Abstract
The CCN family of matricellular proteins is critical for embryonic development and plays important roles in inflammation, wound healing, and injury repair in the adult. Deregulation of their expression or activities contributes to the pathobiology of myriad diseases, many of which may arise when inflammation or tissue injury becomes chronic, including fibrosis, arthrosclerosis, arthritis, diabetic nephropathy and retinopathy, and cancer. Emerging studies indicate that targeting CCN expression or signaling pathways holds promise in the development of diagnostics and therapeutics for such diseases. This review summarizes the biology of CCN proteins, their roles in various pathologies, and potential as therapeutic targets.
INTRODUCTION
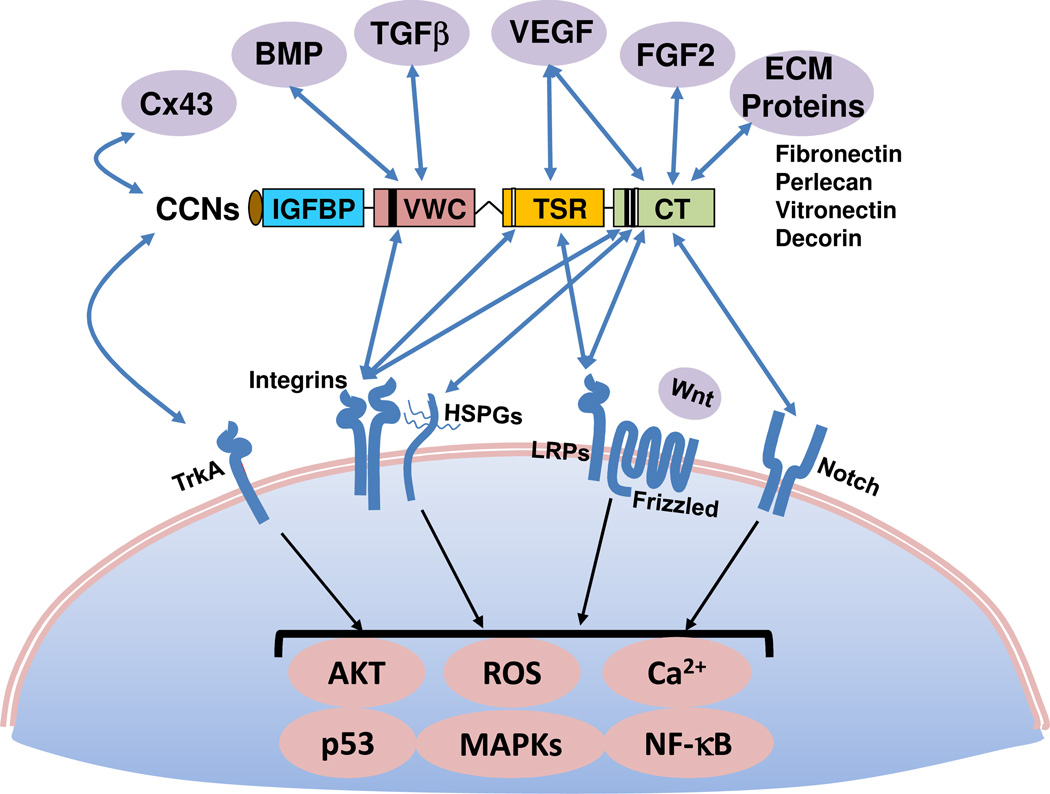
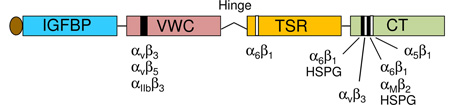
Beyond serving as a scaffold for the organization of cells into tissues, the extracellular matrix (ECM) is also a multifunctional regulator of cellular behavior. ECM proteins can modulate the activity or bioavailability of extracellular signaling molecules such as growth factors, cytokines, chemokines, and extracellular enzymes, or directly bind to and signal through cell surface receptors to regulate cell functions. A subset of ECM proteins, known as matricellular proteins, is dynamically expressed and serves primarily regulatory rather than structural roles1, 2. Among known matricellular proteins are members of the CCN family, a group of highly conserved secreted proteins identified by differential expression screening whose synthesis is regulated by mitogenic growth factors or oncogenic transformation2–4. The acronym CCN is derived from the first three members of the family described, namely CYR61 (cysteine-rich 61/CCN1), CTGF (connective tissue growth factor/CCN2), and NOV (nephroblastoma overexpressed/CCN3). Together with a set of three Wnt-inducible signaling pathway proteins (WISP1/CCN4, WISP2/CCN5, and WISP3/CCN6), they comprise a family of six homologous cysteine-rich proteins in mammals that have been renamed CCN1–6 by international consensus5. CCN proteins share a modular structure, with an N-terminal secretory peptide followed by four conserved domains with sequence homologies to insulin-like growth factor binding proteins (IGFBP), von Willebrand factor type C repeat (VWC), thrombospondin type I repeat (TSR), and a carboxyl-terminal domain (CT) that contains a cysteine-knot motif (Box 1). A non-conserved, protease-sensitive central hinge region bisects the proteins into two halves that bind distinct cell surface receptors. The expression of CCN proteins is exquisitely regulated on transcriptional, post-transcriptional, and translational levels in response to changes in environmental stimuli, including those encountered in tissue injury repair (Box 1, Fig. 1).
Box 1. The CCN family of matricellular proteins.
CCN proteins (except CCN5) are comprised of an N-terminal secretary peptide and four conserved modular domains – IGFBPs, VWC, TSR, and CT – each encoded by a separate exon. A non-conserved central hinge region divides the protein into two halves with different binding capabilities to extracellular proteins and cell surface receptors (see Fig. 2). The locations of identified binding sites for integrin receptors and HSPGs are indicated in the schematic diagram2. CCN proteins contain 38 conserved cysteines located throughout the polypeptide, with the exception of CCN5, which lacks precisely the CT domain but otherwise conserves all cysteines in the remaining three domains, and CCN6, in which four cysteines in the VWC domain are not conserved.
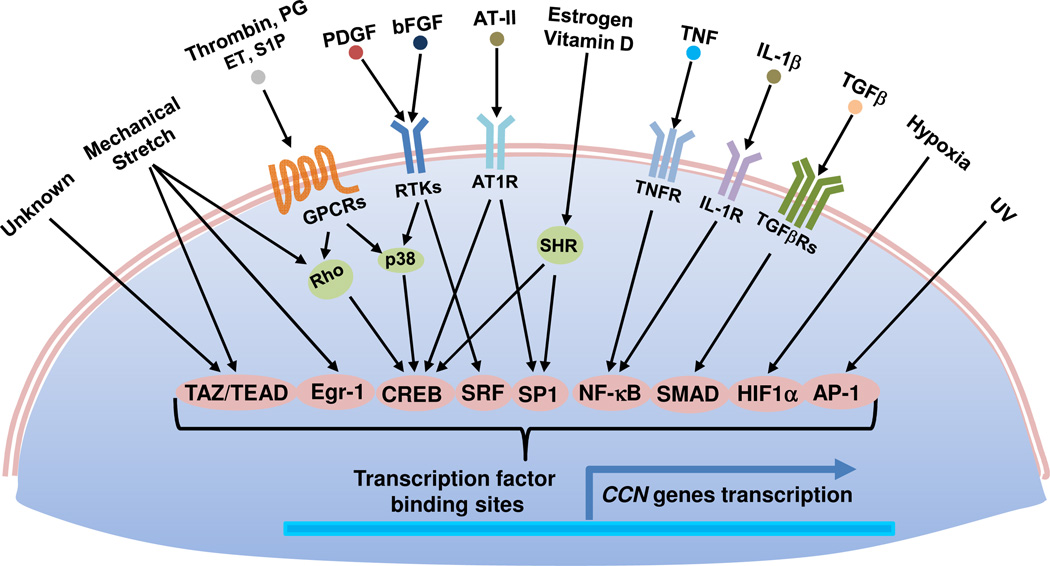
CCN genes are exquisitely sensitive to regulation by mitogenic signals and a wide range of environmental perturbations, including exposure to growth factors, inflammatory cytokines, steroid hormones, oxygen deprivation, UV irradiation, and mechanical forces (Fig. 1). During embryonic development, CCNs are broadly expressed in many organs and tissues, especially in endothelial cells throughout the embryo and in the developing cardiovascular, skeletal, renal, and neuronal systems32, 201. In the adult, expression of CCNs is downregulated in many tissues but becomes redeployed at sites of inflammation, wound healing, and injury repair2, 36.
Identified as immediate-early genes, CCN1 and CCN2 are expressed at a very low level in quiescent fibroblasts, but are transcriptionally activated without requiring de novo protein synthesis within minutes of stimulation by mitogenic growth factors such as platelet-derived growth factor, fibroblast growth factor 2, and transforming growth factor β1 (TGF-β1). Analysis of the Ccn1 and Ccn2 promoters have revealed critical regulatory elements and transcription factors governing their expression2, 3, and identified the essential promoter for apposite regulation of Ccn1 in transgenic mice37. CCN1 and CCN2 are targets of the YAP/TAZ transcriptional co-activators, which interact with the TEAD family of transcription factors to regulate genes related to cell proliferation and survival100 and in response to mechanotransduction202 (see Fig. 2). By contrast, CCN3 and CCN5 are downregulated by mitogenic signals and upregulated under conditions of growth arrest in several cell types61, 113. Additionally, CCN genes are downregulated by specific microRNAs in various cell types, for example CCN2 is regulated by miR-133 and miR-30 in cardiomyocytes and miR-18a in chondrocytes 203, 204, whereas downregulation of CCN1 by miR-155 may contribute to preeclampsia174. The presence of internal ribosome entry sites facilitates the preferential translation of the CCN1 mRNA under conditions of cellular stress, for example after viral infections205, 206.
Structural variants or isoforms of CCN proteins, some of which have been detected in biofluids and may have different activities, can be generated by differential splicing207, 208, proteolysis35, 209, and post-translational modifications210. Alternative splicing typically results in the production of truncated proteins or proteins with either the VWC or TSR domains deleted. These structural variants may potentially greatly expand the functional complexity of CCNs, although how they are regulated and how they impact the biological functions of CCNs in vivo are largely unknown. Whereas CCNs are secreted proteins with N-terminal signal peptides, they may be endocytosed after receptor binding. Several CCNs have been detected in the nucleus and although their nuclear function is unknown, recent studies have suggested the intriguing possibility that CCN1 and CCN5 might regulate transcription211, 212.
At the cellular level, CCN proteins regulate cell adhesion, migration, proliferation, differentiation, apoptosis, survival, senescence, and gene expression. By modulating one or more aspects of these cellular functions in a cell type-specific manner, CCNs coordinate complex biological processes, including cardiovascular and skeletal development during embryogenesis, as well as inflammation, wound healing, and tissue injury repair in the adult. Aberrant CCN expression is associated with a remarkable diversity of seemingly unrelated diseases. A useful albeit simplistic framework with which to rationalize the roles of CCNs in this broad array of diseases is that many pathological conditions arise when inflammation or tissue injury becomes chronic, and in this process CCNs are concomitantly deregulated. Studies in animal models and human patients have confirmed that CCNs are involved in many diseases related to inflammation and injury repair, including arthritis, atherosclerosis, restenosis after vascular injury, fibrosis, cancer, and diabetic nephropathy and retinopathy. Modulating CCN expression or function has yielded significant benefits in animal models of disease, suggesting that CCNs are promising therapeutic targets in these pathologies. Clinical trials targeting CCN2 using a humanized monoclonal antibody (FG-3019) have begun to show encouraging results in several human diseases, such as diabetic nephropathy6. However, the therapeutic potential of other CCN proteins have yet to be evaluated in a clinical setting. In this review, we discuss recent insights into the regulation and function of CCN proteins in various biological processes, summarize the evidence for their participation in disease pathologies, explore their potential as diagnostic markers and therapeutic targets, and discuss various targeting strategies as applied to these proteins.
FUNCTIONS OF CCN PROTEINS AND THEIR MECHANISMS OF ACTION
As members of the CCN family serve both distinct and overlapping biological roles, these highly conserved proteins bind many of the same receptors and function through similar mechanisms to regulate a common set of biological processes. However, the specific biological responses to some of the CCN proteins may be similar in some cell types but rather different in others, and at times diametrically opposed. Nevertheless, some generalizations can be made about how CCNs act. First, many CCN activities are mediated through their direct binding to integrin receptors on the cell surface, with the participation of one or more coreceptors in some contexts. The convergence of signals initiated from multiple receptors in some cases lead to unique biological responses not achieved with a single receptor, and the requirement of multiple receptors for certain CCN functions contributes to target cell specificity. Second, CCNs can regulate the expression of bioactive molecules such as growth factors, cytokines, and MMPs, and physically interact with some of them to modulate their bioavailability and activity (Fig. 2). In addition, CCNs can interact with and tether to other ECM proteins, including decorin, fibronectin, vitronectin, and perlecan, potentially functioning as a local scaffold that coordinates interaction of specific bioactive molecules or ECM proteins with the target cell (Fig. 2). Third, CCNs can profoundly alter the biological activities of cytokines, and possibly growth factors, through signaling crosstalk (Fig. 3). Through these and other mechanisms (see Box 1), CCN proteins integrate and modulate multiple signaling pathways to elicit cell type-specific responses.
Figure 2. Molecular interactions through modular domains of CCN proteins.
CCNs physically interact with a number of ECM proteins (including fibronectin213, perlecan214, vitronectin215, decorin168), growth factors (including such as VEGF34, FGF2216, TGF-β39, and BMPs39), and the gap junction protein connexin43217, 218. The specific modular domains mediating the interactions, where elucidated, are indicated. Whereas fibronectin and perlecan bind the CT domain, whether decorin and vitronectin also bind CT is not as clear. CCNs also bind to and signal through a number of cell surface receptors including several integrins2, 37, which function in concert with HSPGs or LRPs as coreceptors in some contexts. CCN2 also binds TrkA in a complex with β1 integrins13 as co-receptors, and CCN3 can bind Notch14. CCNs can modulate Wnt signaling, in part through binding to the Wnt coreceptor, LRP-611. The activities of the CCN modular domains may interact in a combinatorial manner to induce unique activities and functions4. For example, CCN1 synergism with TNFα requires its binding to both αvβ5 and α6β1 integrins through the VWC and CT domains, respectively, to induce signals from both integrins that converge within the cell7; activation of either integrin alone is insufficient.
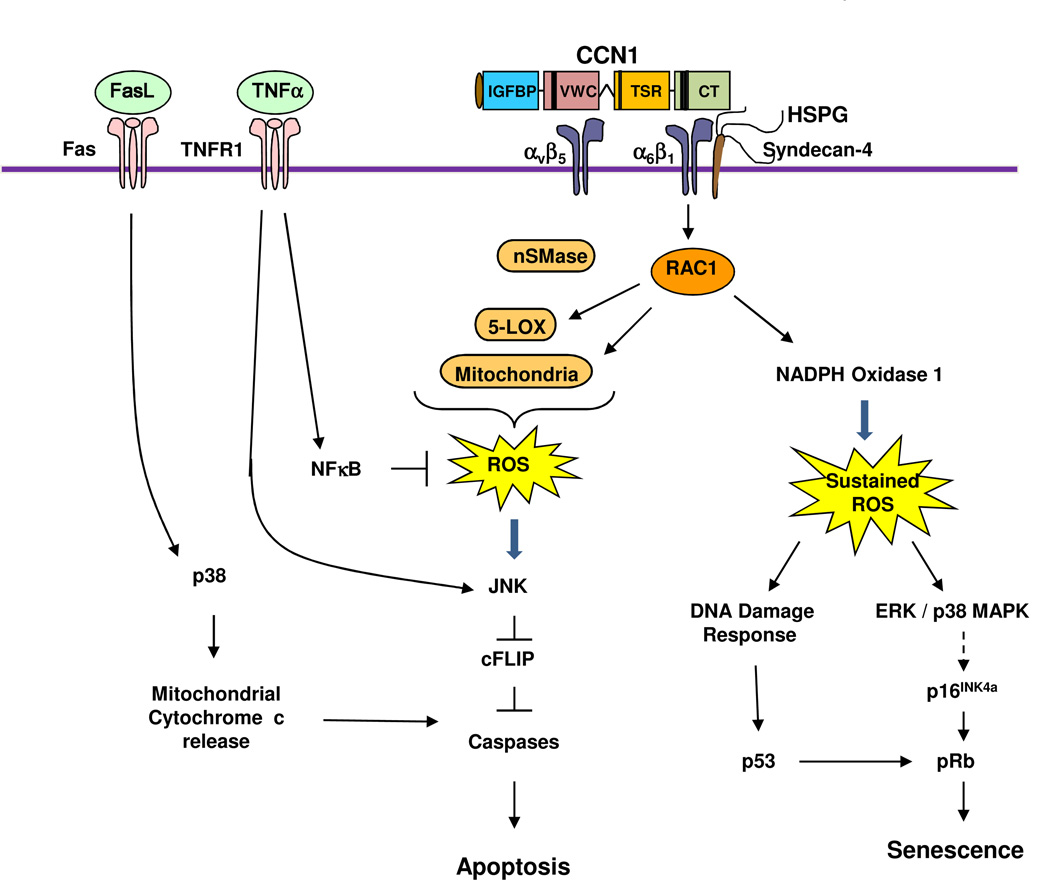
Figure 3. Signaling mechanism of CCN1-induced senescence and crosstalk with TNFα and FasL.
The binding of CCN1 to integrin α6β1-HSPGs (syndecan 4) triggers the activation of RAC-1 and NADPH oxidase 1, leading to a much more robust and sustained level of ROS compared to cell adhesion to other ECM proteins. The sustained ROS induces a DNA damage response and the activation of p53, and triggers the activation of ERK and p38 MAPK, which in turn induces p16INK4a and activates pRb8. Activated p53 and pRb contribute to the induction of cellular senescence. If integrin αvβ5 is also engaged by CCN1, RAC1-dependent ROS accumulation includes contribution from 5-lipoxygenase (5-LOX) and the mitochondria7; neutral sphingomyelinase (nSMase) also contributes to CCN1-induced ROS26. CCN1-induced ROS counteracts the effect of NFκB, which is strongly activated by TNFα and induces the expression of antioxidant proteins. The high level of ROS inhibits cysteine phosphatases that can inactivate MAPKs such as JNK, ERK, and p38 MAPK, leading to a hyperactivation of these kinases219. Activated JNK targets the proteosomal degradation of cFLIP220, an inhibitor of caspase activation, allowing the activation of caspases 8/10 by TNFα to induce apoptosis without blocking de novo protein synthesis or NFκB signaling7. In addition to CCN1, CCN2 and CCN3 also synergize with TNFα to induce apoptosis, presumably through a similar mechanism25. In the presence of FasL, which can trigger apoptosis on its own, CCN1 or CCN2-induced ROS leads to the hyperactivation of p38 MAPK, which enhances cytochrome c release from the mitochondria and thereby increases apoptosis26.
Cell surface receptors
Many activities of CCN proteins are mediated through their direct binding to integrin receptors, with the involvement of coreceptors in some contexts (Table 1). At least eight integrins have been shown to bind CCN proteins2. Since distinct integrins are differentially expressed in various cell types and may mediate disparate activities, CCNs can achieve remarkable functional versatility through their interaction with different integrins in a cell type- and context-specific manner. Although CCN proteins do not contain the canonical RGD sequence that binds several integrins, they interact with integrins through non-canonical binding sites, many of which have been identified (Box 1). Site-directed mutations in these binding sites abolish the specific activities for which the cognate integrins are responsible both in vitro and in vivo7, 8, providing compelling evidence for the role of these integrins in mediating CCN functions.
Table I.
Selected cellular processes stimulated (+) or inhibited (−) by CCN proteins in specific cell types and the cell surface receptors involved
| Cellular responses | CCN Protein | Cells types and cell-surface receptors involved | Ref |
|---|---|---|---|
| Cell adhesion | CCN1, CCN2 (+) | • Fibroblasts – α6β1 and HSPGs | 2 |
| CCN1, CCN2 (+) | • Activated endothelial cells – αvβ3 | 2 | |
| CCN1 (+) | • Smooth muscle cells – α6β1 and HSPGs | 2 | |
| CCN1, CCN2 (+) | • Platelets – αIIbβ3 | 2 | |
| CCN1, CCN2 (+) | • Monocytes – αMβ2, | 2 | |
| CCN1, CCN2 (+) | • Macrophages – αMβ2 | 46 | |
| CCN2 (+) | • Hepatic stellate cells – αvβ3 and LRP | 2 | |
| CCN2 (+) | • Pancreatic stellate cells – α5β1 and HSPGs | 2 | |
| CCN3 (+) | • Endothelial cells – αvβ3, α5β1, α6β1 and HSPGs | 2 | |
| Migration/Invasiveness | CCN1 (+) | • Fibroblasts –αvβ5 | 2 |
| CCN1, CCN2, CCN3 (+) | • Microvascular endothelial cell chemotaxis – αvβ3 | 2 | |
| CCN1 (+) | • Smooth muscle cell chemotaxis – α6β1 and HSPGs | 2 | |
| CCN3 (+) | • Endothelial cells – αvβ3, α5β1 | 2 | |
| CCN3, CCN5 (−) | • Inhibits vascular smooth muscle cell migration | 23, 24 | |
| CCN4 (−) | • Lung cancer cell invasiveness | 111 | |
| DNA synthesis/Proliferation | CCN1 (+) | • Fibroblasts – αvβ3 | 2 |
| CCN1 (+) | • Astrocytoma cells – α5, α6, and β1 | 177 | |
| CCN3, CCN5 (−) | • Smooth muscle cells | 23 | |
| Survival | CCN1, CCN2, CCN3 (+) | • Endothelial cells – αvβ3 | 2 |
| CCN1 (+) | • Breast cancer cells – αvβ3 | 98 | |
| Apoptosis | CCN1 (+) | • Fibroblasts – α6β1 and syndecan 4 | 9 |
| CCN1, CCN2, CCN3 (+) | • Synergism with TNF-α in fibroblasts – α6β1, αvβ5, and syndecan-4 | 7 | |
| CCN1, CCN2 (+) | • Synergism with FasL in fibroblasts – α6β1 and HSPGs | 26 | |
| Differentiation | CCN1 (+) | • Osteoblastic differentiation – αvβ3 | 178 |
| Angiogenesis | CCN1, CCN2, CCN3 (+) | • Endothelial tubule formation – αvβ3 | 2 |
| Inflammation | CCN1 (+) | • Macrophages, NFκB activation and M1 polarization – αMβ2 | 46 |
| CCN1 (+) | • MCF7 breast cancer cells, NFκB activation – αvβ3 | 98 | |
| CCN1 (−) | •Inhibits immune cell infiltration in autoimmune myocarditis | 47 | |
| CCN2 (+) | • Mesangial cells chemokines expression – TrkA and HSPGs | 179 | |
| CCN3 (+) | •Endothelial cells, NFκB activation | 48 | |
| Senescence | CCN1 (+) | • Fibroblasts – α6β1 and HSPGs | 8 |
At least two types of coreceptors have been identified for CCNs. The first of these are cell surface heparan sulfate proteoglycans (HSPGs), of which syndecan-4 has been identified as crucial for CCN functions7, 9. LRPs, which are endocytic receptors that cooperate with many growth factors receptors and integrins to modulate cellular responses10, are the second class of coreceptor11. CCN2 can interact with TrkA in human mesangial cells to enhance TGF-β signaling12 and in glioma cells to activate NFκB13. CCN2 binds to TrkA in a complex with β1 integrins, indicating that TrkA also functions as a coreceptor with integrins13. Additionally, CCN3 can bind Notch and suppress myoblast and osteoblast differentiation14, 15; whether integrins are involved in these signaling events is unknown.
Cell adhesion, migration, and proliferation
As cell adhesive proteins of the ECM, CCNs support cell adhesion and promote cell spreading in many cell types (Table 1). The process of cell adhesion may also lead to other cellular responses, including cell migration, proliferation, and altered gene expression. The adhesion of human skin fibroblasts to CCN1 and CCN2 is mediated through α6β1-HSPGs, and results in cell adhesive signaling events including the rapid formation of α6β1-containing focal adhesion complexes, actin cytoskeleton reorganization, formation of filopodia and lamellipodia, and activation of focal adhesion kinase, paxillin, and RAC16. The effects of CCN proteins on cell proliferation and migration are cell type-specific. In fibroblasts, CCN1, CCN2, and CCN3 have no intrinsic ability to induce mitogenesis on their own, but can enhance DNA synthesis induced by other mitogenic growth factors by acting through integrin αvβ32. Whereas CCN2 alone is capable of promoting DNA synthesis in chondrocytes and osteoblasts17, 18, it also induces a G1-cell cycle arrest in mesangial cells19. CCN1, CCN2, and CCN3 stimulate cell migration or chemotaxis in fibroblasts and endothelial cells, and promote the invasiveness of certain cancer cells2. Although CCN3 inhibits the proliferation of Ewing’s sarcoma cells, it promotes their migration and invasion20. Whereas CCN1 and CCN2 enhance vascular smooth muscle cell (VSMC) proliferation and migration21, 22, CCN3 and CCN5 inhibit them23, 24.
Cell survival, apoptosis, and cellular senescence
Cell adhesion to ECM molecules promotes cell survival, whereas detachment from the ECM induces cell death by anoikis in many cell types. Although adhesion of endothelial cells to CCN1, CCN2, or CCN3 through integrin αvβ3 supports cell survival2, these CCN proteins can also promote apoptosis as cell adhesion substrates in fibroblasts by acting through α6β19. The apoptosis-promoting activity may be most relevant in the context of inflammation, as CCN1, CCN2, and CCN3 all have the unusual ability to enable the inflammatory cytokine TNFα to induce apoptosis without inhibiting NFκB signaling or de novo protein synthesis, conditions required for TNFα cytotoxicity in normal cells in vitro25 (Fig. 3). These CCN proteins also enable the apoptotic activity of lymphotoxin and enhance that of other TNF family cytokines such as FasL and TRAIL26, 27. Knockin mice expressing an apoptosis-defective Ccn1 allele are substantially resistant to TNFα- or Fas-mediated hepatic apoptosis in vivo, indicating that CCN1 is a physiologic regulator of TNFα and FasL cytotoxicity7, 26. By contrast, CCN4 inhibits TNF-induced cell death in cardiomyocytes28, suggesting that the interplay between various CCNs and the TNF family of cytokines may profoundly affect the biological outcome during inflammatory responses.
A recent unexpected finding is that CCN1 can induce cellular senescence in fibroblasts by acting as a cell adhesion molecule8. Through binding to α6β1 and cell surface HSPGs, CCN1 activates the RAC1-dependent NADPH oxidase 1 (NOX1) to induce a robust and sustained level of reactive oxygen species (ROS), which leads to the activation of p53 and pRb, resulting in senescence (Fig. 3). An important feature of senescent cells is the expression of the senescence-associated secretory phenotype (SASP), characterized by the increased expression of ECM-degrading enzymes such as matrix metalloproteinases (MMPs), inflammatory cytokines and chemokines, and down-regulation of ECM components such as collagen, thus imposing a matrix-degrading phenotype29. Consistently, CCN1-induced myofibroblast senescence functions as a mechanism for limiting fibrosis during wound healing30 (see below).
Angiogenesis, chondrogenesis and osteogenesis
CCN1, CCN2, and CCN3 are potent angiogenic inducers, functioning through direct binding to integrin αvβ3 in endothelial cells to promote proliferation and induce chemotaxis and formation of tubules31, 32. They can also regulate the expression33 and activities of other angiogenic factors such as VEGF-A and VEGF-C34, 35. Through their angiogenic activities, these CCNs may be involved in embryonic development, inflammatory diseases, and tumorigenesis32, 36.
Both CCN1 and CCN2 promote chondrogenic and osteoblastic differentiation 37, 38. CCNs can interact with members of the BMP and TGF-β family, most likely through the chordin-like homology found in the VWC domain, and modulate their binding affinity for their respective receptors39. CCN2 and CCN3 can bind BMP2 and inhibit its functions in promoting chondrogenic and osteogenic differentiation15, 40, respectively, whereas CCN4 binds BMP2 and enhances its function in osteogenesis41. CCN2 regulates Wnt signaling by interaction with the Wnt receptor complex through direct binding to the Wnt coreceptor LRP611. CCN1 inhibits osteoclastogenesis42, whereas CCN3 stimulates it through a process that may involve calcium flux43. In transgenic mice, overexpression of CCN2 or CCN3 in osteoblasts antagonizes both BMP and Wnt-signaling and result in osteopenia44, 45.
Inflammation
Accumulating evidence indicate that CCN proteins modulate the inflammatory response36. CCN1 and CCN2 are induced by inflammatory cytokines or upon viral or bacterial infection, and CCNs in turn regulate the activity and expression of cytokines and chemokines. Clear examples include the ability of CCNs to greatly alter the cytotoxicity of TNF family of cytokines both in vitro and in vivo7, 26, and CCN1 to reprogram macrophages toward M1 polarization and activate the expression of proinflammatory cytokines46. CCN1 also regulates immune cell infiltration in vivo in an experimental model of autoimmune myocarditis47. CCN3 inhibits NFκB activation in endothelial cells, suggesting that it may regulate endothelial inflammation48. Furthermore, CCNs are involved in the pathobiology of many inflammatory diseases, as discussed below.
Stem cell differentiation and self-renewal
A recent study shows that CCN2 is sufficient to drive the differentiation of human bone marrow mesenchymal stem cells (MSCs) into α-smooth muscle actin (SMA) negative fibroblasts, which are primed for differentiation into α-SMA+ myofibroblasts when subsequently treated with TGF-β149. Both CCN1 and CCN2 are targets of the canonical Wnt/β-catenin signaling pathway in MSCs and regulate their differentiation into osteoblasts50, 51. CCN3 is essential for the self-renewal of CD34+ hematopoietic stem cells from umbilical cord blood, suggesting its potential utility for promoting stem cell engraftment52. Consistent with a function in stem cell self-renewal, CCN3 inhibits both myogenic and osteoblastic differentiation of MSCs53.
CCN FUNCTIONS IN EMBRYONIC DEVELOPMENT
Targeted disruptions of Ccn genes in mice have been accomplished with the exception of Ccn4, and phenotypes of the knockout mice are listed in Table II. Although the phenotypes of each knockout are distinct, skeletal and vascular defects are among the most commonly observed. Given the importance of several CCNs in ovulation, placentation, and development, the potential risks of targeting their expression or function in women expecting or during pregnancy should be carefully evaluated. Ccn1-null mice are embryonic lethal due to impaired angiogenesis, placental insufficiency, and severe atrioventricular septal defects (AVSD)33, 54. Although Ccn1+/− mice are largely viable, they display persistent ostium primum atrial septal defects in 20% of the adult54. These defects resemble those of human patients with mutations in AVSD1, a susceptibility locus for non-syndromic AVSD identified by linkage analysis55. Since the human CCN1 gene and AVSD1 map to the same chromosomal location, these findings suggest that CCN1 may be a candidate gene for human AVSD37.
Table II.
Phenotypes of Knockout (KO) and knockin (KI) mice
| Mouse Model | Phenotypes | Other Information | Refs. |
|---|---|---|---|
| Ccn1 KO |
|
|
33, 54 |
| Ccn2 KO |
|
|
56–58 |
| Ccn3 KO |
|
|
24, 60 |
| Ccn5 KO |
|
61 | |
| Ccn6 KO |
|
|
62, 160 |
| Ccn1 KI (Ccn1dm/dm) |
|
|
7, 8, 26 |
| Ccn2 floxed |
|
180 | |
|
72 | ||
|
59 | ||
| Ccn2 high to low expression |
|
181 |
Ccn2-null mice are perinatal lethal due to respiratory defects as a secondary consequence of severe skeletal malformations56, consistent with a wealth of data showing the roles of CCN2 in chondrogenesis and endochondrial ossification38. Ccn2-null embryos also exhibit pulmonary hypoplasia57 and decreased β cells in the pancreas58. Cell type-specific deletion of Ccn2 in the ovary and uterus resulted in disrupted follicle development and steroidogenesis, decreased ovulation, and increased numbers of corpus luteum59. By contrast, Ccn3 knockout mice are viable and largely normal, exhibiting only modest and transient sexually dimorphic skeletal abnormalities60. Whereas Ccn5-null mice suffer early embryonic death61, Ccn6 knockout mice display no observable phenotype62.
CCNs IN DISEASES AND TARGETING STUDIES
As CCN proteins are highly expressed and play important roles at sites of inflammation and tissue injury repair, they are often deregulated when these processes become chronic and progress to pathological conditions. For this reason, numerous gene profiling studies have found CCNs aberrantly expressed in a plethora of diseases, many of which are associated with chronic inflammation and tissue repair. Studies in animal models have established that deregulated CCNs indeed contribute to the pathologies of many of these diseases, although their mechanisms of action are not completely understood (Table III). Nevertheless, test-of-principal studies have shown that targeting CCNs can yield significant benefits in animal models. Moreover, the levels of CCNs in biological fluids may serve as non-invasive diagnostic or prognostic markers for certain pathologies. Clinical trials targeting CCN2 have already begun and initial results are encouraging as discussed in a later section, reinforcing the notion that CCNs are emerging as attractive therapeutic targets for a broad spectrum of diseases.
Table III.
Targeting CCNs in animal models of disease
| Disease/ pathology |
CCN proteins | Animal model | Phenotypes/intervention | Refs. |
|---|---|---|---|---|
| Fibrosis | CCN1 | • Wound healing in α6β1-binding defective Ccn1 knockin mice | • Enhanced fibrosis in cutaneous wounds; topical application of CCN1 reverses effect | 8 |
| CCN2 | • Transgenic mice overexpressing CCN2 in hepatocytes | • Exacerbated liver fibrosis induced by CCl4 or bile duct ligation | 182 | |
| • Mouse liver fibrosis (CCl4) | • CCN2 siRNAs reduce fibrosis | 74 | ||
| • N-nitrosodimethylamine-induced liver fibrosis | • CCN2 siRNAs reduce fibrosis | 75 | ||
| • Streptozotocin (STZ)-induced diabetes in podocyte-specific Ccn2 transgenic mice | • Exacerbated renal hypertrophy, proteinuria, and mesangial matrix expansion | 134 | ||
| • Tubulointerstitial fibrosis induced by unilateral ureteral obstruction | • Reduction of ECM induction by CCN2 AS-ODN | 76 | ||
| • Subtotal nephrotectomy in TGF-β1 transgenic mice | • CCN2 AS-ODN blocked interstitial fibrosis in remnant kidney | 78 | ||
| • Cardiomyocyte-specific Ccn2 transgenic rodents; angiotensin II-induced pressure overload heart failure | • No cardiac fibrosis; promotes cardiac hypertrophy and protects against pressure-overload heart failure | 149 | ||
| • Deletion of Ccn2 in fibroblasts and smooth muscle cells | • Resistant to bleomycin-induced skin fibrosis | 72 | ||
| • Fibroblast-specific Ccn2 transgenic mice | • Fibrosis in skin, lung, kidney, and vasculature | 71 | ||
| CCN4 | • Bleomycin-induced pulmonary fibrosis | • Neutralizing antibodies attenuates lung fibrosis | 79 | |
| CCN5 | • Transverse aortic constriction in Ccn5 transgenic mice | • Inhibition of pressure overload-induced cardiac hypertrophy and fibrosis | 82 | |
| Cancer | CCN1 | • Glioma xenografts | • siRNA inhibited pre-established glioma growth | 101 |
| • Pancreatic cancer cells | • shRNA reverses characteristics of EMT and reduced tumorigenicity | 95 94 | ||
| CCN2 | • Pancreatic cancer xenografts | |||
| • Orthotopic and xenografted pancreatic cancer | • Decreased tumor growth with shCCN2 or siCCN2 | 104 | ||
| • FG-3019 attenuates tumor growth and metastasis | 105 | |||
| Cardio-vascular disease | CCN1 | • Rat carotid artery balloon injury | • CCN1 siRNAs suppress neointimal hyperplasia | 145 |
| CCN1 | • Mouse autoimmune myocarditis | • Adenoviral expression of CCN1 attenuates myocarditis | 47 | |
| CCN2 | • Rat carotid artery angioplasty with viral perfusion | • Recombinant CCN2 application increased neointimal thickening | 143 | |
| Nephropathy/Retinopathy | CCN1 | • Mouse oxygen-induced retinopathy (OIR) | • Intravetreal injection of anti-CCN1 antibody reduced retinal neovascularization | 138 |
| CCN2 | • Streptozotocin (STZ)-induced diabetic mice | • Subcutaneous CCN2 AS-ODN decreased proteinuria and albuminuria | 133 | |
| • Reduced basal lamina thickening of retinal capillaries in Ccn2+/− background | 140 | |||
| Wound healing | CCN1 | • Bone fracture (transverse osteotomy) | • Intraperitoneal injection of anti-CCN1 antibody impaired fracture healing | 154 |
| CCN2 | • Rabbit punch wound at ears | • Intradermal CCN2 AS-ODN injection reduced hyperptrophic scarring | 77 | |
AS-ODN, antisense oligonucleotide
Wound healing and fibrotic diseases
Mammalian wound healing and tissue repair occur similarly in virtually all organ systems in three distinct but overlapping phases, initiating with inflammation, followed by granulation tissue formation and ECM deposition, and concluding with matrix remodeling and resolution of the granulation tissue63. At the site of injury, activated resident fibroblasts and recruited fibrocytes differentiate into α-SMA+ myofibroblasts, which proliferate and deposit ECM proteins to promote healing and support tissue integrity while the damaged tissue is being repaired and remodeled. However, excessive ECM deposition may occur in wound repair, leading to fibrosis, scarring, and loss of tissue function. Fibrosis is greatly exacerbated when inflammation or tissue injury becomes chronic and contributes to the pathologies of chronic diseases in many organ systems, for example in the liver as a result of viral infections or alcoholism, in the kidney as a result of diabetes, or in the heart as a result of tissue remodeling following myocardial infarctions63, 64. These pathologies can lead to organ failure and death, yet currently there is no FDA-approved drug available for treating fibrosis. Emerging studies indicate that various CCN proteins are involved in promoting or inhibiting fibrosis in association with injury repair, and provide unique opportunities for the development of novel therapeutics and diagnostics for this pathology.
Upon wounding, platelet α granules release abundant amounts of CCN265, 66 and other growth factors that can induce the expression of CCNs at the site of injury. CCN2 acts synergistically with TGF-β1, a potent profibrotic regulator, to promote matrix protein deposition and fibrogenesis both in vitro and in vivo67. Subcutaneous or intraperitoneal injections of either TGF-β or CCN2 individually does not induce persistent fibrosis, whereas coinjection of both proteins together results in sustained fibrosis68. CCN2 is overexpressed in human fibrotic diseases of virtually every organ or tissue. Correspondingly, serum and biofluid levels of CCN2 correlate with the severity of systemic fibrosis (scleroderma) and fibrosis of the heart, kidney, liver, and lung, suggesting that CCN2 levels in biofluids may be useful as a non-invasive biomarker for many fibrotic diseases69, 70. Despite this close association with fibrosis, overexpression of CCN2 per se in parenchymal cells of most organs does not induce fibrosis in mice, but exacerbates the fibrotic response when challenged with injuries67. However, overexpression of CCN2 specifically in fibroblasts, the major cell type responsible for matrix deposition, is sufficient to drive fibrosis in lung, skin, kidney and arteries71, and mice deleted for Ccn2 in fibroblasts are resistant to bleomycin-induced skin fibrosis72. These observations suggest that CCN2 action in fibroblastic cells may be strongly autocrine in nature. Consistent with the pro-fibrotic function of CCN2, inhibition or downregulation of CCN2 ameliorates fibrosis in many organ systems. Knockdown of CCN2 by anti-sense oligonucleotides or siRNAs reduces CCl473, 74- and N-nitrosodimethylamine75-induced liver fibrosis, ureteral obstruction-induced renal fibrosis76, fibrotic scarring in cutaneous wounds77, and renal interstitial fibrogenesis following partial nephrectomy78. Although less is known about CCN4 actions, it has been shown that CCN4 is upregulated in human patients with idiopathic pulmonary fibrosis and in a mouse model of bleomycin-induced lung fibrosis, and recombinant CCN4 enhances ECM deposition in human fibroblasts79. Correspondingly, orotracheal application of CCN4 neutralizing antibodies to the lung ameliorates bleomycin-induced lung fibrosis79.
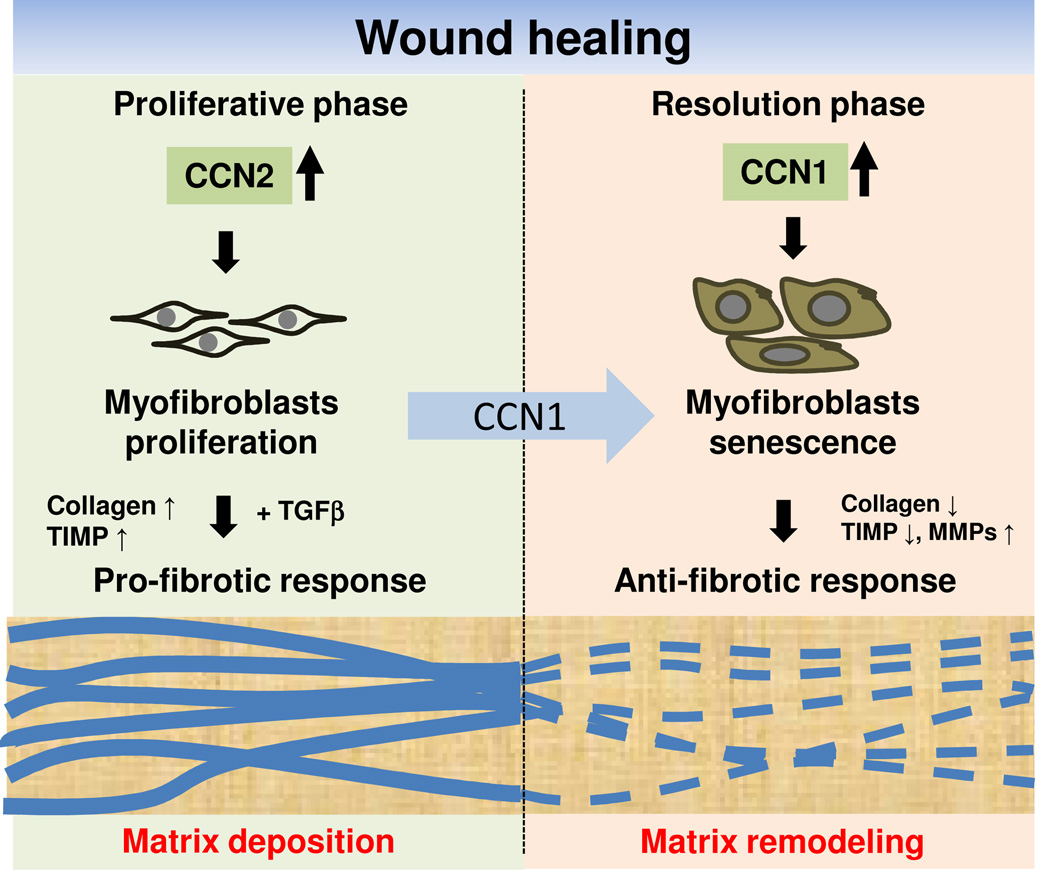
In contrast to the pro-fibrotic CCN2 and CCN4, CCN1 functions to limit fibrosis through a novel mechanism that invokes the induction of cellular senescence in myofibroblasts8. Accumulation of senescent cells is an integral part of the normal wound healing response that functions to curb fibrosis, and this process is controlled by CCN130 (Fig. 4). CCN1 gradually accumulates in the granulation tissue in excisional cutaneous wounds, reaching a level sufficient to trigger senescence in myofibroblasts as the healing process enters the resolution phase, whereupon the senescent cells express the SASP that includes the secretion of MMPs and down-regulation of collagen I and TGF-β18. Thus, CCN1 acts as an anti-fibrotic senescence switch, converting the ECM-synthesizing myofibroblasts into ECM-degrading senescent cells. This mechanism of fibrosis control achieves molecular parsimony by at once halting further ECM production in myofibroblasts, accelerating ECM degradation, and facilitating granulation tissue resolution as senescent cells are cleared by natural killer cells30, 80. In knockin mice expressing senescence-defective CCN1, senescent cells do not accumulate in the granulation tissue and wound healing proceeds with exacerbated fibrosis8. Topical treatment of these wounds with purified CCN1 protein reverses this defect and reduces fibrosis by increasing the number of senescent cells, suggesting a therapeutic potential for CCN1 in the treatment of fibrosis. Interestingly, hepatic stellate cells also undergo senescence to limit fibrosis in chemically-induced liver injury80, indicating that this mechanism of fibrotic control may be general to wound repair in different organs and diverse modes of injuries.
Figure 4. Role of CCNs in wound healing.
CCN1 and CCN2 have distinct roles in wound healing. CCN2 functions in the granulation tissue during the proliferative phase and acts with TGF-β to promote the synthesis ECM, leading to a pro-fibrotic response. As wound healing progresses, CCN1 accumulates to a sufficiently high level to induce an anti-fibrotic senescence switch in myofibroblasts, thereby limiting fibrosis by converting the ECM-synthesizing myofibroblasts into ECM-degrading senescent cells.
Whereas CCN1 inhibits fibrosis by inducing cellular senescence8, CCN3 and CCN5 also appear to have antifibrotic activities, although their antifibrotic mechanism may potentially be distinct. CCN3 suppresses CCN2 and collagen I expression in mesangial cells in vitro81, whereas overexpression of CCN5 reduces cardiac hypertrophy and fibrosis in transgenic mice, possibly by inhibiting TGF-β/SMAD signaling82. Since CCN2 and CCN4 are pro-fibrotic whereas CCN1, CCN3, and CCN5 are anti-fibrotic at least in certain biological contexts, it is critical that reagents targeting members of the CCN family have a high level of specificity. Approaches in this regard are discussed in a later section.
CCN proteins in cancer
It has long been recognized that chronic injuries and deregulated wound healing are risk factors in the development of cancer83. Recent studies have further underscored the importance of inflammation in tumorigenesis and tumor progression84. Inflammation occurring at early stages of neoplastic transformation can accelerate the development of incipient neoplasias into full-blown cancer by releasing mutagenic chemicals such as ROS. Moreover, inflammation is associated with most if not all tumors and contributes to cancer progression by providing a rich arsenal of bioactive molecules to the tumor microenvironment, including factors that promote cell growth, survival, motility, invasion, angiogenesis, and matrix degradation. Aberrant expression of CCNs has been identified in a broad range of tumor types85. With some notable exceptions86, 87, CCN1, CCN2, and CCN4 have been generally associated with promotion of cell proliferation and tumor growth, whereas CCN3, CCN5 and CCN6 have been associated with inhibition of cell proliferation and tumor growth (Table IV).
Table IV.
CCN proteins in cancers
| Tumors (Cancers) |
CCN proteins |
Expression level |
Clinical characterization/experimental observations | Refs. |
|---|---|---|---|---|
| Breast cancer | CCN1 | Higher | • Associated with poor prognosis, tumor stage, metastasis, and mortality | 107 |
| • Overexpression of CCN1 promotes tumorigenesis in xenografts | 90 | |||
| • Mediates taxol resistance | 100 | |||
| CCN2 | Higher | •Associated with poor prognosis and metastasis, mediates taxol resistance | 100, 107 | |
| • Mediates metastasis to bone | 102 | |||
| CCN3 | Higher | • Associated with therapy resistant ER-positive breast cancer | 117 | |
| CCN4 | Higher | • Associated with older patients with advanced tumor | 107 | |
| CCN5 | Higher | • Associated with more differentiated breast cancers with low metastatic potential; inversely correlated with E2-independency and invasive phenotype | 61 | |
| CCN6 | Lower | • Associated with poor prognosis in inflammatory breast cancer | 120 | |
| Endometrial cancer | CCN1 | Higher | • Poor survival of patients with endometrioid subtype | 183 |
| • CCN1 inhibits endometrial cancer cell growth | 184 | |||
| Ovarian cancer | CCN1 | Higher | • Associated with advanced tumor stage; enhanced tumorigenesis in xenografts of CCN1 overexpressing cells | 96 |
| CCN2 | Lower | • Promoter hypermethylation; associated with advanced tumor stage | 185 | |
| Prostate cancer | CCN1 | Higher | • Associated with high grade prostate cancer and lower risk of recurrence after surgery | 176 |
| • Decreased xenografted tumors of DU145 cells with decreased CCN1 | 93 | |||
| CCN2 | • Increased microvessel density and tumor growth in xenografts of CCN2 overexpressing prostate cancer cells | 91 | ||
| Glioma | CCN1& CCN2 | Higher | • Associated with tumor grade, pathology, gender and age at diagnosis | 101 |
| CCN3 | • Reduced tumorgenicity in CCN3 overexpressing cells | 114 | ||
| Lung cancer | CCN1& CCN2 | Lower | • Associated with tumor stage, tumor histology, metastasis, smoking, and family history; expression level correlated with patients’ survival | 109 |
| • Overexpression of CCN1 decreased tumor growth in xenografts | 186 | |||
| CCN4 | • Higher expression in adenosquamous cell carcinoma | 109 | ||
| Pancreatic cancer | CCN1 | Higher | • Expression increases with pancreatic adenocarcinoma progression | 95 |
| CCN2 | Higher | • Highly expressed in pancreatic cancer | 104 | |
| CCN5 | Lower | • Inversely correlated with tumor grade and p53 status | 187 | |
| Colon cancer | CCN1,2 | Higher | • Associated with tumor stage | 188 |
| CCN4 | Higher | • Associated with tumor stage, histologic grade, and lymph node status | 108 | |
| Musculo-skeletal tumor | CCN2 | Higher | • Associated with survival of rhabdomyosarcoma cells | 189 |
| CCN3 | Higher | • Higher risk of metastasis and worse prognosis in Ewing’s sarcoma | 116 | |
| Melanoma | CCN3 | Higher | • Associated with higher metastasis of xenografted CCN3 overexpressing cells | 115 |
| Leukemia | CCN2 | Higher | •Associated with pre-B lineage in ALL | 190 |
| CCN3 | Lower | •Restoring CCN3 expressing sensitizes CML cells to imatimib therapy | 118 | |
CCN1 and CCN2 may enhance tumor growth through their potent angiogenic activity31, 88. Accordingly, forced expression of CCN1 in breast cancer cells promotes tumor growth in xenografts with increased vascularization89, 90 and expression of CCN2 in tumor-reactive stroma enhances microvessel density of xenograft tumor in nude mice91, 92. Consistently, xenograft tumor growth is inhibited by silencing either CCN1 or CCN2 expression in cancer cells of the prostate93, pancreas94, 95, and ovary96. Both CCN1 and CCN2 can promote epithelial-to-mesenchymal transition (EMT)95, 97, enhance survival of some cancer cells through the induction of anti-apoptotic proteins98, 99, and mediate taxol resistance in breast cancer100. Direct intratumoral delivery of CCN1 siRNA into pre-established glioma xenografts decreases tumor growth by up to 40% in a dose-dependent manner, suggesting that targeting CCN1 has therapeutic potential101. Furthermore, CCN2 is a key component of the gene signature that facilitates osteolytic bone metastasis in breast cancer cells102, and treatment with a murine anti-CCN2 monoclonal antibody markedly decreased osteolytic bone metastasis of human breast cancer cell xenografts in nude mice103. Notably, administration of FG-3019, a humanized anti-CCN2 monoclonal antibody, inhibits tumor growth and metastases in xenograft and orthotopic models of pancreatic cancer in mice104, 105. As human pancreatic cancers are largely recalcitrant to therapy, the potential of treatment through targeting CCN2 is particularly encouraging.
Overexpression of CCN4 in normal rat kidney fibroblasts confers the ability to form tumors in nude mice106, and high CCN4 expression is noted in advanced breast and colon cancers107, 108 and adenosquamous cell carcinoma of the lung109. However, CCN4 appears to inhibit metastasis and is preferentially expressed in melanoma cell lines with low metastastic potential110. Lung cancer cells overexpressing CCN4 are less invasive and exhibit reduced metastasis in nude mice, possibly due to its integrin-mediated inhibition of the small GTPase Rac111. A splicing variant of CCN4 lacking the VWC domain has been detected in scirrhous gastric carcinomas, and transfectants expressing this variant enhance the invasive characteristic of co-cultured gastric carcinoma cells112. Therefore, it is possible that CCN4 isoforms expressed as a result of differential splicing might underlie the pro- and anti-invasive activities of CCN4, although this possibility has not been thoroughly investigated.
CCN3, CCN5 and CCN6 have a negative effect on cell proliferation. Although CCN3 inhibits proliferation of cancer cells113, it appears to promote metastasis20. Overexpression of CCN3 results in reduced tumor size in glioma cells xenografts114, but enhances metastatic potential in xenotransplanted melanoma cells115. CCN3 expression is associated with a higher risk of metastasis and worse prognosis in patients with cancers such as Ewing’s sarcoma116, melanoma115, and breast cancer117. In addition to solid tumors, CCN3 is down regulated in chronic myeloid leukemia (CML) as a consequence of the kinase activity of BCR-ABL, a chimeric protein generated through the chromosomal translocation between chromosome 9 and 22118. Patients responding to Imatinib (Gleevec) therapy show an increase in CCN3 expression. Forced expression of CCN3 inhibits proliferation and restores growth control in CML cells, and sensitizes them to Imatinib-induced apoptosis, suggesting that CCN3 may be an alternate targets for novel therapeutics against CML119.
CCN5 has been shown to inhibit the growth of vascular smooth muscle cells, uterine myometrial cells, leiomyoma cells, and estrogen receptor-negative breast cancer cells61. CCN5 expression is significantly decreased in leiomyomas, pancreatic cancers, and salivary gland tumors. CCN6 is expressed in the normal mammary epithelium, but expression is lost or downregulated in 60% of inflammatory breast carcinomas, and restoration of CCN6 expression reduced tumor growth in nude mice120. It has been suggested that CCN6 acts by interfering with the IGF-1 signaling pathway, which promotes the proliferation, survival and metastatic ability of breast cancer cells121.
The picture that emerges is that CCNs can act both positively and negatively in tumorigenesis and tumor progression, depending on the tumor type. Although CCN1 and CCN2 are known angiogenic inducers and may promote the growth of some tumor types, they can suppress tumor growth in some cancers86, 87. CCN1, CCn2, and CCN3 can synergize with cytokines of the TNF family to induce apoptosis, particularly TRAIL, which preferentially kills tumor cells27, and CCN1 can induce cellular senescence8. Both apoptosis and senescence are well-established mechanisms of tumor suppression if triggered in damaged cells at risk of neoplastic transformation122, suggesting that CCNs may inhibit the development of incipient tumors by promoting apoptosis or senescence in damaged cells. It is important to note that most experimental studies on CCN functions in cancer have employed xenograft models and established tumor cell lines, and thus focus on tumor growth and progression rather than the initial stages of tumorigenesis. Whether CCNs exert a positive or negative effect on tumor growth may depend on which integrin is preferentially expressed in the tumor cells (e.g., αvβ3 is growth promoting and α6β1 is growth inhibitory with CCN1 as ligand37), whether angiogenic factors are limiting, and whether conditions that favor apoptosis or senescence (i.e., ROS accumulation) prevail. Despite these uncertainties on how CCNs act in tumorigenesis, targeting CCNs has shown therapeutic promise in specific cancer as described above and these approaches warrant further translational studies.
Diabetic nephropathy and retinopathy
Diabetes is associated with a multitude of metabolic and homeostatic abnormalities, including hyperglycemia and systemic and intraglomerular hypertension, which contribute to microvascular pathologies such as diabetic nephropathy and retinopathy. Substantial evidence from recent studies support the involvement of chronic inflammation in the pathogenesis of diabetic nephropathy and retinopathy123, 124. Chronic hyperglycemia, the hallmark of diabetes, is a pro-inflammatory condition that leads to multiple biochemical alterations including the formation of advanced glycation endproducts (AGEs), which are thought to trigger or amplify many aspects of diabetic pathology125. Exposure to high glucose stimulates CCN2 expression in human mesangial cells126, and both CCN1 and CCN2 are induced by AGEs in vitro and in vivo127, 128. In addition, mechanical stretch and hemodynamic forces simulating hypertension have been shown to induce the expression of CCN1 and CCN2 in vascular endothelial cells and smooth muscle cells, and CCN2 in renal mesangial cells129. Thus, hyperglycemia and hypertension are both factors that contribute to the progression of diabetic disease with associated induction of CCN1 and CCN2.
Diabetic nephropathy is characterized by hypertrophy of glomeruli and tubules, thickening of their associated basement membrane, and glomerular and interstitial fibrosis, all of which compromise kidney function130. Microalbuminuria at the incipient stage of diabetic nephropathy progresses to overt proteinuria as glomerular filtration deteriorates. In diabetic patients, CCN2 expression is increased in both glomerular podocytes and mesangial cells130, whereas CCN1 expression in podocytes is downregulated131. CCN2 induces pro-fibrotic responses in cultured human mesangial cells and promotes the infiltration of inflammatory cells into the renal interstitium130, 132. Treatment of diabetic rats with aminoguanidine to inhibit AGE formation concomitantly block the induction of Ccn2 and albuminuria127. Knockdown of Ccn2 expression by antisense oligonucleotides attenuates progression of nephropathy in mouse models of diabetes133, whereas transgenic overexpression of CCN2 in podocytes enhances urinary albuminuria134. Current treatment of diabetic nephropathy focuses on the control of renal hypertension using angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs)135, thus targeting CCN2 may provide an alternative or complementary therapy. Moreover, plasma and urinary levels of CCN2 correlate with clinical parameters associated with the severity of diabetic nephropathy, suggesting that CCN2 can be a useful biomarker for this disease136, 137.
Retinopathy is a common microvascular complication of diabetes caused by enhanced angiogenesis and fibrosis in the vitreous cavity of the eye, resulting in loss of vision. CCN1 appears to be involved in retinal angiogenesis in streptozotocin (STZ)-induced diabetes or oxygen-induced retinopathy (OIR) in rodents, and in proliferative diabetic retinopathy in humans128, 138. Intravitreal injection of a neutralizing anti-CCN1 antibody in the OIR model significantly reduces retinal neovascularization with no apparent toxicity or inflammation138. CCN2 is highly expressed in microvascular pericytes of the human diabetic retina, and its levels correlate with the degree of fibrosis in vitroretinal disorders139. Heterozygous Ccn2+/− mice subjected to STZ-induced diabetes exhibit significantly lower basal membrane thickening of retinal capillaries compared to wild type mice140. These results are consistent with the notion that targeting CCNs may help ameliorate the pathology of diabetic retinopathy.
Cardiovascular diseases
CCN proteins have been implicated in such vascular pathologies as atherosclerosis, restenosis, thrombosis, and hypertension. Atherosclerosis is a chronic inflammatory disease of the arterial wall triggered by the prolonged elevation of lipids in the bloodstream (hyperlipidemia), whereas proliferative restenosis is a common response to vascular injury after balloon angioplasty. CCN1 and CCN2 promote neointimal hyperplasia after vascular injury, whereas CCN3 and CCN5 inhibit it. Both CCN1 and CCN2 are detected at high levels in rupture-prone atherosclerotic plaques, particularly in areas of neovascularization or areas that are infiltrated with inflammatory cells141, 142, and in the neointima in restenosis after balloon injuries22, 143. Inhibiting CCN1 expression by either its negative regulator FOXO3a144 or siRNA145 effectively reduced neoinitimal hyperplasia following balloon injury in the rat carotid artery. As neoinitimal hyperplasia is a common complication in the treatment of atherosclerosis, targeting CCN proteins may be beneficial. By contrast, CCN3 expression is substantially reduced following balloon injury. CCN3 inhibits smooth muscle cell proliferation in culture, and Ccn3-null mice suffer enhanced neointimal thickening when challenged with vascular injury, indicating that CCN3 inhibits neoinitimal hyperplasia24. Likewise, CCN5 inhibits vascular smooth muscle cell proliferation and motility, and its expression is greatly reduced in arteries after balloon injury23.
CCN1 is upregulated in inflammatory cardiomyopathy in humans, and CCN1 gene transfer in animal models attenuates autoimmune myocarditis by inhibiting the infiltration of spleen macrophages and lymphocytes47. The effects of CCN1 on immune cells are partially mimicked by cyclic RGD peptides, which bind αv integrins and are in clinical trials as cancer therapeutics146. Both CCN1 and CCN2 are highly induced in cardiomyocytes of patients with ischemic cardiomyopathy and in cardiac remodeling after myocardial infarction147, 148. Cardiomyocyte-specific expression of CCN2 in transgenic mice did not by itself induce fibrosis, but exacerbates pressure overload-induced cardiac fibrosis82 and leads to cardiomyocyte hypertrophy by 7 months of age149. By contrast, CCN5 inhibits the CCN2-induced hypertrophic responses in cardiomyocytes, and transgenic expression of CCN5 in cardiomyocytes reduces pressure overload-induced cardiac fibrosis82. These results indicate that CCN2 and CCN5 play opposing roles in cardiac hypertrophy and fibrosis. CCN5 is the only member of the family that lacks the CT domain. Deleting the CT domain in CCN2 renders it CCN5-like in inhibiting cardiomyocyte hypertrophic responses in vitro, and fusing the CT domain from CCN2 to CCN5 transforms it into a CCN2-like pro-hypertrophic molecule82, indicating a pro-fibrotic function in the CCN2 CT domain.
Arthritis and other inflammatory diseases
Osteoarthritis (OA) is a common and debilitating degenerative joint disease for which the principal forms of treatment provide only temporary pain management, and surgery may be required if the joint degeneration is severe. Both CCN2 and CCN4 are strongly upregulated in cartilage of human OA patients150, 151. CCN4 contributes to cartilage damage by inducing the production of MMPs and aggrecanase in macrophages and chondrocytes151. CCN2 promotes the proliferation and differentiation of chondrocytes without inducing calcification of articular cartilage38. Direct injection of CCN2 into the joint cavity stimulates cartilage repair in an experimental OA model in rats, suggesting that CCN2 may have therapeutic value in treating OA152. CCN1 and CCN2 are highly expressed in hypertrophic and proliferative chondrocytes during fracture healing, and blockade of CCN1 by neutralizing antibodies inhibits bone fracture healing in mice153, 154.
CCN1 and CCN2 are both highly expressed in rheumatoid arthritis (RA), an inflammatory joint disorder that leads to the destruction of articular cartilage155, 156. CCN1 may contribute to hyperplasia of the synovial lining, resulting in joint destruction in RA. CCN2 synergizes with M-CSF/sRANKL to enhance osteoclastogenesis and contributes to bone destruction in RA155. Current treatment for recalcitrant RA includes monoclonal antibodies (infliximab, golimumab, adalimumab, and certolizumb pegol) against TNFα, which can regulate the expression of CCN genes and functionally interact with CCN proteins7, 36. In this regard, it is interesting to note that TNFα antagonists are also effective therapies in inflammatory bowel disease and CCN1 is upregulated 10–20 fold in patients with Crohn’s disease or ulcerative colitis157, suggesting that CCN1 and TNFα may functionally interact in these diseases as well.
In the central nervous system (CNS), chronic inflammation-like glial responses cause neurodegenerative events including plaque formation, dystrophic neurite growth, and excessive tau phosphorylation. Thus, neuroinflammation has been implicated in pathological conditions of the CNS such as Alzheimer’s disease (AD), Parkinson’s disease, amyotrophic lateral sclerosis, or multiple sclerosis. Elevated CCN2 expression is observed in AD brain neurons and astrocytes, and its expression level correlates with the progression of clinical dementia and the deposition of neuritic plaques and neurofibrillary tangles158. Moreover, CCN2 promotes amyloid precursor protein (APP) processing and subsequent amyloid-β-peptide (Aβ) generation in vitro159, suggesting that CCN2 may contribute to AD progression by enhancing β-amyloidosis. However, currently little is known about the normal functions of CCN proteins in the CNS.
Genetic mutations and polymorphisms in human diseases
Of the six members of the CCN gene family, only CCN6 has been associated with a human disease with Mendelian inheritance. Mutations in CCN6 cause the autosomal recessive skeletal disease progressive pseudorheumatoid dysplasia (PPD), a juvenile-onset degenerative disease of the joint160. Alleles leading to PPD harbor loss-of-function mutations that include deletion, frameshift, nonsense, and missense mutations160. The development of DNA-based prenatal diagnosis targeting CCN6 may be useful for at-risk families, as symptoms of PPD are not evident until years after birth. Gene disruption of Ccn6 in mice fails to recapitulate the human disease and presents no discernible phenotype62, although in zebra fish Ccn6 is shown to modulates the canonical BMP and Wnt signaling pathways critical for cartilage homeostasis161.
DNA sequence polymporphisms in CCN genes associated with various diseases have been identified (Table V). Notably, polymorphisms in the human CCN2 gene are found to be significantly associated with scleroderma162–164, although some of these results may be controversial165. Other polymorphisms of CCN genes have been associated with susceptibility to hypertension, type I diabetes, colorectal cancer, hepatic fibrosis, etc. How these polymorphisms affect CCN gene expression or function is largely unknown and warrant further studies.
Table V.
Mutation and polymorphism associated with human diseases
| CCN | Identified mutation & polymorphism | Associated human diseases | Refs. |
|---|---|---|---|
| CCN1 | • rs3753794, rs3753793 and rs2297140, rs2297141 and IVS 2+50 | • Plasma HDL-cholesterol levels in obese individuals | 191 |
| • rs954353 | • Colorectal cancer (CRC) | 192 | |
| CCN2 | • −945 (G to C) substitution | • Susceptibility to systemic sclerosis | 162 |
| • rs9399005 | • Susceptibility to systemic sclerosis | 164 | |
| • rs6918698 | • Susceptibility to systemic sclerosis | 163 | |
| • rs9402373 and rs12526196 | • Severity of hepatic fibrosis (HF) | 193 | |
| • −945 GG genotype | • Cardiovascular events of stroke and myocardial infarction (MI) in surviving hemodialysis (HD) patients | 194 | |
| • −447C | • Higher degree of calcification in patients with symptomatic aortic stenosis | 195 | |
| • −20 GG genotype | • Susceptibility to nephropathy in type I diabetes | 196 | |
| CCN3 | N/A | ||
| CCN4 | • 2364 (A to G) substitution | • Prevalence of hypertension | 197 |
| • 2365 AA genotype | • Spinal osteoarthritis with high endplate sclerosis | 198 | |
| CCN5 | N/A | ||
| CCN6 | • Germline mutations (likely loss of function) | • Autosomal recessive disorder progressive pseudorheumatoid dyaplasia (PPD) | 160 |
| • Somatic mutation (frameshifts at a polyadenosine tract) | • Gastrointestinal tumors from patients with mutation in the mismatch repair pathway | 199 | |
| • 84 AA genotype | • Susceptibility to juvenile idiopathic arthritis (JIA) | 200 | |
THERAPEUTIC APPROACHES AND CLINICAL TRIALS
Much of the clinical potential of targeting CCNs in therapy is still largely unexplored, as translational studies are still in early stages. Since CCNs are secreted proteins, humanized monoclonal antibodies are particularly well suited as blocking agents for their activities and thus hold promise as potential therapeutics. In this regard, the hinge region encoded by the same exon as the VWC domain (Box 1) may be a good choice of antigen, since this region is unique to each CCN family member and contains an abundance of charged amino acids, enhancing its antigenicity. Other targeting strategies such as the use of siRNA or antisense oligonucleotides to down-regulate the expression of specific CCN genes have been successful in animal models166. These approaches can also afford a high degree of target gene specificity if possible off-target effects are minimized through careful oligonucleotide sequence selection. The use of synthetic peptides as targeting agents for CCNs is less well explored but nevertheless has some potential. For example, recent studies show that decorin can bind CCN2 directly through its leucine-rich repeat and inhibit the pro-fibrotic activity of CCN2167, suggesting the possibility of targeting CCN2 using a binding peptide. However, decorin is also known to bind other members of the CCN family and may not act specifically on CCN2168. Since CCNs interact with integrin αvβ3 to mediate angiogenic functions31, 169, peptides that bind and inhibit αvβ3 signaling may thwart CCN1-promoted angiogensis in tumor growth. However, this strategy of targeting αvβ3 and is not specific for CCN proteins. Other peptides have been identified to block CCN binding to integrin α6β1-HSPGs and inhibit activities mediated through these receptors170, 171, although the utility of these peptides in blocking CCN functions have not been investigated in vivo.
A number of clinical trials have been conducted or are underway targeting CCN2. A phase 1 study to evaluate the safety and tolerability of FG-3019172, a humanized monoclonal antibody directed against the VWC domain, was performed in patients with mild to moderate idiopathic pulmonary fibrosis found it safe and well tolerated173. A follow up phase 2 study is currently in progress (NCT01262001). Another phase 1b study showed that treatment of patients with type I or type II diabetes and microalbuminuria using FG-3019 was well tolerated6. Particularly encouraging is the observation that the urinary albumin/creatinine ratio (ACR) was reduced by more than 50% by the end of the treatment, suggesting that blockade of CCN2 is associated with a decrease in albuminuria. A similar phase 1 study on diabetic patients with more severe macroalbuminuria with a background of treatment for hypertension has also been completed (NCT00754143). On the strength of these observations, a phase 2 study was initiated in patients with type 2 diabetes and advanced kidney disease to evaluate the effect of FG-3019 on renal function and associated cardiovascular co-morbidities (NCT00913393). Another ongoing phase 1 study is evaluating FG-3019 therapy in combination with gemcitabine (nucleoside analog chemotherapeutic drug) and erlotinib (tyrosine kinase inhibitor) for patients with locally advanced or metastatic pancreatic cancer (NCT01181245). In addition, a phase 2 study has just begun in patients with liver fibrosis due to chronic hepatitis B infection in which the efficacy of FG-3019 for reversing fibrosis is being evaluated (NCT01217632).
Aside from monoclonal antibodies, a 2-O-methoxyethyl (2-MOE) modified antisense oligonucleotide (EXC-001) designed to inhibit CCN2 expression has been used in several phase II clinical trials to study its effects in controlling scarring after breast surgery (NCT01037413) or abdominoplasty surgery (NCT01037985; NCT01038297). Results show a reduction in scarring with no significant drug-related adverse effects (http://www.excaliard.com/news/PressReleaseExcaliardJanuary2011.pdf). Additionally, RXI-109, a self-delivering RNAi compound that reduces CCN2 expression, is being prepared for potential clinical trial (RXi Pharmaceuticals).
It is encouraging that no side effects have been observed in phase 1 trials using humanized monoclonal antibody or antisense oligonucleotide targeting CCN2. Since CCN proteins are generally downregulated in many tissues in the adult and are highly expressed only at sites of inflammation and injury repair, it is likely that therapeutic targeting of CCN proteins will preferentially affect the sites of pathology and have minimal impact on functions of normal organs and tissues. An exception might be pregnancy, since systemic targeting of certain CCN proteins may increase the risk of preeclampsia174 or inhibit placentation33.
CONCLUSIONS AND FUTURE DIRECTIONS
The CCN family of matricellular proteins plays important roles in modulating inflammation and tissue injury repair, and is often deregulated in a broad spectrum of pathologies that develop when these processes become chronic. Emerging studies have identified opportunities in which levels of CCN proteins in biofluids may serve as non-invasive diagnostic or prognostic tools, including systemic fibrosis69, 175, diabetic nephropathy137 and renal hypertension136, and some forms of cancer87, 116, 176. The potential of monitoring CCNs as a method of non-invasive assessment of fibrosis is of particular interest clinically. Studies in animal models of disease have also demonstrated therapeutic value in targeting relevant CCN genes in such diseases as cancer, diabetic nephropathy, and fibrosis of the liver, kidney, and skin. Clinical trials targeting CCN2 have begun and initial results are encouraging. We surmise that as studies on CCNs progress, more pathologies will be identified for which CCNs may serve as potential diagnostic or therapeutic targets.
Among the key challenges in future research is to elucidate the specific role of CCNs in disease etiology. In only a few cases have there been limited functional insight into how CCNs might be triggering or contributing to disease pathology. Understanding the critical pathological role of CCNs and their action mechanisms will help identify relevant targets for therapeutic intervention as the key signaling steps are identified. Since some members of the CCN family often play opposing roles, expression of a specific CCN protein may be either deleterious or beneficial in a particular pathological context. Therefore, the specificity of reagents targeting members of the CCN family is of paramount importance. Furthermore, how various CCN proteins act to provoke antithetical effects in some contexts is currently not well understood. Future studies that clarify how opposing CCN proteins function mechanistically may present further prospects for combinatorial therapies that simultaneously downregulate and upregulate the expression or function of rival CCN members, potentially magnifying the therapeutic impact.
Although CCNs represent unusual opportunities as therapeutic targets in a broad spectrum of diseases, their potential has remained relatively underexplored to date. Currently clinical trials are being conducted and planned targeting only CCN2, whereas other members of the family have not been investigated despite their apparent roles in many of the same pathologies in which CCN2 plays a role. This may be due in part to the lack of coordinated efforts to develop humanized neutralizing monoclonal antibodies against other members of the CCN family, although studies using other targeting strategies can be initiated. As the relative efficacies of targeting distinct members of the CCN family in the same pathologies remain to be determined, future studies that compare the full range of CCNs as therapeutic targets will be of considerable interest.
Figure 1. Transcriptional regulation of CCN genes.
Multiple extracellular and environmental stimuli rapidly induce CCN genes through activation of various transcription factors, usually without requiring de novo protein synthesis. These include PDGF, FGF2, TGF-β, IL1β, TNFα, angiotensin II (AT-II)2, 3, agonists of G protein-coupled receptors (GPCRs)177 such as thrombin, prostaglandins (PE), endothelin-1 (ET), and sphingosine-1-phosphate (S1P), hormones (estrogen and vitamin D) that which bind steroid hormone receptors (SHR), hypoxia, UV, and mechanical stretch37, 129. The locations of various transcription factor bindings sites vary for each CCN gene.
ACKNOWLEDGEMENT
We apologize to colleagues whose primary papers were not cited owing to space constraints. This work was supported in part by grants from the National Institutes of Health (AR61791, GM78492, HL81390) to L.F.L.
GLOSSARY
- Matricellular proteins
Dynamically expressed extracellular matrix proteins with a modular structure that serve regulatory roles and are often involved in wound healing.
- Integrins
A family of heterodimeric cell surface signaling receptors that mediate cell-cell and cell-matrix interactions, and are comprised of 18 α and 8 β subunits to constitute 24 known integrin heterodimers in mammals.
- LRP
Members of the low density lipoprotein receptor-related protein family are cell surface receptors that bind a broad spectrum of ligands, facilitating endocytosis and signaling of associated receptors.
- Immediate-early genes
Genes that are transcriptionally activated with rapid kinetics without requiring de novo protein synthesis by a broad range of stimuli, including viral infection or various extracellular signals.
- Cellular senescence
A state of irreversible cell cycle arrest induced by various forms of cellular stresses including DNA damage, oncogene activation, oxidative stress, chromatin disruption, and telomere erosion.
- SASP
One of the characteristics of senescent cells is expression of the senescence-associated secretory phenotype, which includes increased secretion of inflammatory cytokines and matrix degrading enzymes, and downregulation of ECM proteins such as collagen.
- MMPs
Matrix metalloproteinases comprise a family of >20 zinc-dependent endopeptidases involved in the degradation of ECM proteins, cleavage of cell surface receptors, release of apoptotic ligands and activation of chemokines/cytokines.
- Platelet α granules
Cytoplasmic granules containing many proteins factors involved in inflammation and wound repair synthesized predominantly in megakaryocytes, including cell adhesive proteins, growth and coagulation factors, and protease inhibitors, that are released upon platelet activation at sites of vessel injury.
- Epithelial-to-mesenchymal transition
A process in which epithelial cells lose their cell adhesive properties and gain cell mobility, typically involving repression of E-cadherin expression. EMT is essential for many developmental processes and occurs in oncogenic transformation.
- Microalbuminuria
A condition in which small amounts of albumin leaks into the urine upon injury or damage to the kidney, indicating abnormal permeability in the renal glomerulus.
- Balloon angioplasty
A method of mechanically widening an obstructed blood vessel, typically a result of atherosclerosis, by passing a collapsed balloon catheter through the obstructed area and inflating the balloon to open up the blood vessel as the catheter is withdrawn.
Reference List
- 1.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 2.Chen C-C, Lau LF. Functions and Mechanisms of Action of CCN Matricellular Proteins. Int. J. Biochem. Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 4.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem. Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perbal B, Takigawa M. CCN Proteins: A New Family of Cell Growth and Differentiaion Regulators. London: Imperial College Press; 2005. [Google Scholar]
- 6.Adler SG, et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes microalbuminuria. Clin. J. Am. Soc. Nephrol. 2010;5:1420–1428. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C-C, et al. Cytotoxicity of TNFα is regulated by Integrin-Mediated Matrix Signaling. EMBO J. 2007;26:1257–1267. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jun J-I, Lau LF. The Matricellular Protein CCN1/CYR61 Induces Fibroblast Senescence and Restricts Fibrosis in Cutaneous Wound Healing. Nat. Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todorovic V, Chen C-C, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J. Cell Biol. 2005;171:559–568. doi: 10.1083/jcb.200504015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- 12.Wahab NA, Weston BS, Mason RM. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J. Am. Soc. Nephrol. 2005;16:340–351. doi: 10.1681/ASN.2003100905. [DOI] [PubMed] [Google Scholar]
- 13.Edwards LA, et al. Effect of Brain- and Tumor-Derived Connective Tissue Growth Factor on Glioma Invasion. J. Natl. Cancer Inst. 2011;103:1162–1178. doi: 10.1093/jnci/djr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamoto K, et al. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J. Biol. Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- 15.Minamizato T, et al. CCN3/NOV inhibits BMP-2-induced osteoblast differentiation by interacting with BMP and Notch signaling pathways. Biochem. Biophys. Res. Commun. 2007;354:567–573. doi: 10.1016/j.bbrc.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Chen C-C, Chen N, Lau LF. The angiogenic factors Cyr61 and CTGF induce adhesive signaling in primary human skin fibroblasts. J. Biol. Chem. 2001;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi T, et al. Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology. 2000;141:264–273. doi: 10.1210/endo.141.1.7267. [DOI] [PubMed] [Google Scholar]
- 18.Safadi FF, et al. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J. Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- 19.abdel-Wahab N, Weston BS, Roberts T, Mason RM. Connective tissue growth factor and regulation of the mesangial cell cycle: role in cellular hypertrophy. J. Am. Soc. Nephrol. 2002;13:2437–2445. doi: 10.1097/01.asn.0000031828.58276.02. [DOI] [PubMed] [Google Scholar]
- 20.Benini S, et al. In Ewing’s sarcoma CCN3(NOV) inhibits proliferation while promoting migration and invasion of the same cell type. Oncogene. 2005;24:4349–4361. doi: 10.1038/sj.onc.1208620. [DOI] [PubMed] [Google Scholar]
- 21.Fan WH, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur. J. Cell Biol. 2000;79:915–923. doi: 10.1078/0171-9335-00122. [DOI] [PubMed] [Google Scholar]
- 22.Grzeszkiewicz TM, Lindner V, Chen N, Lam SCT, Lau LF. The angiogenic factor CYR61 supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin α6β1 and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143:1441–1450. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- 23.Lake AC, Bialik A, Walsh K, Castellot JJ., Jr. CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am. J. Pathol. 2003;162:219–231. doi: 10.1016/S0002-9440(10)63813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoyama T, et al. CCN3 inhibits neointimal hyperplasia through modulation of smooth muscle cell growth and migration. Arterioscler. Thromb. Vasc. Biol. 2010;30:675–682. doi: 10.1161/ATVBAHA.110.203356. [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Lau LF. Deadly liaisons: fatal attraction between CCN matricellular proteins and the tumor necrosis factor family of cytokines. J. Cell Commun. Signal. 2010;4:63–69. doi: 10.1007/s12079-009-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juric V, Chen CC, Lau LF. Fas-Mediated Apoptosis is Regulated by the Extracellular Matrix Protein CCN1 (CYR61) in vitro and in vivo. Mol. Cell. Biol. 2009;29:3266–3279. doi: 10.1128/MCB.00064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franzen CA, et al. The Matrix Protein CCN1 is Critical for Prostate Carcinoma Cell Proliferation and TRAIL-Induced Apoptosis. Mol. Cancer Res. 2009;7:1045–1055. doi: 10.1158/1541-7786.MCR-09-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatachalam K, et al. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNF-alpha)-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J. Biol. Chem. 2009;284:14414–14427. doi: 10.1074/jbc.M809757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodier F, Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging (Albany. NY) 2010;2:627–631. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, product of a growth factor-inducible immediate-early gene, promotes angiogenesis and tumor growth. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubota S, Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis. 2007;10:1–11. doi: 10.1007/s10456-006-9058-5. [DOI] [PubMed] [Google Scholar]
- 33.Mo FE, et al. CYR61 (CCN1) Is Essential for Placental Development and Vascular Integrity. Mol. Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoki I, et al. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 35.Dean RA, et al. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol. Cell Biol. 2007;27:8454–8465. doi: 10.1128/MCB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The CCN family: A new class of inflammation modulators? Biochimie. 2010;93:377–388. doi: 10.1016/j.biochi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell. Mol. Life Sci. 2011;68:3149–3163. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubota S, Takigawa M. The role of CCN2 in cartilage and bone development. J. Cell Commun. Signal. 2011;5:209–217. doi: 10.1007/s12079-011-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeda A, et al. CCN family 2/connective tissue growth factor modulates BMP signalling as a signal conductor, which action regulates the proliferation and differentiation of chondrocytes. J. Biochem. 2009;145:207–216. doi: 10.1093/jb/mvn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono M, Inkson CA, Kilts TM, Young MF. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J. Bone Miner. Res. 2011;26:193–208. doi: 10.1002/jbmr.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crockett JC, et al. The matricellular protein CYR61 inhibits osteoclastogenesis by a mechanism independent of αvβ3 and αvβ5. Endocrinology. 2007;148:5761–5768. doi: 10.1210/en.2007-0473. [DOI] [PubMed] [Google Scholar]
- 43.Ouellet V, et al. CCN3 impairs osteoblast and stimulates osteoclast differentiation to favor breast cancer metastasis to bone. Am. J. Pathol. 2011;178:2377–2388. doi: 10.1016/j.ajpath.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rydziel S, et al. Nephroblastoma overexpressed (Nov) inhibits osteoblastogenesis and causes osteopenia. J. Biol. Chem. 2007;282:19762–19772. doi: 10.1074/jbc.M700212200. [DOI] [PubMed] [Google Scholar]
- 45.Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Canalis E. Skeletal overexpression of connective tissue growth factor (ctgf) impairs bone formation and causes osteopenia. Endocrinology. 2008;149:4374–4381. doi: 10.1210/en.2008-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai T, Chen C-C, Lau LF. The matricellular protein CCN1 activates a pro-inflammatory genetic program in murine macrophages. J. Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rother M, et al. Matricellular signaling molecule CCN1 attenuates experimental autoimmune myocarditis by acting as a novel immune cell migration modulator. Circulation. 2010;122:2688–2698. doi: 10.1161/CIRCULATIONAHA.110.945261. [DOI] [PubMed] [Google Scholar]
- 48.Lin Z, et al. A novel role of CCN3 in regulating endothelial inflammation. J. Cell Commun. Signal. 2010;4:141–153. doi: 10.1007/s12079-010-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo Q, et al. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J. Biol. Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 51.Si W, et al. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A–induced osteoblast differentiation of mesenchymal stem cells. Mol. Cell Biol. 2006;26:2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta R, Hong D, Iborra F, Sarno S, Enver T. NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science. 2007;316:590–593. doi: 10.1126/science.1136031. [DOI] [PubMed] [Google Scholar]
- 53.Katsuki Y, et al. Inhibitory effect of CT domain of CCN3/NOV on proliferation and differentiation of osteogenic mesenchymal stem cells, Kusa-A1. Biochem. Biophys. Res. Commun. 2008;368:808–814. doi: 10.1016/j.bbrc.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Mo F-E, Lau LF. The matricellular protein CCN1 is essential for cardiac development. Circ. Res. 2006;99:961–969. doi: 10.1161/01.RES.0000248426.35019.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheffield VC, et al. Identification of a complex congenital heart defect susceptibility locus by using DNA pooling and shared segment analysis. Hum. Mol. Genet. 1997;6:117–121. doi: 10.1093/hmg/6.1.117. [DOI] [PubMed] [Google Scholar]
- 56.Ivkovic S, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baguma-Nibasheka M, Kablar B. Pulmonary hypoplasia in the connective tissue growth factor (Ctgf) null mouse. Dev. Dyn. 2008;237:485–493. doi: 10.1002/dvdy.21433. [DOI] [PubMed] [Google Scholar]
- 58.Crawford LA, et al. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol. Endocrinol. 2009;23:324–336. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagashima T, et al. Connective tissue growth factor is required for normal follicle development and ovulation. Mol. Endocrinol. 2011;25:1740–1759. doi: 10.1210/me.2011-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Canalis E, et al. Nephroblastoma Overexpressed (Nov) Inactivation Sensitizes Osteoblasts to Bone Morphogenetic Protein-2, But Nov Is Dispensable for Skeletal Homeostasis. Endocrinology. 2010;151:221–233. doi: 10.1210/en.2009-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russo JW, Castellot JJ. CCN5: biology and pathophysiology. J. Cell Commun. Signal. 2010;4:119–130. doi: 10.1007/s12079-010-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kutz WE, Gong Y, Warman ML. WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol. Cell Biol. 2005;25:414–421. doi: 10.1128/MCB.25.1.414-421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 64.Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Invest Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 65.Cicha I, Garlichs CD, Daniel WG, Goppelt-Struebe M. Activated human platelets release connective tissue growth factor. Thromb. Haemost. 2004;91:755–760. doi: 10.1160/TH03-09-0602. [DOI] [PubMed] [Google Scholar]
- 66.Kubota S, et al. Abundant retention and release of connective tissue growth factor (CTGF/CCN2) by platelets. J. Biochem. (Tokyo) 2004;136:279–282. doi: 10.1093/jb/mvh126. [DOI] [PubMed] [Google Scholar]
- 67.Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J. Cell Commun. Signal. 2009;4:1–4. doi: 10.1007/s12079-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mori T, et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J. Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 69.Leask A, Parapuram SK, Shi-wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J. Cell Commun. Signal. 2009;3:89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phanish MK, Winn SK, Dockrell ME. Connective tissue growth factor-(CTGF, CCN2)--a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp. Nephrol. 2010;114:e83–e92. doi: 10.1159/000262316. [DOI] [PubMed] [Google Scholar]
- 71.Sonnylal S, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu S, Shi-wen X, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63:239–246. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 73.Uchio K, Graham M, Dean NM, Rosenbaum J, Desmouliere A. Down-regulation of connective tissue growth factor and type I collagen mRNA expression by connective tissue growth factor antisense oligonucleotide during experimental liver fibrosis. Wound. Repair Regen. 2004;12:60–66. doi: 10.1111/j.1067-1927.2004.012112.x. [DOI] [PubMed] [Google Scholar]
- 74.Li G, et al. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J. Gene Med. 2006;8:889–900. doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- 75.George J, Tsutsumi M. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther. 2007;14:790–803. doi: 10.1038/sj.gt.3302929. [DOI] [PubMed] [Google Scholar]
- 76.Yokoi H, et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2004;15:1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- 77.Sisco M, et al. Antisense inhibition of connective tissue growth factor (CTGF/CCN2) mRNA limits hypertrophic scarring without affecting wound healing in vivo. Wound. Repair Regen. 2008;16:661–673. doi: 10.1111/j.1524-475X.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 78.Okada H, et al. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J. Am. Soc. Nephrol. 2005;16:133–143. doi: 10.1681/ASN.2004040339. [DOI] [PubMed] [Google Scholar]
- 79.Konigshoff M, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J. Clin. Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riser BL, et al. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am. J. Pathol. 2009;174:1725–1734. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon PO, et al. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J. Mol. Cell Cardiol. 2010;49:294–303. doi: 10.1016/j.yjmcc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 83.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 84.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 85.Dhar A, Ray A. The CCN family proteins in carcinogenesis. Exp. Oncol. 2010;32:2–9. [PubMed] [Google Scholar]
- 86.Dobroff AS, et al. Silencing cAMP-response element binding protein (CREB) identifies cysteine-rich protein 61 (CYR61) as a tumor suppressor gene in melanoma. J. Biol. Chem. 2009;284:26194–26206. doi: 10.1074/jbc.M109.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin BR, et al. Connective tissue growth factor acts as a therapeutic agent and predictor for peritoneal carcinomatosis of colorectal cancer. Clin. Cancer Res. 2011;17:3077–3088. doi: 10.1158/1078-0432.CCR-09-3256. [DOI] [PubMed] [Google Scholar]
- 88.Shimo T, et al. Involvement of CTGF, a hypertrophic chondrocyte-specific gene product, in tumor angiogenesis. Oncology. 2001;61:315–322. doi: 10.1159/000055339. [DOI] [PubMed] [Google Scholar]
- 89.Xie D, et al. Breast cancer. Cyr61 is overexpressed, estrogen-inducible, and associated with more advanced disease. J. Biol. Chem. 2001;276:14187–14194. doi: 10.1074/jbc.M009755200. [DOI] [PubMed] [Google Scholar]
- 90.Tsai MS, Bogart DF, Castaneda JM, Li P, Lupu R. Cyr61 promotes breast tumorigenesis and cancer progression. Oncogene. 2002;21:8178–8185. doi: 10.1038/sj.onc.1205682. [DOI] [PubMed] [Google Scholar]
- 91.Yang F, et al. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- 92.Yin D, et al. Connective tissue growth factor associated with oncogenic activities and drug resistance in glioblastoma multiforme. Int. J. Cancer. 2010;127:2257–2267. doi: 10.1002/ijc.25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun ZJ, et al. Involvement of Cyr61 in growth, migration, and metastasis of prostate cancer cells. Br. J. Cancer. 2008;99:1656–1667. doi: 10.1038/sj.bjc.6604712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bennewith KL, et al. The role of tumor cell-derived connective tissue growth factor (CTGF/CCN2) in pancreatic tumor growth. Cancer Res. 2009;69:775–784. doi: 10.1158/0008-5472.CAN-08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haque I, et al. Cyr61/CCN1 signaling is critical for epithelial-mesenchymal transition and stemness and promotes pancreatic carcinogenesis. Mol. Cancer. 2011;10:8. doi: 10.1186/1476-4598-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gery S, et al. Ovarian carcinomas: CCN genes are aberrantly expressed and CCN1 promotes proliferation of these cells. Clin. Cancer Res. 2005;11:7243–7254. doi: 10.1158/1078-0432.CCR-05-0231. [DOI] [PubMed] [Google Scholar]
- 97.Shi Y, et al. Homologous peptide of connective tissue growth factor ameliorates epithelial to mesenchymal transition of tubular epithelial cells. Cytokine. 2006;36:35–44. doi: 10.1016/j.cyto.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 98.Lin MT, et al. Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-κB-dependent XIAP up-regulation. J. Biol. Chem. 2004;279:24015–24023. doi: 10.1074/jbc.M402305200. [DOI] [PubMed] [Google Scholar]
- 99.Wang MY, et al. Connective tissue growth factor confers drug resistance in breast cancer through concomitant up-regulation of Bcl-xL and cIAP1. Cancer Res. 2009;69:3482–3491. doi: 10.1158/0008-5472.CAN-08-2524. [DOI] [PubMed] [Google Scholar]
- 100.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the Hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 101.Goodwin CR, et al. Cyr61 mediates hepatocyte growth factor-dependent tumor cell growth, migration, and Akt activation. Cancer Res. 2010;70:2932–2941. doi: 10.1158/0008-5472.CAN-09-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 103.Shimo T, et al. Pathogenic role of connective tissue growth factor (CTGF/CCN2) in osteolytic metastasis of breast cancer. J. Bone Miner. Res. 2006;21:1045–1059. doi: 10.1359/jbmr.060416. [DOI] [PubMed] [Google Scholar]
- 104.Dornhofer N, et al. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816–5827. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 105.Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol. Cancer Ther. 2006;5:1108–1116. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- 106.Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- 107.Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917–8923. [PubMed] [Google Scholar]
- 108.Tian C, et al. Overexpression of connective tissue growth factor WISP-1 in Chinese primary rectal cancer patients. World J. Gastroenterol. 2007;13:3878–3882. doi: 10.3748/wjg.v13.i28.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen PP, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS. ONE. 2007;2:e534. doi: 10.1371/journal.pone.0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hashimoto Y, et al. expression of the Elm1 gene, a nove gene of the CCN (Connective tissue growth factor, Cyr61/Cef10, and neuroblastoma overexpressed gene) family, suppresses in vivo tumor growth and metastasis of K-1735 murine melanoma cells. J. Exp. Med. 1998;187:289–296. doi: 10.1084/jem.187.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soon LL, et al. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J. Biol. Chem. 2003;278:11465–11470. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]
- 112.Tanaka S, et al. A novel variant of WISP1 lacking a Von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene. 2001;20:5525–5532. doi: 10.1038/sj.onc.1204723. [DOI] [PubMed] [Google Scholar]
- 113.Bleau AM, et al. Antiproliferative activity of CCN3: involvement of the C-terminal module and post-translational regulation. J. Cell Biochem. 2007;101:1475–1491. doi: 10.1002/jcb.21262. [DOI] [PubMed] [Google Scholar]
- 114.Gupta N, et al. Inhibition of glioma cell growth and tumorigenic potential by CCN3 (NOV) Mol. Pathol. 2001;54:293–299. doi: 10.1136/mp.54.5.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vallacchi V, et al. CCN3/nephroblastoma overexpressed matricellular protein regulates integrin expression, adhesion, and dissemination in melanoma. Cancer Res. 2008;68:715–723. doi: 10.1158/0008-5472.CAN-07-2103. [DOI] [PubMed] [Google Scholar]
- 116.Perbal B, et al. Prognostic relevance of CCN3 in Ewing sarcoma. Hum. Pathol. 2009;40:1479–1486. doi: 10.1016/j.humpath.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 117.Ghayad SE, et al. Identification of TACC1, NOV, and PTTG1 as new candidate genes associated with endocrine therapy resistance in breast cancer. J. Mol. Endocrinol. 2009;42:87–103. doi: 10.1677/JME-08-0076. [DOI] [PubMed] [Google Scholar]
- 118.McCallum L, et al. A novel mechanism for BCR-ABL action: stimulated secretion of CCN3 is involved in growth and differentiation regulation. Blood. 2006;108:1716–1723. doi: 10.1182/blood-2006-04-016113. [DOI] [PubMed] [Google Scholar]
- 119.McCallum L, et al. CCN3 suppresses mitogenic signalling and reinstates growth control mechanisms in Chronic Myeloid Leukaemia. J. Cell Commun. Signal. 2011 Jul 20; doi: 10.1007/s12079-011-0142-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kleer CG, et al. WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene. 2002;21:3172–3180. doi: 10.1038/sj.onc.1205462. [DOI] [PubMed] [Google Scholar]
- 121.Lorenzatti G, Huang W, Pal A, Cabanillas AM, Kleer CG. CCN6 (WISP3) decreases ZEB1-mediated EMT and invasion by attenuation of IGF-1 receptor signaling in breast cancer. J. Cell Sci. 2011;124:1752–1758. doi: 10.1242/jcs.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmitt CA. Senescence, apoptosis and therapy--cutting the lifelines of cancer. Nat. Rev. Cancer. 2003;3:286–295. doi: 10.1038/nrc1044. [DOI] [PubMed] [Google Scholar]
- 123.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de FM, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev. Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 124.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin. Pract. Endocrinol. Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- 126.Murphy M, et al. Suppression Subtractive Hybridization Identifies High Glucose Levels as a Stimulus for Expression of Connective Tissue Growth Factor and Other Genes in Human Mesangial Cells. J. Biol Chem. 1999;274:5830–5834. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- 127.Twigg SM, et al. Renal connective tissue growth factor induction in experimental diabetes is prevented by aminoguanidine. Endocrinology. 2002;143:4907–4915. doi: 10.1210/en.2002-220619. [DOI] [PubMed] [Google Scholar]
- 128.Hughes JM, et al. Advanced glycation end products cause increased CCN family and extracellular matrix gene expression in the diabetic rodent retina. Diabetologia. 2007;50:1089–1098. doi: 10.1007/s00125-007-0621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273:3639–3649. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 130.Mason RM. Connective tissue growth factor(CCN2), a pathogenic factor in diabetic nephropathy. What does it do? How does it do it? J. Cell Commun. Signal. 2009;3:95–104. doi: 10.1007/s12079-009-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sawai K, et al. Expression of CCN1 (CYR61) in developing, normal, and diseased human kidney. Am. J. Physiol Renal Physiol. 2007;293:F1363–F1372. doi: 10.1152/ajprenal.00205.2007. [DOI] [PubMed] [Google Scholar]
- 132.Sanchez-Lopez E, et al. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-kappaB. J. Am. Soc. Nephrol. 2009;20:1513–1526. doi: 10.1681/ASN.2008090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21:3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- 134.Yokoi H, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 135.Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 2005;67:799–812. doi: 10.1111/j.1523-1755.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 136.Jaffa AA, et al. Connective Tissue Growth factor and Susceptibility to Renal and Vascular Disease Risk in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2008;93:1893–1900. doi: 10.1210/jc.2007-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nguyen TQ, et al. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care. 2008;31:1177–1182. doi: 10.2337/dc07-2469. [DOI] [PubMed] [Google Scholar]
- 138.You JJ, Yang CH, Chen MS, Yang CM. Cysteine-rich 61, a member of the CCN family, as a factor involved in the pathogenesis of proliferative diabetic retinopathy. Invest Ophthalmol. Vis. Sci. 2009;50:3447–3455. doi: 10.1167/iovs.08-2603. [DOI] [PubMed] [Google Scholar]
- 139.Kuiper EJ, et al. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch. Ophthalmol. 2006;124:1457–1462. doi: 10.1001/archopht.124.10.1457. [DOI] [PubMed] [Google Scholar]
- 140.Kuiper EJ, et al. Connective tissue growth factor is necessary for retinal capillary basal lamina thickening in diabetic mice. J. Histochem. Cytochem. 2008;56:785–792. doi: 10.1369/jhc.2008.950980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hilfiker A, et al. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation. 2002;106:254–260. doi: 10.1161/01.cir.0000021426.87274.62. [DOI] [PubMed] [Google Scholar]
- 142.Cicha I, et al. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler. Thromb. Vasc. Biol. 2005;25:1008–1013. doi: 10.1161/01.ATV.0000162173.27682.7b. [DOI] [PubMed] [Google Scholar]
- 143.Kundi R, et al. Arterial gene transfer of the TGF-beta signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF. Cardiovasc. Res. 2009;84:326–335. doi: 10.1093/cvr/cvp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee HY, et al. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 2007;100:372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- 145.Matsumae H, et al. CCN1 Knockdown Suppresses Neointimal Hyperplasia in a Rat Artery Balloon Injury Model. Arterioscler. Thromb. Vasc. Biol. 2008;28:1077–1083. doi: 10.1161/ATVBAHA.108.162362. [DOI] [PubMed] [Google Scholar]
- 146.Reynolds AR, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 147.Hilfiker-Kleiner D, et al. Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation. 2004;109:2227–2233. doi: 10.1161/01.CIR.0000127952.90508.9D. [DOI] [PubMed] [Google Scholar]
- 148.Dean RG, et al. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J. Histochem. Cytochem. 2005;53:1245–1256. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 149.Panek AN, et al. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS. ONE. 2009;4:e6743. doi: 10.1371/journal.pone.0006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Omoto S, et al. Expression and localization of connective tissue growth factor (CTGF/Hcs24/CCN2) in osteoarthritic cartilage. Osteoarthritis. Cartilage. 2004;12:771–778. doi: 10.1016/j.joca.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 151.Blom AB, et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- 152.Nishida T, et al. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J. Bone Miner. Res. 2004;19:1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- 153.Nakata E, et al. Expression of connective tissue growth factor/hypertrophic chondrocyte-specific gene product 24 (CTGF/Hcs24) during fracture healing. Bone. 2002;31:441–447. doi: 10.1016/s8756-3282(02)00846-3. [DOI] [PubMed] [Google Scholar]
- 154.Athanasopoulos AN, et al. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J. Biol. Chem. 2007;282:26746–26753. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nozawa K, et al. Connective tissue growth factor promotes articular damage by increased osteoclastogenesis in patients with rheumatoid arthritis. Arthritis Res. Ther. 2009;11:R174. doi: 10.1186/ar2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Q, et al. A critical role of Cyr61 in interleukin-17-dependent proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2009;60:3602–3612. doi: 10.1002/art.24999. [DOI] [PubMed] [Google Scholar]
- 157.Koon HW, et al. Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis. Am. J. Pathol. 2008;173:400–410. doi: 10.2353/ajpath.2008.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ueberham U, Ueberham E, Gruschka H, Arendt T. Connective tissue growth factor in Alzheimer’s disease. