Several new agents—cabazitaxel, abiraterone acetate, enzalutamide, and radium-223—are changing the treatment options and management of patients with metastatic castration-resistant prostate cancer. This review article is summarizes the latest data on novel agents and current treatment strategies for patients with metastatic castration-resistant prostate cancer.
Keywords: Metastatic castration-resistant, Prostate cancer, Treatment, Docetaxel, Cabazitaxel, Abiraterone acetate, Enzalutamide
Abstract
The arrival of several new agents—cabazitaxel, abiraterone acetate, enzalutamide, and radium-223—is changing the treatment options and management of patients with metastatic castration-resistant prostate cancer (mCRPC). Many other novel agents are also being investigated. As new drugs become approved, new treatment strategies and markers to best select which patients will best respond to which drug are needed. This review article is a summary of a European Treatment Practices Meeting, which was convened to discuss these latest data on novel agents and current treatment strategies in the mCRPC setting.
Implications for Practice:
With so many novel agents available to treat patients with metastatic castration-resistant prostate cancer (mCRPC), a better understanding of factors to consider when assessing the clinical utility of treatment options for patients is needed. This review article discusses treatment strategies for mCRPC in the first- and second-line setting, and highlights the role of clinical markers, patient history, and assessing fitness for treatment when making treatment decisions. Prostate cancer is a heterogeneous disease, therefore, treatment must consider the characteristics of the disease as it manifests in an individual patient. In addition, assessments of patient response to treatment should reflect the mechanism of the drug. Further study is needed to identify predictive biomarkers to indicate patient response to novel agents.
Introduction
Aside from skin cancer, prostate cancer is reported to be the most common cancer in men living in the U.S. and Europe [1–3]. In 2013, the age-standardized mortality rate for prostate cancer is predicted to be 10.5 per 100,000 men, and rates are predicted to begin a steady decline across the European Union [4]. Metastatic castration-resistant prostate cancer (mCRPC) is an advanced form of the disease characterized by disease progression following surgical or medical castration. The European Association of Urology defines mCRPC as castrate levels of serum testosterone, three consecutive increases in prostate-specific antigen (PSA) resulting in two 50% increases above the nadir, antiandrogen withdrawal for at least 4 weeks, PSA progression despite secondary hormonal manipulations, and/or progression of osseous or soft-tissue lesions [5].
Approximately 10%–20% of patients with prostate cancer develop mCRPC within 5 years of follow-up after initial therapy. More than 80% of patients have bone metastases at castration-resistant prostate cancer (CRPC) diagnosis [6]. The process by which prostate cancer cells become castrate resistant is unclear, but it is characterized by overexpression or hyperactivation of the androgen receptor (AR) despite castrate levels of androgen. Mechanisms by which the AR remains activated are varied and include amplification [7–10] or mutation [11–13] of the AR gene, gene fusion [14, 15], overexpression of coactivators [16, 17], cytokine signaling [18, 19], crosstalk with transcription factors [20], synthesis of intracrine androgens by the tumor [21, 22], and constitutive activation of splice variants [23–25]. In CRPC, tumor characteristics such as morphology, immunophenotype, and genotype are typically heterogeneous both between patients and within the same patient [26, 27].
Patients with mCRPC have a poor prognosis and are expected to survive up to 18–19 months [28–30]. As the disease progresses, quality of life deteriorates and, until recently, few treatment options were available. Several new therapies have shown an improvement in overall survival (OS) rates for patients with mCRPC who have already received chemotherapy with docetaxel [28, 29, 31, 32]. The impact of these new data on daily clinical practice, treatment sequencing, and best care for individual patients is not yet fully understood. A European expert meeting was convened to discuss the effect of these recent data on treatment strategies in mCRPC. The results of those discussions are summarized in this article.
Current Treatment Options in Metastatic CRPC
First-Line Treatment
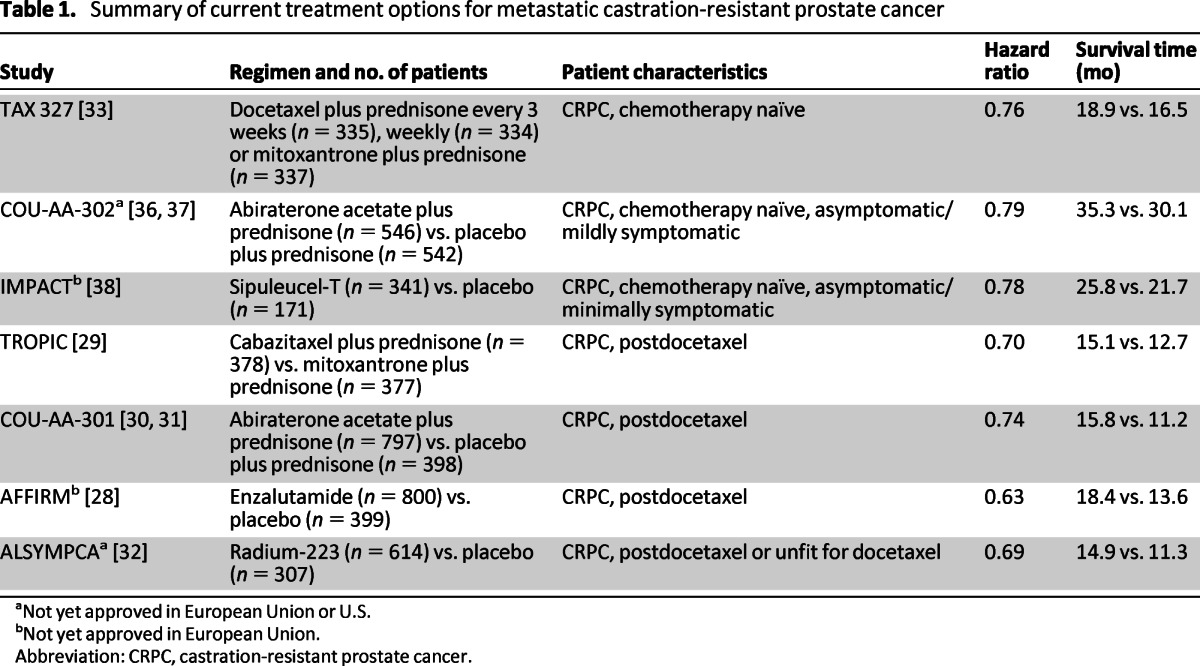
Between 2004 and 2010, when patients began to progress on androgen deprivation therapy (ADT) despite castrate levels of testosterone, chemotherapy with docetaxel plus prednisone was the main option for treatment of mCRPC [33–35] (Table 1). However, some patients may never receive docetaxel due to poor performance status, comorbidities, concerns about tolerability, and in some cases patient preference [40, 41]. It has been reported that around only 37% of patients received chemotherapy and the remainder received best supportive care [42, 43].
