B2): Funnel plot for heterogeneity. (B3): Vitamin D and calcium versus calcium, excluding the studies with high heterogeneity. (C1): Calcium versus no intervention. (C2): Funnel plot for heterogeneity.
Abbreviations: Ca, calcium; CI, confidence interval; OR, odds ratio; Vit D, vitamin D.
Hypocalcemia Data
The definition of hypocalcemia varied between the studies. Five studies defined hypocalcemia as calcium levels <0.8 mmol/L [9, 18–19, 21, 25]. In comparison, Testa et al. used calcium levels <2.10 mmol/L as a cutoff [22]. Tartaglia et al. and Kurukahvecioglu et al. defined hypocalcemia as symptoms that required calcium supplementation [20, 24] (Table 2).
Serum calcium and PTH readings at 1 day, 2 days, 3 days, 1 week, and 1 month postoperatively were collected in all the studies (Table 5). A compression of the different treatments showed that there were significant statistical differences in serum calcium level on the first and second days as well as 1 week after surgery. However, the only significant statistical difference for the serum PTH was at 1 month after surgery, even though all values were within normal limits (Table 6).
Table 5.
Postoperative serum calcium and parathyroid hormone readings
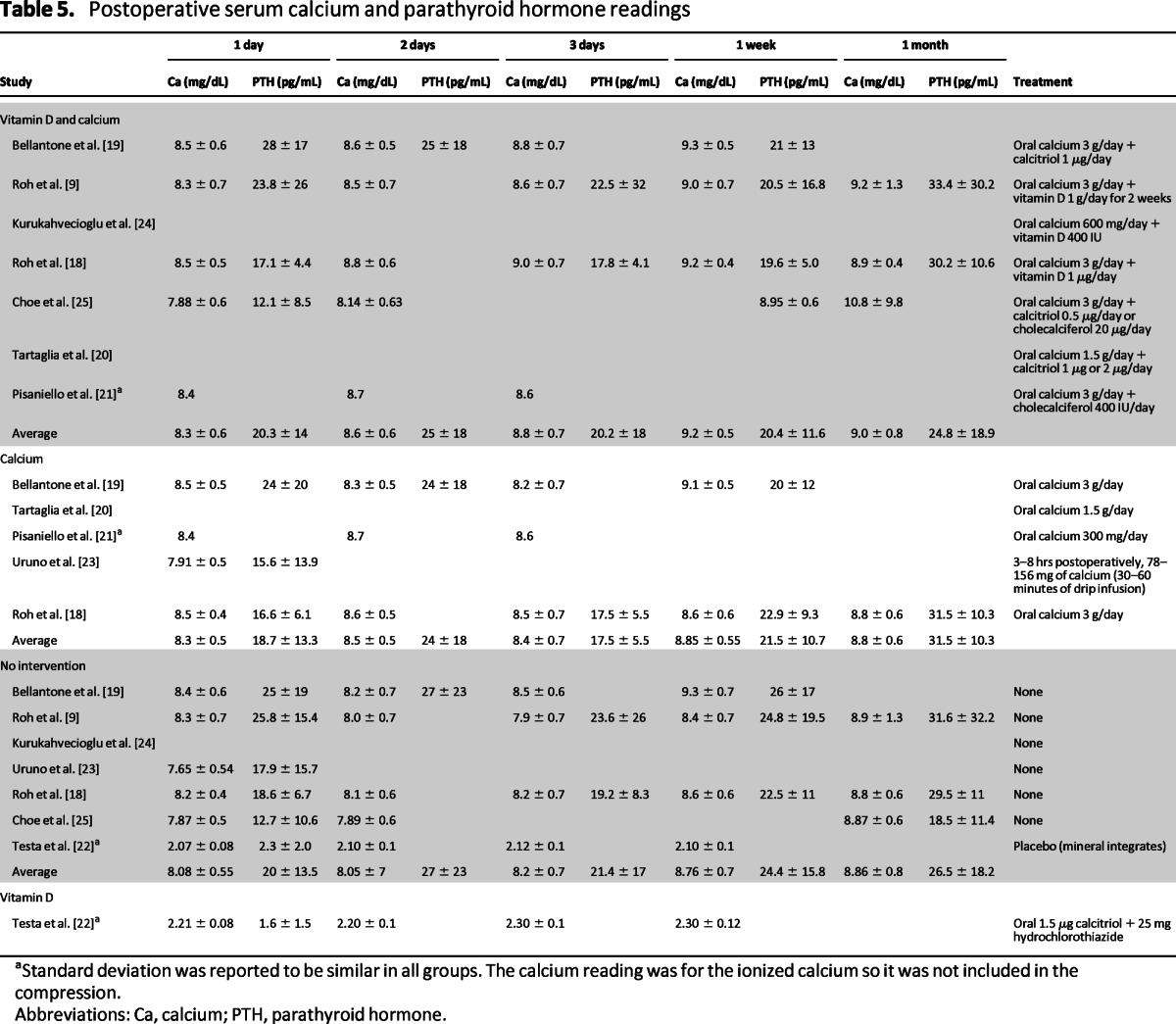
aStandard deviation was reported to be similar in all groups. The calcium reading was for the ionized calcium so it was not included in the compression.
Abbreviations: Ca, calcium; PTH, parathyroid hormone.
Table 6.
Postoperative serum calcium and parathyroid hormone readings: Compression
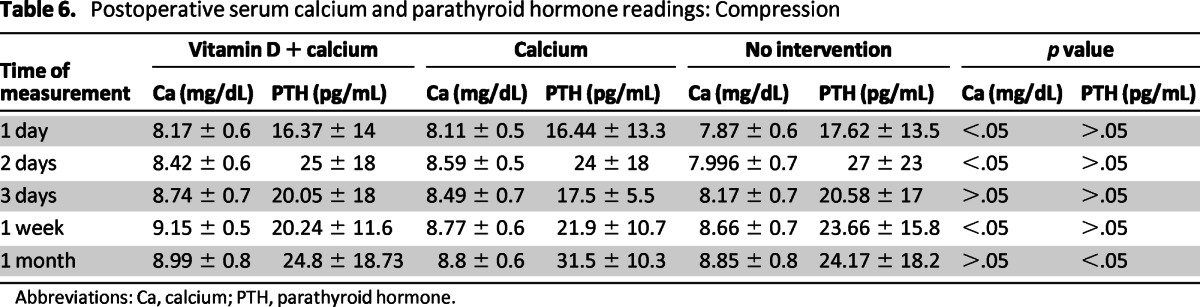
