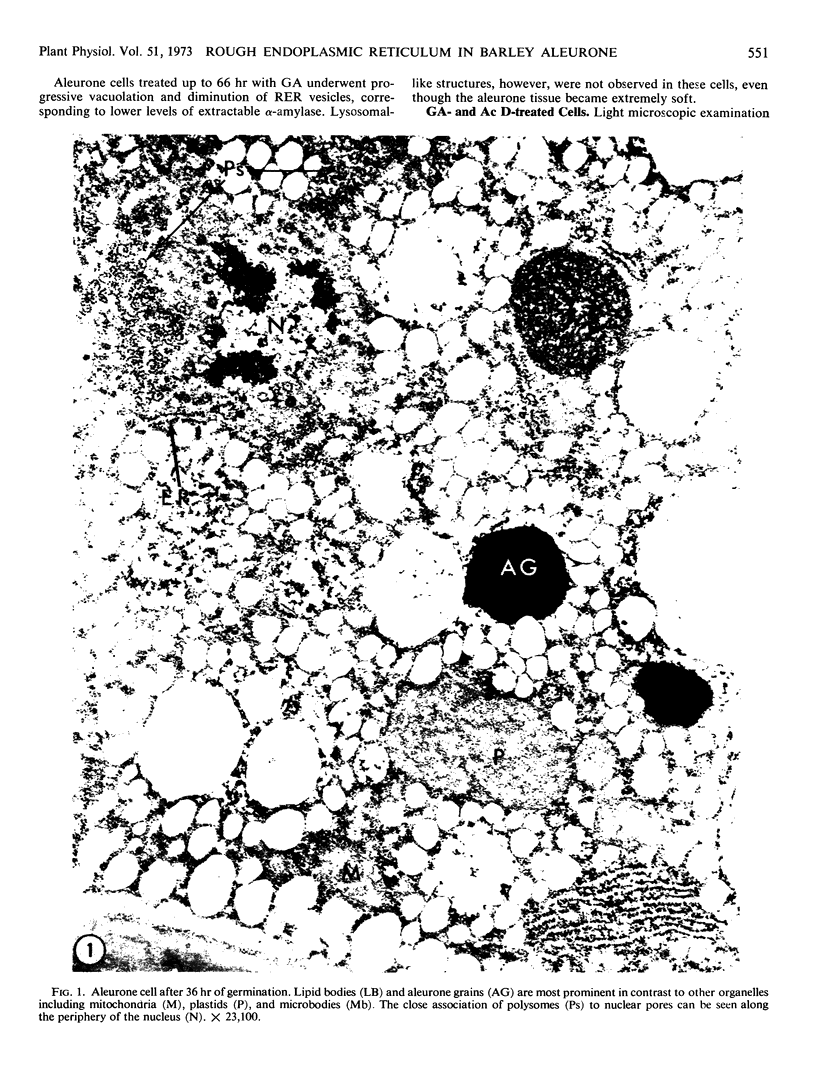
Abstract
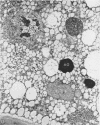
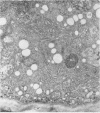
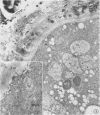
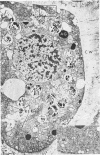
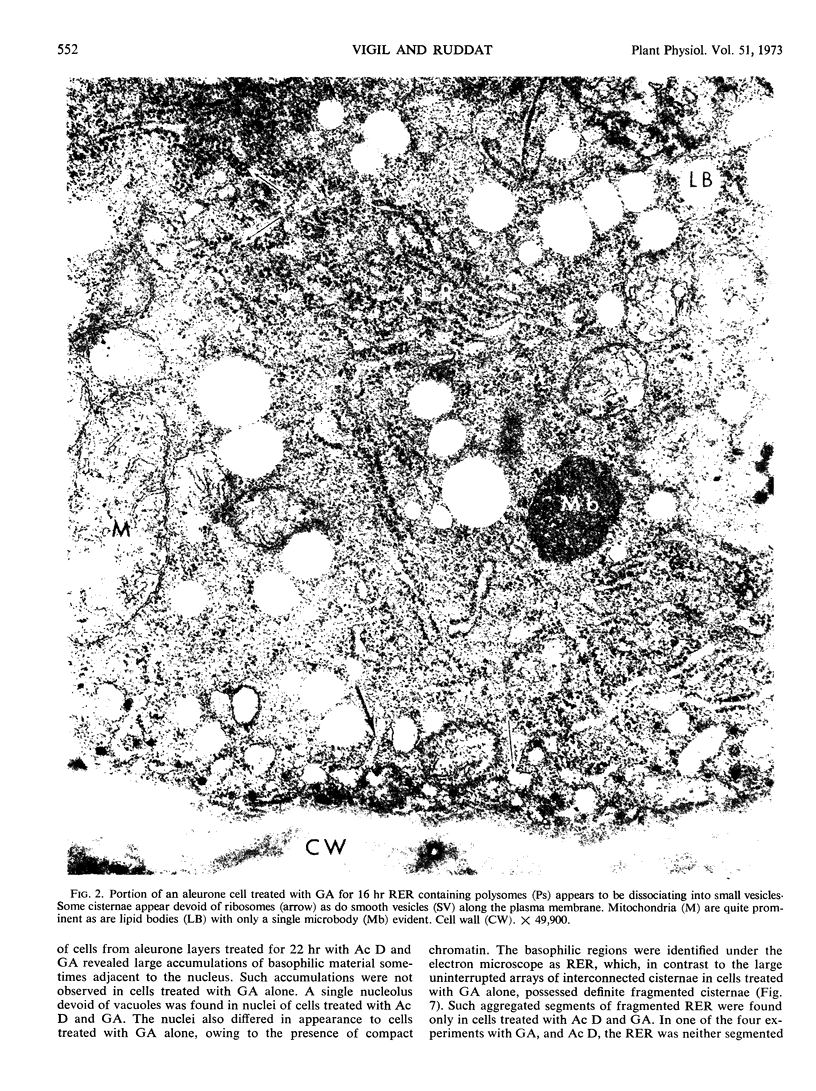
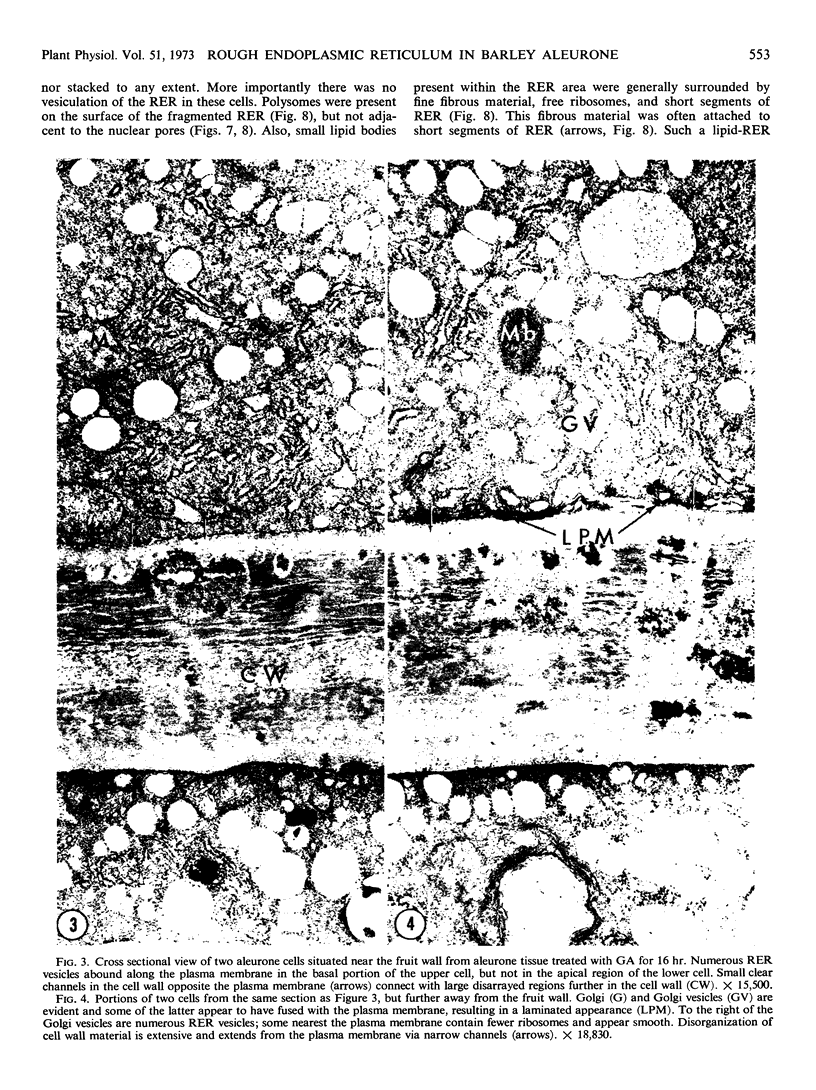
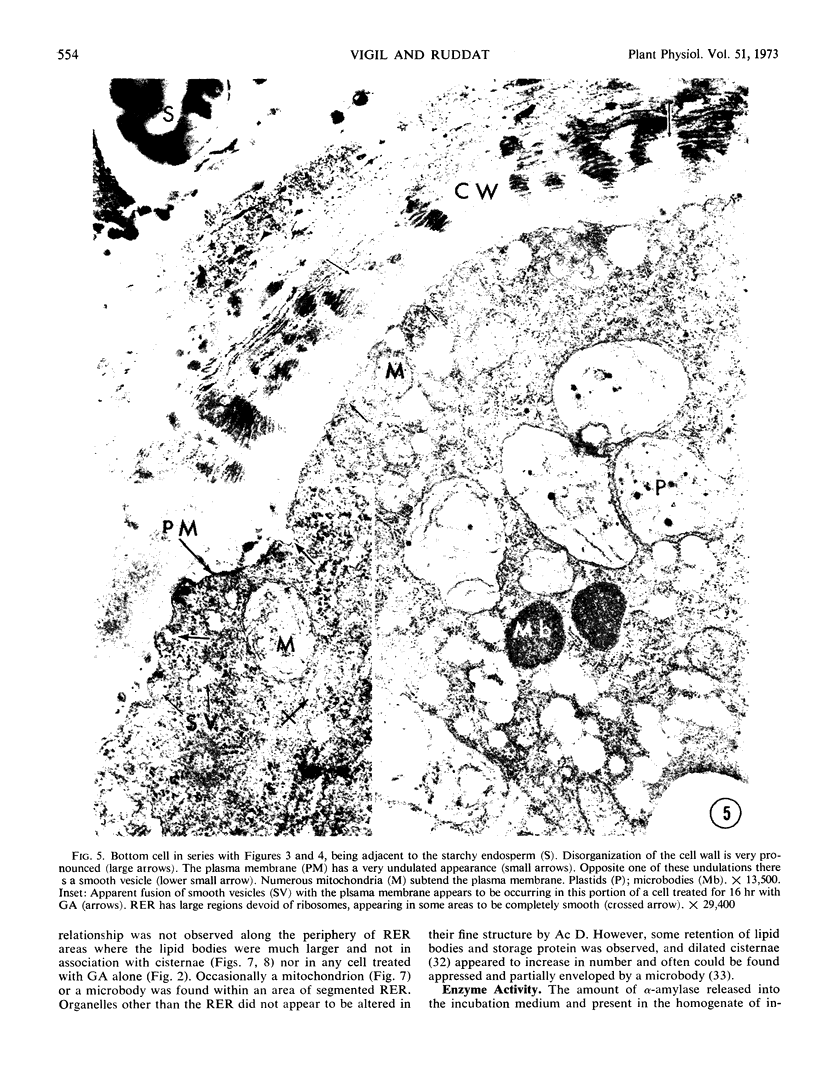
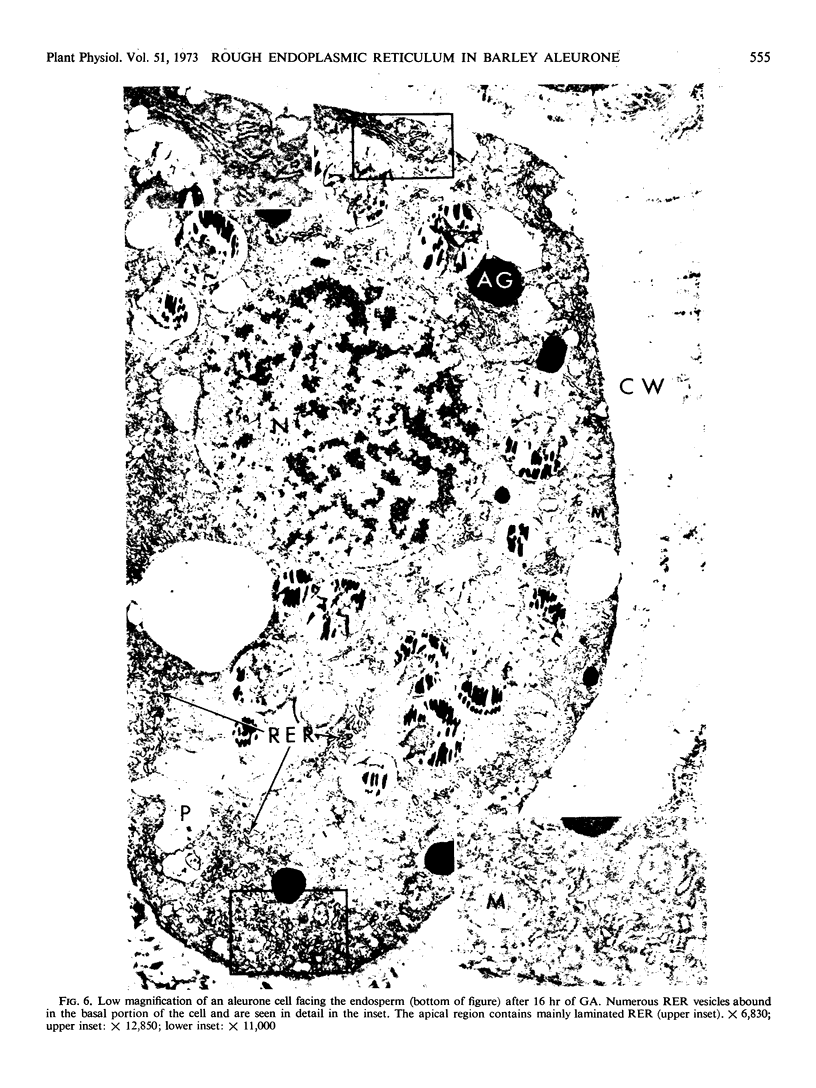
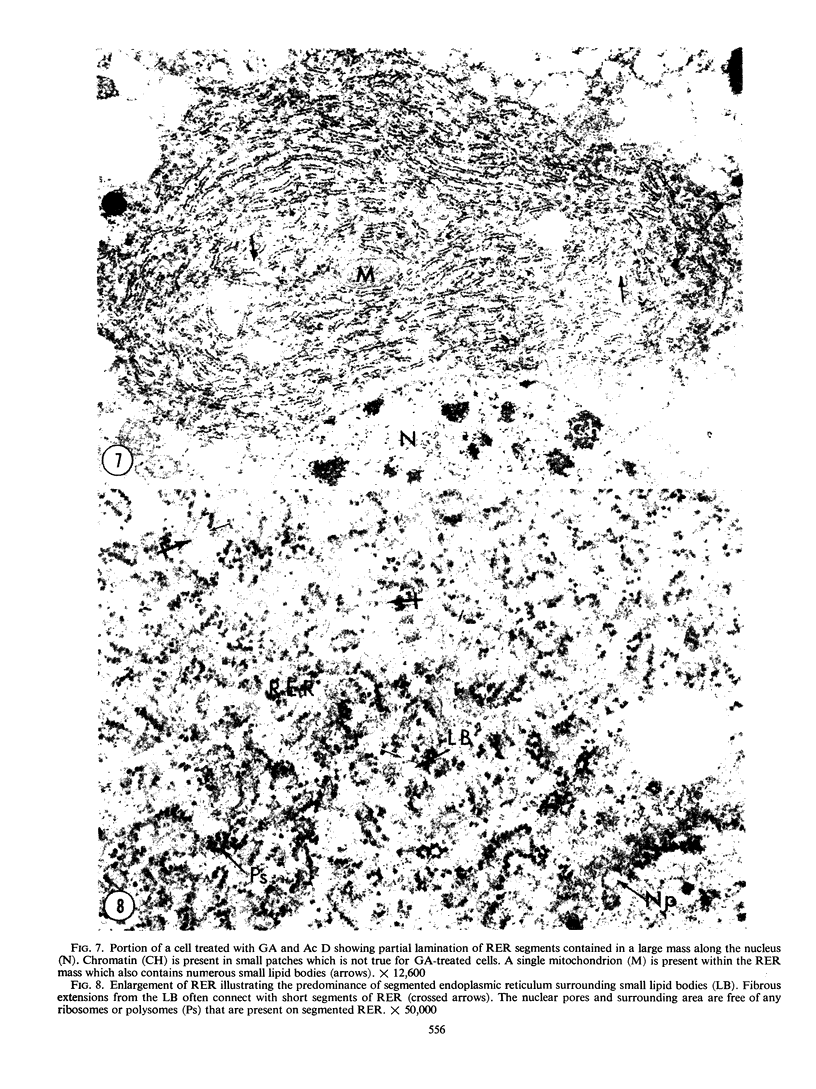
Analysis of structural changes in barley aleurone cells during germination or following incubation of isolated layers in gibberellic acid with or without actinomycin D revealed extensive development of rough endoplasmic reticulum. Following the assembly of stacked rough endoplasmic reticulum, vesiculation occurred mainly in basal