Table 1.
Summary of current treatment options for metastatic castration-resistant prostate cancer
aNot yet approved in European Union or U.S.
bNot yet approved in European Union.
Abbreviation: CRPC, castration-resistant prostate cancer.
Sipuleucel-T is an autologous immunotherapy evaluated in asymptomatic or minimally symptomatic patients with mCRPC in the Identification of Men With a Genetic Predisposition to Prostate Cancer (IMPACT) study. The study reported a median survival of 25.8 months with sipuleucel-T compared with 21.7 months for the control arm, and consequently, the drug was approved in 2010 by the U.S. Food and Drug Administration (FDA). The most frequently reported adverse events in the IMPACT study were chills, fever, and headache in the sipuleucel-T arm [38]; however, after approval the FDA requested a postmarketing analysis to evaluate the risk of stroke (NCT01306890) [44]. Furthermore, it has been speculated that the survival difference reported in the IMPACT study could reflect, at least in part, a detrimental effect of the control intervention rather than a beneficial effect of sipuleucel-T [45]. Because the drug is not yet approved in Europe, a phase II open-label study of sipuleucel-T (NCT01477749) has begun to assess whether the drug can be safely manufactured and effectively administered to European patients with mCRPC [46]. A course of three infusions of sipuleucel-T costs approximately $93,000 and is approved by Medicare [47]; however, if approved in Europe, the drug may not be widely reimbursed.
Second-Line Treatment
Cabazitaxel is a second-generation tubulin-binding taxane indicated for patients who have previously received docetaxel. In the TROPIC study, which enrolled 755 patients with mCRPC, chemotherapy with cabazitaxel plus prednisone resulted in a median OS time of 15.1 months compared with 12.7 months for patients receiving mitoxantrone plus prednisone (hazard ratio [HR]: 0.70, 95% confidence interval [CI]: 0.59–0.83; p < .0001) [29]. In 2011, abiraterone acetate was the first androgen biosynthesis inhibitor to be approved for treatment of patients with mCRPC who have progressed on or after treatment with docetaxel. Abiraterone acetate is an oral drug that inhibits CYP17 and is given in combination with prednisone. In the final analysis of the COU-AA-301 phase III study involving 1,195 patients who had previously received treatment with docetaxel, OS times were 15.8 months with abiraterone acetate plus prednisone and 11.2 months for placebo plus prednisone (HR: 0.74, 95% CI: 0.64–0.86; p < .0001) [30, 31].
It has been speculated that the survival difference reported in the IMPACT study could reflect, at least in part, a detrimental effect of the control intervention rather than a beneficial effect of sipuleucel-T. Because the drug is not yet approved in Europe, a phase II open-label study of sipuleucel-T (NCT01477749) has begun to assess whether the drug can be safely manufactured and effectively administered to European patients with mCRPC.
Bone-Targeting Agents
More than 90% of patients with CRPC have bone metastases [33]. Bone lesions are associated with elevated osteoclast activity that releases tumor-growth stimulating factors from the bone. The cycle of bone destruction and tumor growth continues, leading to skeletal-related events (SREs) such as spinal cord compression, pathological fracture, and the need for surgery or external beam radiotherapy [48, 49]. Bone metastases are a major cause of death, disability, and decreased quality of life; they also increase the cost of treatment [50].
To date, zoledronic acid is the only bisphosphonate that has been shown to reduce both pain and the number of SREs in patients with CRPC and bone metastases compared with placebo [51, 52]. Bisphosphonates are antiresorptive agents that block pathological resorption by inhibiting osteoclast activation and function [53]. Denosumab is a monoclonal antibody against the receptor activation of nuclear factor kappa-B ligand; it is licensed in the U.S. and Europe to treat men at risk of bone loss or fracture associated with hormonal therapy (age >70 years with osteopenia or history of osteoporotic fracture) [54]. In a recent study of 1,432 patients with mCRPC, denosumab significantly increased bone metastasis-free survival by a median of 4.2 months compared with placebo (median: 29.5 vs. 25.2 months; HR: 0.85, 95% CI: 0.73–0.98, p = .028). In addition, denosumab also significantly delayed the time to first bone metastasis (33.2 vs. 29.5 months; HR: 0.84, 95% CI: 0.71–0.98, p = .032) [55]. In a further phase III study, median time to first on-study SRE was 20.7 months with denosumab compared with 17.1 months with zoledronic acid (HR: 0.82, 95% CI: 0.71–0.95; p = .0002 for noninferiority; p = .008 for superiority) [56].
Radium-223 is a radiopharmaceutical that acts as a calcium mimic, targeting new bone growth in and around bone metastases via heavy alpha particles that have an ultrashort range of less than 100 μm. It may take only a single alpha particle to kill a cancer cell, and the short penetration results in highly localized tumor-cell killing with minimal damage to surrounding healthy cells. In the updated analysis of the ALSYMPCA study, which included 921 patients with CRPC, the median OS times were 14.9 months with radium-223 compared with 11.3 months with placebo (HR: 0.695, 95% CI: 0.581–0.8732; p < .0001) [32]. Radium-223 also significantly delayed median time to SREs: 15.6 months with radium-223 versus 9.8 months with placebo (p < .001; HR: 0.66; 95% CI: 0.52–0.83) [57].
Cabozantinib is another promising bone-targeting agent that inhibits both vascular endothelial growth factor and mesenchymal-epithelial transition factor (MET) [58]. MET is upregulated in several tumors and has been shown to drive invasive and aggressive tumors leading to metastases [59, 60]. MET-driven metastasis may be further stimulated by hypoxic conditions in the tumor environment. Furthermore, MET expression has been associated with bone metastases [61]. In phase II studies, cabozantinib (100 mg daily) was given to patients who had previously received docetaxel for treatment of mCRPC; it was associated with high rates of bone scan resolution, pain relief, and overall disease control. However, PSA changes were discordant and not consistent with other measures of tumor activity [61, 62]. Interim results were also reported for 51 patients receiving cabozantinib at 40 mg/ daily, showing that the lower dose is also effective; magnetic resonance imaging results confirmed the antitumor effect [63].
Making Treatment Decisions in the Management of Metastatic CRPC
There is a growing armamentarium of effective treatment options in mCRPC after docetaxel treatment [28–32]. The benefit of these treatments must be carefully balanced with tolerability and also cost. Because prostate cancer is a heterogeneous disease, biomarkers may identify those men who will most benefit from specific therapies and may help to identify markers for early response or progression, thus optimizing treatment outcomes [64].
Biomarkers are either prognostic, predictive, or surrogate markers, or they may have a combination of these characteristics. A prognostic biomarker provides evidence for a patient's potential outcome from a disease independent of therapy, whereas predictive biomarkers estimate the likelihood of response/benefit to a specific therapy [65, 66]. Most biomarkers reported in mCRPC are prognostic rather than predictive (reviewed by Armstrong et al. [64]). Although these biomarkers are helpful, predictive and surrogate biomarkers would be of greater benefit in making treatment decisions.
PSA is the most common marker used in daily clinical practice because it is easy to measure and has been used historically when monitoring patients receiving chemotherapy; however, it is not a surrogate marker for OS. PSA flare (an initial rise) after starting therapy happens in a minority of patients. Furthermore, some novel agents may not influence PSA levels [61, 62, 67] and some subgroups of prostate cancer do not produce PSA. For example, a very small subset of patients with either low PSA or undetectable PSA may have anaplastic small cell tumors. In some cases, this may be in addition to adenocarcinoma and will require a change of treatment (e.g., platinum-based chemotherapy in combination with hormonal therapy) [68]. PSA doubling time (DT) is prognostic of OS, and rapid PSA DT may indicate the need for aggressive therapy [69]; however, to date, few studies include PSA kinetics as a surrogate endpoint [70].
Urine N-telopeptide and bone alkaline phosphatase are markers of bone turnover that have been linked to survival in several data sets; they can be used to support interpretation of bone scans when differentiating between bone flare and bone progression [32, 62, 67, 71]. However, patients with visceral or node disease may have normal levels of bone markers [72, 73].
Enumeration of circulating tumor cells (CTCs) is a prognostic biomarker that has been incorporated into recently designed clinical studies to assess its suitability as a surrogate marker (NCT01718353, NCT01347788) [74]. A recent study assessed CTCs for AR expression—namely AR-on, AR-off, or AR-mixed, which was a simultaneous expression of AR-induced and AR-suppressed markers; this technology may be further developed to assess a patient's sensitivity to endocrine therapy [75].
Elevated lactate dehydrogenase (LDH) levels can indicate either extent of tumor burden or presence of an aggressive phenotype [76]. Anemia may be either disease- or treatment-related and may also be a result of a comorbidity, but it can be easily monitored. It is a strong prognostic factor for PSA decline following therapy with docetaxel, tumor response rate, and OS in mCRPC. Disease-related anemia is also associated with aggressive disease [76–78]. Lastly, C-reactive protein (CRP) is a marker of systemic inflammation that may also be prognostic of survival in patients previously treated with docetaxel [79].
In daily clinical practice, the following markers are often used to identify those patients who may have aggressive disease: short PSA DT; increases in LDH, AP, CRP, and carcinoembryonic antigen; and high Gleason scores (9/10). The number of visceral metastases may also suggest aggressive disease [80]; however, as patients treated with novel therapies are living longer, more patients may be developing visceral metastases. In addition, a short duration of response to docetaxel (or ADT), rapid development of resistance to docetaxel, or rapid clinical progression (often seen as an increase in pain or number of bone lesions) can all indicate an aggressive phenotype [71, 80, 81].
When selecting which patient should receive which treatment, it may also be important to consider the dynamic biological changes that have been suggested to evolve during the progression of CRPC [82]. Androgen dependence in prostate cancer evolves through four states of the AR, as recently outlined by Nelson [83]. In the first state (endocrine androgen dependence), the AR is stimulated by testicular testosterone, which allows the disease to be effectively controlled by luteinizing hormone-releasing hormone agonists (castration-sensitive prostate cancer). In the second state (intracrine androgen dependence), the AR is activated by androgens of intracellular origin, which are synthesized either de novo or from adrenal precursors. The CYP17 enzyme is crucially implicated in the process, which is consistent with the effectiveness of CYP17 inhibitors, such as abiraterone, in patients with CRPC. Importantly, increased intracellular expression of CYP17 in biopsied metastatic lesions in patients with CRPC who were treated with abiraterone was associated with longer time on treatment; it may serve as a tool to predict sensitivity to this class of agents [84].
Activation of the AR appears to be independent of ligand binding in the third state (ligand independence, AR dependence), due to the expression of an AR splice variant and crosstalk with other signal transduction pathways such as HER2/neu, IL-6 and Src kinase. In the fourth state (androgen independence and AR independence), AR signaling is not responsible for neoplastic proliferation, so any AR-directed treatment is likely to be ineffective. This model, developed to better understand the biology in the different phases of CRPC, may also be used to help decide the best therapeutic option and treatment combinations for patients [83].
Patients Progressing After First-Line Chemotherapy
Docetaxel is currently the only therapy indicated for first-line treatment of symptomatic mCRPC in Europe. To date, there are no clinical data providing evidence on when to initiate therapy and how best to sequence treatments following docetaxel in mCRPC [40, 64, 85]. In Europe, budgetary considerations are a major limiting factor affecting the sequence or combination of drugs that can be offered to the patient. In many cases, the budget available for treatment will vary from country to country and from region to region within one country (see Bourke et al. [86] for a discussion of budgetary considerations for endocrine therapy in the U.K.). Physicians not only need to decide how best to sequence available treatments, but also which tests to perform before and after treatment to assess disease status, how to interpret test results, and how to use the results to guide management decisions. The Prostate Cancer Clinical Trials Working Group Two considered treatment outcomes as being twofold: The first outcome is to control, relieve, or eliminate disease manifestations present at the beginning of treatment, and the second is to prevent or delay future manifestations of the disease [71, 87].
Treatment is rarely initiated, stopped, or switched on the basis of increases in PSA alone; however, any clinically relevant change in parameters, such as PSA kinetics (e.g., PSA DT), presence of pain, increase or change in the pattern of metastases, and deterioration in disease-related performance status can be indicators for treatment initiation or change of regimen [88]. Monitoring individual outcomes of the disease (e.g., PSA, pain, and metastases) requires careful interpretation as the significance of a change in a single measure differs depending on the mode of action of drug being prescribed. This approach ensures that the drug is given sufficient time to affect the disease without discontinuing treatment prematurely [87].
Following first-line treatment with docetaxel, patients may receive further chemotherapy with docetaxel rechallenge if they responded well to first-line therapy (providing they have a good performance status and a good life expectancy [40, 89–91]), cabazitaxel or abiraterone acetate; however, to date there are no clear guidelines indicating which treatment should be prescribed first or which patients would respond best to the different treatment options. Figure 1 provides an example of the algorithm of factors that might be considered when deciding how to treat a patient with mCRPC who has progressed following 6–8 cycles of docetaxel.
Figure 1.
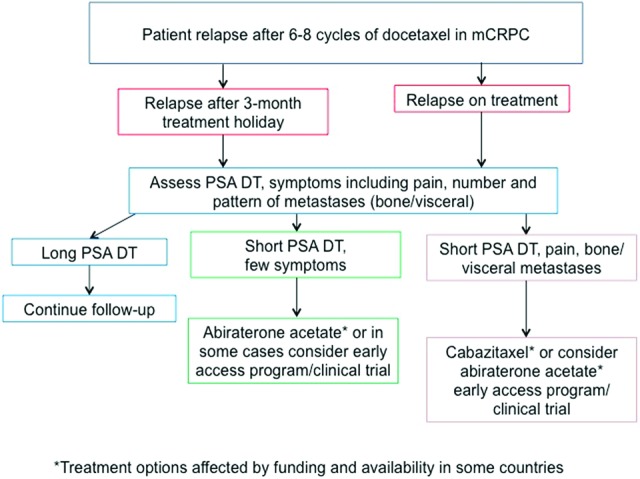
Treatment algorithm for patients with metastatic castration-resistant prostate cancer who relapse after 6–8 cycles of docetaxel. Asterisks indicate treatment options that are affected by funding and availability in some countries.
Abbreviations: mCRPC, metastatic castration-resistant prostate cancer; PSA DT, prostate-specific antigen doubling time.
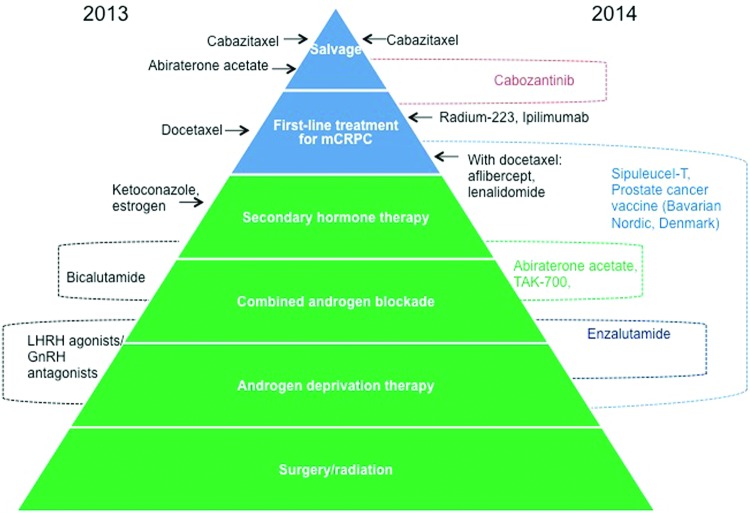
As more novel agents become available, appropriate sequencing of therapies will provide greater benefit for patients if there are validated biomarkers to best select which patients will respond best to which drug. Beyond 2013, the number of treatments available is likely to increase [92] and some treatment options are likely to be used earlier in the treatment pathway (Fig. 2).
Figure 2.
Current and possible future sequencing of drugs in castration-resistant prostate cancer in Europe.
Abbreviations: GNRH, gonadotropin-releasing hormone; LHRH, luteinizing hormone-releasing hormone; mCRPC, metastatic castration-resistant prostate cancer.
Recommendations in the Treatment of Frail or Elderly Patients
Elderly patients are often treated on the basis of age rather than fitness. In a survey of U.K. oncologists, patients of advanced age were considered ineligible for treatment with chemotherapy [40]. The life expectancy of older patients is often underestimated. Therefore, many older patients who are more likely to be—but are not necessarily—vulnerable or frail are undertreated.
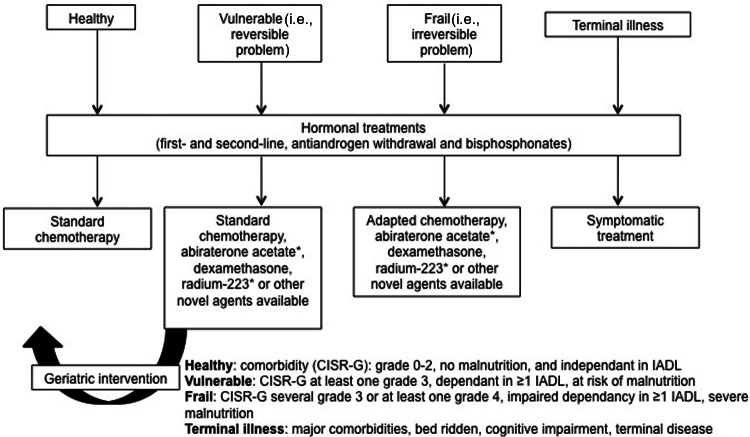
Fit and healthy older men should be treated with the same treatment as younger men [93]. Ideally, older patients (≥70 years of age) would be treated on the basis of their ability to independently engage with life and their level of fitness. The International Society of Geriatric Oncology provides clear guidelines on how to treat older patients, assessing them on the basis of those who are fit, unfit, or vulnerable (have comorbidities or other complications for treatment; Fig. 3) [93]. Generally, comorbidities are less of an issue for patients in the postchemotherapy setting compared with the prechemotherapy setting because the symptoms associated with mCRPC generally outweigh the comorbidities. Furthermore, if the patient is fit, systemic treatment may be the best form of palliation [28, 29, 31, 35, 94, 95].
Figure 3.
Treatment algorithm for older men with metastatic castration-resistant prostate cancer (≥70 years of age). Adapted from [93] with permission to include novel treatment options.*
Abbreviations: CSIR-G, Cumulative Scoring Index Rating–Geriatrics; IADL, Instrumental Activities of Independent Daily Living.
Emerging Treatment Options
Numerous new treatment options are currently being developed for the treatment of mCRPC in phase III studies (Table 2). Two phase III studies have reported improvements in OS in the postdocetaxel setting and are being reviewed by the regulatory authorities. Enzalutamide is an oral AR signaling inhibitor that was assessed in 1,199 patients with CRPC who previously received docetaxel. The median OS time for patients who received enzalutamide was 18.4 months compared with 13.6 months for those patients receiving the placebo (HR: 0.631, 95% CI: 0.529–0.752; p < .0001) [28]. The drug was approved by the FDA and is currently being reviewed by the European Medicines Agency.
Table 2.
Summary of ongoing phase III clinical studies of novel agents for metastatic castration-resistant prostate cancer
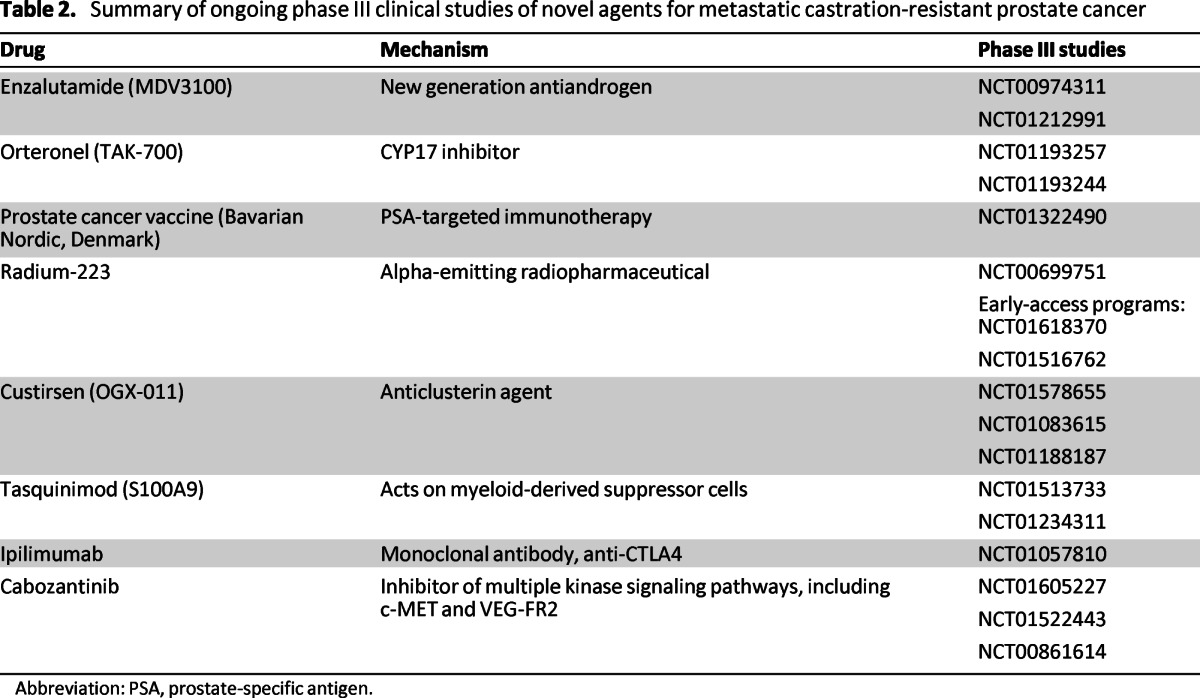
Abbreviation: PSA, prostate-specific antigen.
Abiraterone acetate plus prednisone has also been investigated earlier in the treatment pathway. In the COU-AA-302 study, abiraterone acetate plus prednisone was given to asymptomatic or mildly symptomatic chemotherapy-naïve patients with mCRPC. Results from the updated interim analysis indicated a significant improvement in radiographic progression-free survival in the abiraterone acetate plus prednisone arm compared with the placebo plus prednisone arm (8.3 months for placebo plus prednisone and 16.5 months for abiraterone acetate plus prednisone; HR: 0.53, 95% CI: 0.45–0.62; p < .0001). Median OS times were 35.3 months in the abiraterone acetate arm and 30.1 months in the control arm, but the prespecified level of significance was not yet reached [36, 37]. Abiraterone acetate plus prednisone was approved by the FDA for treatment of men with mCRPC prior to receiving chemotherapy in December 2012. In contrast to Europe, abiraterone acetate plus prednisone may now be given earlier in the disease and enzalutamide is also available for patients after docetaxel as an alternative to cabazitaxel in the U.S.
The negative result from the READY study adds to the growing list of drugs that have failed to report an OS benefit. Difficulty in translating activity of new drugs observed in phase I and II studies into an improvement in OS in phase III studies may reflect the heterogeneity of the disease.
Many more drugs are currently being investigated in phase I/II studies of CRPC including tyrosine kinase inhibitors (sunitinib [96], sorafinib [97]), estrogens [98, 99], antisense molecules (OGX-011 [99]), immunotherapies (Biosante Pharmaceuticals, USA [101], Bavarian Nordic, Denmark [102]), a selective inhibitor of CYP17 (orteronel [103]), novel antiandrogens (ARN-509 [104], ODM-201 [105]), antiangiogenesis agents (lenalidomide [106–108]), and epothilones [109]. In addition, Src kinase inhibitors are being investigated for mCRPC; however, when dasatanib was added to docetaxel in the phase III READY study, no improvement in OS was observed [110].
The negative result from the READY study adds to the growing list of drugs that have failed to report an OS benefit [111–114]. Difficulty in translating activity of new drugs observed in phase I and II studies into an improvement in OS in phase III studies may reflect the heterogeneity of the disease. However, study design also requires careful consideration. Some new drugs do not target the androgenic axis; therefore, to accurately assess efficacy, study endpoints must reflect the target of the drug. For example, with bone-targeting drugs such as cabozantinib and immunotherapies such as sipuleucel-T, PSA is not an accurate marker [55, 62, 67, 115], but with hormonal therapies, there is a clear PSA response [28, 30, 31, 36] in responding patients. In addition, changes in PSA might not be consistent with other markers of response/progression. For example, in the COU-AA-301 study, time to PSA progression was 8.5 months, whereas time to radiographic progression was 5.6 months in the abiraterone acetate plus prednisone treatment arm [30].
It is possible to select study endpoints that are specific to drug activity and include biomarkers in study protocols to better understand patient outcomes. As there is no universal definition of progression, protocols to assess progression must also be reliable, consistent, and feasible for inclusion in large randomized controlled phase III studies to ensure that there are no interlaboratory or intercenter effects that may confound results. In a phase III study of atrasentan, there was a difference in the time to disease progression between the placebo and atrasentan treatment arms reported by U.S. and non-U.S. sites (U.S. sites: 590 days with atrasentan vs. 671 days with placebo, HR: 1.15, 95% CI: 0.86–1.54, p = .17; non-U.S. sites: 847 days with atrasentan vs. 667 days with placebo, HR: 0.80, 95% CI: 0.63–1.00, p = .02) [114]. Ensuring that patients receive on-study treatment long enough for efficacy to be determined by using clear protocols (e.g., parameters to distinguish bone flare from bone progression) is also essential for successful clinical studies [78].
Summary
There are many exciting developments, both recent and anticipated, in the treatment of mCRPC in the postdocetaxel setting. An increased understanding of the mechanisms behind the development of CRPC has led to the development of several new targeted agents that have improved treatment outcomes for patients. However, mCRPC remains a chronic disease with poor expected survival. We have yet to determine in which sequence to use novel agents and how to select the patients most likely to respond well. Because of pending approval from regulatory authorities and budgetary constraints, physicians practicing in Europe have fewer drugs available for sequencing or combining treatments than their U.S. colleagues. It is hoped that future studies will identify markers and the best assessment tools for novel agents to further improve both survival and quality of life of patients with mCRPC.
Acknowledgments
This article is based on an advisory board meeting supported by Janssen Pharmaceutical Companies of Johnson & Johnson in Europe, the Middle East, and Africa. This work was supported by an educational grant from Janssen to assist with editorial support.
We thank Cheryl Jenkins (Rocket Science Medical Communications, supported by Janssen) for medical writing assistance, as well as the members of the European Treatment Practices group for their contribution to discussions that have been captured in this article: Gerhardt Attard, Valentina Baldazzi, Sergio Barroso, Alfredo Berruti, Joan Carles, Noel Clarke, Maria De Santis, Giuseppe Di Lorenzo, Ignacio Duran, Karim Fizazi, Wolfgang Loidl, Xavier Maldonado, Nicolas Mottet, Pieter Mulders, Christos Papandreou, Heather Payne, and Giuseppe Procopio.
Author Contributions
Conception/Design: Axel S. Merseburger, Joaquim Bellmunt, Cheryl Jenkins, Chris Parker, John M. Fitzpatrick
Collection and/or assembly of data: Axel S. Merseburger, Joaquim Bellmunt, Cheryl Jenkins, Chris Parker, John M. Fitzpatrick
Data analysis and interpretation: Axel S. Merseburger, Joaquim Bellmunt, Cheryl Jenkins, Chris Parker, John M. Fitzpatrick
Manuscript writing: Axel S. Merseburger, Joaquim Bellmunt, Cheryl Jenkins, Chris Parker, John M. Fitzpatrick
Final approval of manuscript: Axel S. Merseburger, Joaquim Bellmunt, Cheryl Jenkins, Chris Parker, John M. Fitzpatrick
Disclosures
Axel S. Merseburger: AstraZeneca, Pfizer, Janssen, Astellas, Teva, Ipsen (C/A); AstraZeneca, Pfizer, Janssen, Astellas, Teva, Ipsen, Pierre Fabre, Novartis, GlaxoSmithKline (H); Bayer, Astellas, Janssen, Teva (RF); Cheryl Jenkins: Janssen (C/A); Chris Parker: Bayer, BNIT (C/A); Astellas, Bayer, Janssen, Sanofi-Aventis, Takeda (H); John M. Fitzpatrick: Sanofi, Astellas, Janssen (C/A, H). The other author reported no financial relationships.
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792–800. doi: 10.1093/annonc/mdt010. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bastian PJ, Bellmunt J, et al. European Association of Urology: Guidelines on prostate cancer 2012. [Accessed April 5, 2013]. Available at http://www.uroweb.org/guidelines/online-guidelines/
- 6.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: A systematic review. Int J Clin Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 7.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 8.Palmberg C, Koivisto P, Kakkola L, et al. Androgen receptor gene amplification at primary progression predicts response to combined androgen blockade as second line therapy for advanced prostate cancer. J Urol. 2000;164:1992–1995. [PubMed] [Google Scholar]
- 9.Ford OH, III, Gregory CW, Kim D, et al. Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J Urol. 2003;170:1817–1821. doi: 10.1097/01.ju.0000091873.09677.f4. [DOI] [PubMed] [Google Scholar]
- 10.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Palma JF, Agus DB, et al. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem. 2010;56:1492–1495. doi: 10.1373/clinchem.2010.143297. [DOI] [PubMed] [Google Scholar]
- 12.Steinkamp MP, O'Mahony OA, Brogley M, et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009;69:4434–4442. doi: 10.1158/0008-5472.CAN-08-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchanan G, Greenberg NM, Scher HI, et al. Collocation of androgen receptor gene mutations in prostate cancer. Clin Cancer Res. 2001;7:1273–1281. [PubMed] [Google Scholar]
- 14.Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 15.Bastus NC, Boyd LK, Mao X, et al. Androgen-induced TMPRSS2:ERG fusion in nonmalignant prostate epithelial cells. Cancer Res. 2010;70:9544–9548. doi: 10.1158/0008-5472.CAN-10-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comuzzi B, Lambrinidis L, Rogatsch H, et al. The transcriptional co-activator cAMP response element-binding protein-binding protein is expressed in prostate cancer and enhances androgen- and anti-androgen-induced androgen receptor function. Am J Pathol. 2003;162:233–241. doi: 10.1016/S0002-9440(10)63814-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Culig Z, Comuzzi B, Steiner H, et al. Expression and function of androgen receptor coactivators in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:265–271. doi: 10.1016/j.jsbmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Ueda T, Mawji NR, Bruchovsky N, et al. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002;277:38087–38094. doi: 10.1074/jbc.M203313200. [DOI] [PubMed] [Google Scholar]
- 19.Seaton A, Scullin P, Maxwell PJ, et al. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis. 2008;29:1148–1156. doi: 10.1093/carcin/bgn109. [DOI] [PubMed] [Google Scholar]
- 20.Stope MB, Ronnau C, Schubert T, et al. Transforming growth factor beta in prostate cancer: Cellular effects and basic molecular mechanisms. Urologe A. 2013;52:378–383. doi: 10.1007/s00120-012-3049-5. [DOI] [PubMed] [Google Scholar]
- 21.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon CG, Locke JA, Adomat HH, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70:390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 23.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71:1656–1667. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: Lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 27.Aryee MJ, Liu W, Engelmann JC, et al. DNA methylation alterations exhibit intraindividual stability and interindividual heterogeneity in prostate cancer metastases. Sci Transl Med. 2013;5:169ra10. doi: 10.1126/scitranslmed.3005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 29.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 30.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 31.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker C, Nilsson S, Heinrich D, et al. Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA) J Clin Oncol. 2012;30:LBA4512. [Google Scholar]
- 33.Tannock IF, de WR, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 34.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 35.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 36.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim analysis of COU-AA-302, a randomized phase III study of abiraterone acetate in patients wiht metastatic castration-resistant prostate cancer without prior chemotherapy. J Clin Oncol. 2013;31:5. [Google Scholar]
- 38.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 39.de Bono JS, Fizazi K, Saad F, et al. Primary, secondary and quality of life endpoint results from the phase III AFFIRM study of MDV3100, an androgen receptor signalling inhibitor. J Clin Oncol. 2012;30:4519. [Google Scholar]
- 40.Payne H, Bahl A, Mason M, Troup J, De BJ. Optimizing the care of patients with advanced prostate cancer in the UK: Current challenges and future opportunities. BJU Int. 2012;110:658–667. doi: 10.1111/j.1464-410X.2011.10886.x. [DOI] [PubMed] [Google Scholar]
- 41.Fitzpatrick JM. Optimizing the management of prostate cancer in senior adults: call to action. The Oncologist. 2012;17(Suppl 1):1–3. doi: 10.1634/theoncologist.2012-S1-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berruti A, Tucci M, Mosca A, et al. Predictive factors for skeletal complications in hormone-refractory prostate cancer patients with metastatic bone disease. Br J Cancer. 2005;93:633–638. doi: 10.1038/sj.bjc.6602767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–583. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 44.U.S. Food and Drug Administration. Clinical team leader review memo regarding SGE patient representative: Provenge, April 26, 2010. [Accessed April 5, 2013]. Available at http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm213559.htm.
- 45.Huber ML, Haynes L, Parker C, et al. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104:273–279. doi: 10.1093/jnci/djr514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dendreon. Sipuleucel-T manufacturing demonstration study: NCT01477749. [Accessed April 5, 2013]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01477749.
- 47.Sonpavde G, Di LG, Higano CS, et al. The role of sipuleucel-T in therapy for castration-resistant prostate cancer: A critical analysis of the literature. Eur Urol. 2012;61:639–647. doi: 10.1016/j.eururo.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Lipton A. Implications of bone metastases and the benefits of bone-targeted therapy. Semin Oncol. 2010;37(Suppl 2):S15–S29. doi: 10.1053/j.seminoncol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 50.Lange PH, Vessella RL. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 1998;17:331–336. doi: 10.1023/a:1006106209527. [DOI] [PubMed] [Google Scholar]
- 51.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 52.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 53.Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 54.Aapro M, Saad F. Bone-modifying agents in the treatment of bone metastases in patients with advanced genitourinary malignancies: A focus on zoledronic acid. Ther Adv Urol. 2012;4:85–101. doi: 10.1177/1756287212441234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogelzang NJ, Parker C, Nilsson S, et al. Updated analysis of radium-223 dichloride (RA-223) impact on skeletal-related events (SRE) in patients with castration-resistant prostate cancer (CRPC) and bone metastases from the phase III randomized trial (ALSYMPCA) J Clin Oncol. 2013;31:11. [Google Scholar]
- 58.Sartor O, Michels RM, Massard C, et al. Novel therapeutic strategies for metastatic prostate cancer in the post-docetaxel setting. The Oncologist. 2011;16:1487–1497. doi: 10.1634/theoncologist.2010-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eder JP, Vande Woude GF, Boerner SA, et al. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 61.Hussain M, Smith MR, Sweeney C, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. J Clin Oncol. 2011;29:4516. [Google Scholar]
- 62.Smith MR, Sweeney C, Rathkopf DE, et al. Cabozantinib (XL184) in chemotherapy-pretreated metastatic castration-resistant prostate cancer: Results from a phase II nonrandomized expansion cohort. J Clin Oncol. 2012;30:4513. doi: 10.1200/JCO.2013.54.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Bono JS, Smith MR, Rathkopf DE, et al. Cabozantinib (XL184) at 40 mg in patients with metastatic castration-resistant prostate cancer: Results of a phase 2 non-randomized expansion cohort (NRE) Ann Oncol. 2012;23 Abstract 8970. [Google Scholar]
- 64.Armstrong AJ, Eisenberger MA, Halabi S, et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol. 2012;61:549–559. doi: 10.1016/j.eururo.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dancey JE, Dobbin KK, Groshen S, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16:1745–1755. doi: 10.1158/1078-0432.CCR-09-2167. [DOI] [PubMed] [Google Scholar]
- 66.Merseburger AS, Hennenlotter J, Simon P, et al. Activation of the PKB/Akt pathway in histological benign prostatic tissue adjacent to the primary malignant lesions. Oncol Rep. 2006;16:79–83. [PubMed] [Google Scholar]
- 67.Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: Results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubenstein JH, Katin MJ, Mangano MM, et al. Small cell anaplastic carcinoma of the prostate: Seven new cases, review of the literature, and discussion of a therapeutic strategy. Am J Clin Oncol. 1997;20:376–380. doi: 10.1097/00000421-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 69.Oudard S, Banu E, Scotte F, et al. Prostate-specific antigen doubling time before onset of chemotherapy as a predictor of survival for hormone-refractory prostate cancer patients. Ann Oncol. 2007;18:1828–1833. doi: 10.1093/annonc/mdm332. [DOI] [PubMed] [Google Scholar]
- 70.Colloca G. Prostate-specific antigen kinetics as a surrogate endpoint in clinical trials of metastatic castration-resistant prostate cancer: A review. Cancer Treat Rev. 2012;38:1020–1026. doi: 10.1016/j.ctrv.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonpavde G, Pond GR, Berry WR, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012;30:607–613. doi: 10.1016/j.urolonc.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 74.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 75.Miyamoto DT, Lee RJ, Stott SL, et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2012;2:995–1003. doi: 10.1158/2159-8290.CD-12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 77.Armstrong AJ, Garrett-Mayer ES, Yang YC, et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: A TAX327 study analysis. Clin Cancer Res. 2007;13:6396–6403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong AJ, Garrett-Mayer E, de WR, et al. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–211. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- 79.Ito M, Saito K, Yasuda Y, et al. Prognostic impact of C-reactive protein for determining overall survival of patients with castration-resistant prostate cancer treated with docetaxel. Urology. 2011;78:1131–1135. doi: 10.1016/j.urology.2011.07.1416. [DOI] [PubMed] [Google Scholar]
- 80.Logothetis CJ, Millikan R. Chemotherapy for advanced prostate cancer: 25 years later. J Clin Oncol. 2008;26:2423–2424. doi: 10.1200/JCO.2007.14.7819. [DOI] [PubMed] [Google Scholar]
- 81.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 82.Rescigno P, Buonerba C, Bellmunt J, et al. New perspectives in the therapy of castration resistant prostate cancer. Curr Drug Targets. 2012;13:1676–1686. doi: 10.2174/138945012803529956. [DOI] [PubMed] [Google Scholar]
- 83.Nelson PS. Molecular states underlying androgen receptor activation: A framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol. 2012;30:644–646. doi: 10.1200/JCO.2011.39.1300. [DOI] [PubMed] [Google Scholar]
- 84.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yap TA, Swanton C, de Bono JS. Personalization of prostate cancer prevention and therapy: Are clinically qualified biomarkers in the horizon? EPMA J. 2012;3:3. doi: 10.1007/s13167-011-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bourke L, Kirkbride P, Hooper R, et al. Endocrine therapy in prostate cancer: Time for reappraisal of risks, benefits and cost-effectiveness? Br J Cancer. 2013;108:9–13. doi: 10.1038/bjc.2012.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scher HI, Morris MJ, Basch E, et al. End points and outcomes in castration-resistant prostate cancer: From clinical trials to clinical practice. J Clin Oncol. 2011;29:3695–3704. doi: 10.1200/JCO.2011.35.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sartor AO, Fitzpatrick JM. Urologists and oncologists: Adapting to a new treatment paradigm in castration-resistant prostate cancer (CRPC) BJU Int. 2012;110:328–335. doi: 10.1111/j.1464-410X.2011.10818.x. [DOI] [PubMed] [Google Scholar]
- 89.Caffo O, Pappagallo G, Brugnara S, et al. Multiple rechallenges for castration-resistant prostate cancer patients responding to first-line docetaxel: Assessment of clinical outcomes and predictive factors. Urology. 2012;79:644–649. doi: 10.1016/j.urology.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 90.Loriot Y, Massard C, Gross-Goupil M, et al. The interval from the last cycle of docetaxel-based chemotherapy to progression is associated with the efficacy of subsequent docetaxel in patients with prostate cancer. Eur J Cancer. 2010;46:1770–1772. doi: 10.1016/j.ejca.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 91.Heck MM, Thalgott M, Retz M, et al. Rational indication for docetaxel rechallenge in metastatic castration-resistant prostate cancer. BJU Int. 2012;110:E635–E640. doi: 10.1111/j.1464-410X.2012.11364.x. [DOI] [PubMed] [Google Scholar]
- 92.Crawford ED, Flaig TW. Optimizing outcomes of advanced prostate cancer: Drug sequencing and novel therapeutic approaches. Oncology (Williston Park) 2012;26:70–77. [PubMed] [Google Scholar]
- 93.Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: Recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106:462–469. doi: 10.1111/j.1464-410X.2010.09334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Logothetis CJ, de Bono JS, Molina A, et al. Effect of abiraterone acetate (AA) on pain control and skeletal-related events (SRE) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) post docetaxel (D): Results from the COU-AA-301 phase III study. J Clin Oncol. 2011;29:4520. [Google Scholar]
- 95.Nilsson S, Strang P, Aksnes AK, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48:678–686. doi: 10.1016/j.ejca.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 96.Dror MM, Regan MM, Oh WK, et al. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol. 2009;20:913–920. doi: 10.1093/annonc/mdp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zengerling F, Streicher W, Schrader AJ, et al. Effects of sorafenib on C-terminally truncated androgen receptor variants in human prostate cancer cells. Int J Mol Sci. 2012;13:11530–11542. doi: 10.3390/ijms130911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oh WK, Kantoff PW, Weinberg V, et al. Prospective, multicenter, randomized phase II trial of the herbal supplement, PC-SPES, and diethylstilbestrol in patients with androgen-independent prostate cancer. J Clin Oncol. 2004;22:3705–3712. doi: 10.1200/JCO.2004.10.195. [DOI] [PubMed] [Google Scholar]
- 99.Aggarwal R, Weinberg V, Small EJ, et al. The mechanism of action of estrogen in castration-resistant prostate cancer: Clues from hormone levels. Clin Genitourin Cancer. 2009;7:E71–E76. doi: 10.3816/CGC.2009.n.027. [DOI] [PubMed] [Google Scholar]
- 100.Saad F, Hotte S, North S, et al. Randomized phase II trial of Custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG trial P-06c. Clin Cancer Res. 2011;17:5765–5773. doi: 10.1158/1078-0432.CCR-11-0859. [DOI] [PubMed] [Google Scholar]
- 101.Santegoets SJ, Stam AG, Lougheed SM, et al. T cell profiling reveals high CD4(+)CTLA-4 (+) T cell frequency as dominant predictor for survival after Prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother. 2013;62:245–256. doi: 10.1007/s00262-012-1330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Petrylak D, Gandhi JG, Clark WR, et al. A phase I/II study of safety and efficacy of orteronel (TAK-700), an oral, investigational, nonsteroidal 17,20-lyase inhibitor, with docetaxel and prednisone (DP) in metastatic castration-resistant prostate cancer (mCRPC): Updated phase II results. J Clin Oncol. 2013;31:59. [Google Scholar]
- 104.Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fizazi K, Massard C, James ND, et al. ODM-21, a new generation androgen receptor inhibitor for castration-resistant prostate cancer: Preclinical and phase I data. J Clin Oncol. 2013;31:65. [Google Scholar]
- 106.Henry JY, Lu L, Adams M, et al. Lenalidomide enhances the anti-prostate cancer activity of docetaxel in vitro and in vivo. Prostate. 2012;72:856–867. doi: 10.1002/pros.21488. [DOI] [PubMed] [Google Scholar]
- 107.Keizman D, Zahurak M, Sinibaldi V, et al. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: Results of a phase I/II double-blinded, randomized study. Clin Cancer Res. 2010;16:5269–5276. doi: 10.1158/1078-0432.CCR-10-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dahut WL, Aragon-Ching JB, Woo S, et al. Phase I study of oral lenalidomide in patients with refractory metastatic cancer. J Clin Pharmacol. 2009;49:650–660. doi: 10.1177/0091270009335001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beer TM, Smith DC, Hussain A, et al. Phase II study of first-line sagopilone plus prednisone in patients with castration-resistant prostate cancer: A phase II study of the Department of Defense Prostate Cancer Clinical Trials Consortium. Br J Cancer. 2012;107:808–813. doi: 10.1038/bjc.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Araujo JC, Trudel GC, Saad F, et al. Overall survival (OS) and safety of dasatinib/docetaxel versus docetaxel in patients with metastatic castration-resistant prostate cancer (mCRPC): Results from the randomized phase III READY trial. J Clin Oncol. 2013;31:LBA8. [Google Scholar]
- 111.Miller K, Moul JW, Gleave M, et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:187–192. doi: 10.1038/pcan.2013.2. [DOI] [PubMed] [Google Scholar]
- 112.Sternberg CN, Petrylak DP, Sartor O, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: The SPARC trial. J Clin Oncol. 2009;27:5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 113.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or with-out bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30:1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–2487. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Migano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]