Abbreviations: Ca, calcium; PTH, parathyroid hormone.
In the 2006 Roh et al. study [9], there were minimal hypocalcemic symptoms in the group who received both vitamin D and calcium but more severe symptoms in the group with no intervention, including carpopedal spasms in three patients. Intravenous calcium was administered to five patients in the group who received no intervention but not to any patients in the supplement group (p = .022). In the Choe et al. study [25], most hypocalcemic symptoms were mild, with moderate or severe symptoms occurring in only 13 (4.3%) patients. Intravenous calcium injections were necessary in 43 (14.1%) patients; 34 (11%) patients needed only one injection, whereas 3 (1%) patients required more than three injections.
In the 2009 Roh et al. study [18], hypocalcemic symptoms were minimal in the oral calcium and vitamin D group and in the noncentral neck dissection group, but they were more severe in the group who received no intervention. Intravenous calcium was administered to seven (7%) patients in the no intervention group, compared with no patients in the oral calcium and vitamin D group (p = .096). Kurukahvecioglu et al. [24] showed that minor symptoms occurred in 4 patients in the group who received no intervention and 10 patients in the treated group. Intravenous calcium administration was required for seven patients in the group who received no intervention, but in none of the patients in the treated group (p < .001). The mean hospital stay in the control group was 2.9 ± 0.9 days (range: 2–7 days) and 1.2 ± 0.4 days (range: 1–2 days) in the treated group (p < .001).
Bellantone et al. [19] showed that 11 patients (40.7%) in the group who received no intervention experienced symptoms of hypocalcemia and required oral calcium and vitamin D administration. Two patients (7.7%) in the oral calcium group required vitamin D administration because of persistent hypocalcemia. Two patients (7.4%) in the group who received no intervention had carpopedal spasms and nine patients (33.3%) had perioral numbness with positive Chvostek sign. Patients who were symptomatic in the oral calcium and vitamin D groups experienced minor symptoms (mild perioral tingling, with a positive Chvostek sign). No patients in the oral calcium and vitamin D groups and two patients (7.4%) in the group who received no intervention required intravenous calcium administration (p < .01).
Pisaniello et al. [21] demonstrated that four (6.6%) patients in the oral calcium group and one patient (1.6%) in the oral calcium and vitamin D group experienced symptomatic hypocalcemia that required intravenous calcium (p = .36). The patients experienced muscle cramps and tingling. Testa et al. [22] reported that symptomatic hypocalcemia (muscle cramps and tingling of the face and extremities) developed in four (20%) patients (7.4%) in the group who received no intervention but in no patients in the oral calcium and vitamin D group (p < .05).
Discussion
Postoperative hypocalcemia is one of the most immediate and common surgical complications of total thyroidectomy [27]. To minimize the symptoms of postoperative hypocalcemia, some surgeons prefer to give calcium treatment empirically, whereas others prefer to educate patients about symptoms so that they can return to the hospital if these symptoms arise [28]. Moreover, it has been reported that administration of post-thyroidectomy oral calcium with vitamin D did not appear to inhibit the function of normal parathyroid glands [29].
Several studies have investigated the role of postoperative PTH as a predictor of hypocalcemia. It has been suggested that calcium and/or vitamin D supplementation may be dictated by the postoperative PTH level [6]. Postoperative PTH levels were shown to have a high sensitivity and specificity for predicting hypocalcemia [16–17, 29–30]. In a meta-analysis of four Australian studies, Grodski et al. [31, 32] found that an undetectable PTH level 4 hours after surgery predicted hypocalcemia with a sensitivity of 48.4%, specificity of 96.7%, and positive predictive value of 83.8%, but that 16% of patients with an undetectable PTH level remained normocalcemic without treatment. Noordzij et al. analyzed the pooled data from 9 studies and found that the PTH level 6 hours after surgery had the highest accuracy for predicting hypocalcemia; in addition, a greater than 65% decline from the baseline serum PTH level 6 hours postoperatively predicted hypocalcemia with a sensitivity of 96.4% and a specificity of 91.4% [34]. Youngwirth et al. demonstrated that postoperative PTH may be used to decrease the incidence of hypocalcemia and emergency room visits [8]. Nevertheless, there is no consensus on the role and benefits of routine calcium and/or vitamin supplementation following thyroidectomy.
The meta-analysis conducted in this study showed a significant decrease in the rate of symptomatic postoperative hypocalcemia between the groups treated with vitamin D or its metabolites along with calcium as compared to no prophylaxis or calcium alone. The results showed that the combination vitamin D or metabolites with calcium is the most effective strategy. Our results are similar to the meta-analysis conducted by Sanabria et al., who evaluated the routine postoperative administration of vitamin D and calcium after total thyroidectomy in studies published from January 1980 to April 2008 [14]. In their analysis, four studies—Roh et al. [9], Bellantone et al. [19], Pisaniello et al. [21], and Tartaglia et al. [22]—were included with 706 patients. Calcitriol and calcium were compared to no treatment and only calcium treatment, with a decreased rate of hypocalcemia identified for both comparisons (odds ratio [OR] = 0.32, 95% confidence interval [CI]: 0.13–0.79; and OR = 0.31, 95% CI: 0.14–0.70, respectively). Despite wide confidence intervals, the results are statistically significant. Our study, in comparison, included 9 studies of 2,285 patients, 4 groups, and 3 comparisons; the demonstrated OR was more significant with a narrower CI (Fig. 2).
The meta-analysis conducted in this study showed a significant decrease in the rate of symptomatic postoperative hypocalcemia between the groups treated with vitamin D or its metabolites along with calcium as compared to no prophylaxis or calcium alone. The results showed that the combination vitamin D or metabolites with calcium is the most effective strategy.
The studies included in this study showed a wide range of variability in patient characteristics. For example, the malignancy rate varied from 8% to 100%. Similar variability is evident by the inclusion of patients with radical neck dissection, Graves' disease, surgery type, parathyroid identification, and autotransplantation. However, randomization of the studies balanced these differences. Importantly, there were some weaknesses and limitations in some of the included studies. The blind evaluation of results and allocation concealment reporting were inadequate, which may have biased the results. The potential adverse events of the treatment of all patients were not adequately evaluated and could represent an increase in costs not considered in this analysis. None of the included studies reported or denied any conflict of interest, apart from Roh et al., who specifically reported none [18], and Testa et al., who mentioned a grant provided by the Ministero Della Pubblica Istruzione edella Ricerca Scientifica [22].
Multiple other specific limitations should be noted. Choe et al. did not investigate the effect of preoperative vitamin D deficiency on the postoperative hypocalcemia [24]. The study by Tartaglia et al. was not blinded and did not evaluate the costs of calcitonin and ionized calcium measurement [20]. The 2006 Roh et al. study [9] did not observe for hypercalcemia or other side effects. The prevalence of hypocalcemia in the Testa et al. study is high because they used the “biochemical definition,” and 70% of the patients presented predisposing factors for hypoparathyroidism (voluminous and/or toxic goiter) [22]. Their study has several other limitations such as small sample size, and their inclusion criteria may have introduced bias to it [22]. Pisaniello et al. did not report the point at which treatment was suspended [21]. The 2009 Roh et al. study [18] did not provide any information about the rate of permanent hypoparathyroidism. These study limitations should be noted but do not affect the validity of our meta-analysis results.
Although a cost analysis is beyond the scope of this study, it has been shown that routine oral calcium with or without vitamin D reduces patients' length of stay at the hospital. The economic profitability of thyroid surgery is improved when mean length of stay is reduced to 2 days through a fast-track protocol. Decreasing the duration of hospitalization was more effective than decreasing operative duration in controlling overall costs [34]. Moreover, routine oral calcium and calcitriol supplementation in patients after thyroidectomy seems to be less expensive and results in higher patient utility than selective supplementation [31]. Routine calcium supplementation would save $29,365 per quality-adjusted life year (QALY), with a savings of $62 per patient and an incremental benefit of 0.002 QALYs [36]. Singer et al. reported that the cost of a 3-week regimen of calcium carbonate is approximately $15, which is less expensive than either the cost of overnight admission or published laboratory protocols that are designed to predict the risk of hypocalcemia [37].
Overall Study Recommendations
The analysis of our results suggests several recommendations that may result in decreased incidence of hypocalcemia, hypocalcemic symptoms, and hospital length of stay. Although the serum calcium values were higher for the patients who received calcium and vitamin D than for those who received calcium only, all values were within normal limits. Based on these data, it is reasonable to recommend that calcium supplementation is enough to decrease the risk of post-thyroidectomy hypocalcemia. Vitamin D supplementation should be reserved for patients who have a higher risk of developing postoperative hypocalcemia, including patients who undergo central lymph node dissection, recurrent neck surgery, intraoperative parathyroid gland injury; patients who have intraoperative or postoperative low PTH; and patients who are known to have a vitamin D deficiency.
Postoperative oral calcium at a minimum dose of 3 g/day should be routinely given to all patients for 2 weeks following thyroid surgery. Patients who are at increased risk of postoperative hypocalcemia should be treated with the same calcium regimen, along with the addition of calcitriol at a dose of 1 μg/day. This analysis does not support routine use of higher doses of calcium or vitamin D; however, no clear recommendations can be made against increasing the initial calcium dosage for patients who are at increased risk of postoperative hypocalcemia.