regions of the cell, resulting in a polar distribution of rough endoplasmic reticulum vesicles. It is postulated that these vesicles are involved in protein secretion, because smooth vesicles, derived from the rough endoplasmic reticulum, apparently become appressed to the plasma membrane. The increased α-amylase in the ambient medium and in cell homogenates correlated directly with formation and subsequent vesiculation of the rough endoplasmic reticulum. Furthermore, when cells were treated with actinomycin D and gibberellic acid, α-amylase synthesis was inhibited by 45% and secretion by 63%. These cells were characterized cytologically by large areas of disarrayed segments of fragmented rough endoplasmic reticulum, corresponding to a high intracellular level of α-amylase. In addition, small lipid bodies common to the segmented regions of rough endoplasmic reticulum were surrounded by fine fibrous material, short segments of rough endoplasmic reticulum, and free ribosomes, suggesting that actinomycin D had interfered with development and organization of rough endoplasmic reticulum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Hormonal control of enzyme synthesis: on the mode of action of gibberellic Acid and abscisin in aleurone layers of barley. Plant Physiol. 1967 Jul;42(7):1008–1016. doi: 10.1104/pp.42.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins W. H., Varner J. E. Hormonal control of polyribosome formation in barley aleurone layers. Plant Physiol. 1972 Mar;49(3):348–352. doi: 10.1104/pp.49.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley alpha-amylase induced by gibberellic acid. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1520–1526. doi: 10.1073/pnas.58.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. A., Paleg L. G. Lysosomal nature of hormonally induced enzymes in wheat aleurone cells. Biochem J. 1972 Jun;128(2):367–375. doi: 10.1042/bj1280367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips S. H., Avissar Y. Plant-leaf microbodies as the intracellular site of nitrate reductase and nitrite reductase. Eur J Biochem. 1972 Aug 18;29(1):20–24. doi: 10.1111/j.1432-1033.1972.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Oka T., Topper Y. J. Hormone-dependent accumulation of rough endoplasmic reticulum in mouse mammary epithelial cells in vitro. J Biol Chem. 1971 Dec 25;246(24):7701–7707. [PubMed] [Google Scholar]
- Pastan I., Friedman R. M. Actinomycin D: inhibition of phospholipid synthesis in chick embryo cells. Science. 1968 Apr 19;160(3825):316–317. doi: 10.1126/science.160.3825.316. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Mense R. M. Characteristics of the process of enzyme release from secretory plant cells. Plant Physiol. 1972 Feb;49(2):187–189. doi: 10.1104/pp.49.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yomo H., Varner J. E. Hormonal control of a secretory tissue. Curr Top Dev Biol. 1971;6(6):111–144. doi: 10.1016/s0070-2153(08)60639-0. [DOI] [PubMed] [Google Scholar]