Postoperative oral calcium at a minimum dose of 3 g/day should be routinely given to all patients for 2 weeks following thyroid surgery. Patients who are at increased risk of postoperative hypocalcemia should be treated with the same calcium regimen, along with the addition of calcitriol at a dose of 1 μg/day.
Conclusions
This meta-analysis demonstrated a decrease in postoperative hypocalcemia rates for patients who received calcium supplementation routinely. This rate decreased further with administration of vitamin D. The results of this study support a change in clinical practice and suggest that all patients undergoing thyroidectomy should be discharged with continued calcium and/or vitamin D supplementation for 1–2 weeks. Despite the significant results presented in this analysis, further investigation is needed to evaluate the exact dose and route of supplementation, as well as whether supplements should be given to patients preoperatively or postoperatively. Cost-effectiveness studies should also be considered.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
The Ministry of Higher Education of Saudi Arabia and King Faisai Specialist Hospital and Research Center, Riyadh, Saudi Arabia, through a fellowship with the first author, provided financial support for this study.
We thank Glen E. Leverson and Paul L. Chee, biostatisticians at the Department of Surgery, University of Wisconsin, Madison, Wisconsin, for assistance with statistical analysis.
Author Contributions
Conception/Design: Amal Alhefdhi, Herbert Chen
Provision of study material or patients: Amal Alhefdhi, Haggi Mazeh, Herbert Chen
Collection and/or assembly of data: Amal Alhefdhi, Haggi Mazeh
Data analysis and interpretation: Amal Alhefdhi, Herbert Chen
Manuscript writing: Amal Alhefdhi, Haggi Mazeh
Final approval of manuscript: Amal Alhefdhi, Haggi Mazeh, Herbert Chen
Disclosures
The authors indicated no financial relationships.
Section editor: Stan Sidhu: None
Reviewer “A”: None
Reviewer “B”: None
Reviewer “C”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Karamanakos SN, Markou KB, Panagopoulos K, et al. Complications and risk factors related to the extent of surgery in thyroidectomy. Results from 2,043 procedures. Hormones. 2010;9:318–325. doi: 10.14310/horm.2002.1283. [DOI] [PubMed] [Google Scholar]
- 2.Ho TW, Shaheen AA, Dixon E, et al. Utilization of thyroidectomy for benign disease in the United States: A 15-year population-based study. Am J Surg. 2011;201:570–574. doi: 10.1016/j.amjsurg.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Mehanna HM, Jain A, Randeva H, et al. Postoperative hypocalcemia: The difference a definition makes. Head Neck. 2010;32:279–283. doi: 10.1002/hed.21175. [DOI] [PubMed] [Google Scholar]
- 4.Wiseman JE, Mossanen M, Ituarte PH, et al. An algorithm informed by the parathyroid hormone level reduces hypocalcemic complications of thyroidectomy. World J Surg. 2010;34:532–537. doi: 10.1007/s00268-009-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanzada TW, Samad A, Memon W, et al. Post thyroidectomy complications: The Hyderabad experience. J Ayub Med Coll Abbottabad. 2010;22:65–68. [PubMed] [Google Scholar]
- 6.Adler J, Sippel RS, Schaefer S, Chen H. Preserving function and quality of life after thyroid and parathyroid surgery. Lancet Oncol. 2008;9:1069–1075. doi: 10.1016/S1470-2045(08)70276-6. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Chung MK, Son YI. Reliable early prediction for different types of post-thyroidectomy hypocalcemia. Clin Exp Otorhinolaryngol. 2011;4:95–100. doi: 10.3342/ceo.2011.4.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youngwirth L, Benavidez J, Sippel R, et al. Postoperative parathyroid hormone testing decreases symptomatic hypocalcemia and associated emergency room visits after total thyroidectomy. Surgery. 2010;148:841–844. doi: 10.1016/j.surg.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Roh JL, Park CI. Routine oral calcium and vitamin D supplements for prevention of hypocalcemia after total thyroidectomy. Am J Surg. 2006;192:675–678. doi: 10.1016/j.amjsurg.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Sabour S, Manders E, Steward DL. The role of rapid PACU parathyroid hormone in reducing post-thyroidectomy hypocalcemia. Otolaryngology. 2009;141:727–729. doi: 10.1016/j.otohns.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Khan MI, Waguespack SG, Hu MI. Medical management of postsurgical hypoparathyroidism. Endocr Pract. 2011;17(1):18–25. doi: 10.4158/EP10302.RA. [DOI] [PubMed] [Google Scholar]
- 12.Hessman C, Fields J, Schuman E. Outpatient thyroidectomy: Is it a safe and reasonable option? Am J Surg. 2011;201(5):565–568. doi: 10.1016/j.amjsurg.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Tredici P, Grosso E, Gibelli B, et al. Identification of patients at high risk for hypocalcemia after total thyroidectomy. Acta Otorhinolaryngol Ital. 2011;31(3):144–148. [PMC free article] [PubMed] [Google Scholar]
- 14.Sanabria A, Dominguez LC, Vega V, et al. Routine postoperative administration of vitamin D and calcium after total thyroidectomy: A meta-analysis. Int J Surg. 2011;9(1):46–51. doi: 10.1016/j.ijsu.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Abboud B, Sleilaty G, Zeineddine S, et al. Is therapy with calcium and vitamin D and parathyroid autotransplantation useful in total thyroidectomy for preventing hypocalcemia? Head Neck. 2008;30:1148–1154. doi: 10.1002/hed.20836. [DOI] [PubMed] [Google Scholar]
- 16.Sywak MS, Palazzo FF, Yeh M, et al. Parathyroid hormone assay predicts hypocalcemia after total thyroidectomy. Aust NZ J Surg. 2007;77(8):667–670. doi: 10.1111/j.1445-2197.2007.04183.x. [DOI] [PubMed] [Google Scholar]
- 17.McLeod IK, Arciero C, Noordzij JP, et al. The use of rapid parathyroid hormone assay in predicting postoperative hypocalcemia after total or completion thyroidectomy. Thyroid. 2006;16(3):259–265. doi: 10.1089/thy.2006.16.259. [DOI] [PubMed] [Google Scholar]
- 18.Roh JL, Park JY, Park CI. Prevention of postoperative hypocalcemia with routine oral calcium and vitamin D supplements in patients with differentiated papillary thyroid carcinoma undergoing total thyroidectomy plus central neck dissection. Cancer. 2009;115(2):251–258. doi: 10.1002/cncr.24027. [DOI] [PubMed] [Google Scholar]
- 19.Bellantone R, Lombardi CP, Raffaelli M, et al. Is routine supplementation therapy (calcium and vitamin D) useful after total thyroidectomy? Surgery. 2002;132(6):1109–1112. doi: 10.1067/msy.2002.128617. [DOI] [PubMed] [Google Scholar]
- 20.Tartaglia F, Giuliani A, Sgueglia M, et al. Randomized study on oral administration of calcitriol to prevent symptomatic hypocalcemia after total thyroidectomy. Am J Surg. 2005;190(3):424–429. doi: 10.1016/j.amjsurg.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Pisaniello D, Parmeggiani D, Piatto A, et al. Which therapy to prevent post-thyroidectomy hypocalcemia? G Chir. 2005;26(10):357–361. [PubMed] [Google Scholar]
- 22.Testa A, Fant V, De Rosa A, et al. Calcitriol plus hydrochlorothiazide prevents transient post-thyroidectomy hypocalcemia. Horm Metab Res. 2006;38(12):821–826. doi: 10.1055/s-2006-956504. [DOI] [PubMed] [Google Scholar]
- 23.Uruno T, Miyauchi A, Shimizu K, et al. A prophylactic infusion of calcium solution reduces the risk of symptomatic hypocalcemia in patients after total thyroidectomy. World J Surg. 2006;30(3):304–308. doi: 10.1007/s00268-005-0374-5. [DOI] [PubMed] [Google Scholar]
- 24.Kurukahvecioglu O, Karamercan A, Akin M, et al. Potential benefit of oral calcium/vitamin D administration for prevention of symptomatic hypocalcemia after total thyroidectomy. Endocr Regul. 2007;41(1):35–39. [PubMed] [Google Scholar]
- 25.Choe JH, Kim WW, Lee SK, et al. Comparison of calcitriol versus cholecalciferol therapy in addition to oral calcium after total thyroidectomy with central neck lymph node dissection: A prospective randomized study. Head Neck. 2011;33(9):1265–1271. doi: 10.1002/hed.21619. [DOI] [PubMed] [Google Scholar]
- 26.Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004;17(1):59–67. doi: 10.3122/jabfm.17.1.59. [DOI] [PubMed] [Google Scholar]
- 27.Mitra I, Nichani JR, Yap B, et al. Effect of central compartment neck dissection on hypocalcemia incidence after total thyroidectomy for carcinoma. J Laryngol Otol. 2011;125(5):497–501. doi: 10.1017/S0022215110002471. [DOI] [PubMed] [Google Scholar]
- 28.Coiro V, Zanardi G, Jotti S, et al. High-calcium mineral water as a calcium supplementing measure for post-thyroidectomy hypocalcemia. Minerva Endocrinol. 2008;33(1):7–13. [PubMed] [Google Scholar]
- 29.Hackman KL, Gagnon C, Briscoe RK, et al. Efficacy and safety of oral continuous low-dose versus short-term high-dose vitamin D: A prospective randomised trial conducted in a clinical setting. Med J Aust. 2010;192(12):686–689. doi: 10.5694/j.1326-5377.2010.tb03702.x. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi C, Raffaelli M, Princi P, et al. Early prediction of post-thyroidectomy hypocalcemia by one single iPTH measurement. Surgery. 2004;136(6):1236–1241. doi: 10.1016/j.surg.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 31.Vescan A, Witterick I, Freeman J. Parathyroid hormone as a predictor of hypocalcemia after thyroidectomy. Laryngoscope. 2005;115(12):2105–2108. doi: 10.1097/01.MLG.0000181504.69230.87. [DOI] [PubMed] [Google Scholar]
- 32.Grodski S. Australian Endocrine Surgeons Guidelines AES06/01. Postoperative parathyroid hormone measurement and early discharge after total thyroidectomy: Analysis of Australian data and management recommendations. ANZ J Surg. 2007;77(4):199–202. doi: 10.1111/j.1445-2197.2007.04018.x. [DOI] [PubMed] [Google Scholar]
- 33.Grodski S, Lundgren CI, Sidhu S., et al. Postoperative PTH measurement facilitates day 1 discharge after total thyroidectomy. Clin Endocrinol (Oxford) 2009;70(2):322–325. doi: 10.1111/j.1365-2265.2008.03317.x. [DOI] [PubMed] [Google Scholar]
- 34.Noordzij JP, Lee SL, Bernet VJ, et al. Early prediction of hypocalcemia after thyroidectomy using parathyroid hormone: An analysis of pooled individual patient data from nine observational studies. J Am Coll Surg. 2007;205(6):748–754. doi: 10.1016/j.jamcollsurg.2007.06.298. [DOI] [PubMed] [Google Scholar]
- 35.D'Hubert E, Proske JM. How to optimize the economic viability of thyroid surgery in a French public hospital? J Visc Surg. 2010;147(4):e259–e263. doi: 10.1016/j.jviscsurg.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Wang TS, Cheung K, Roman SA, et al. To supplement or not to supplement: A cost-utility analysis of calcium and vitamin D repletion in patients after thyroidectomy. Ann Surg Oncol. 2011;18(5):1293–1299. doi: 10.1245/s10434-010-1437-x. [DOI] [PubMed] [Google Scholar]
- 37.Singer MC, Bhakta D, Seybt MW, et al. Calcium management after thyroidectomy: A simple and cost-effective method. Otolaryngol Head Neck Surg. 2012;146(3):362–365. doi: 10.1177/0194599811433557. [DOI] [PubMed] [Google Scholar]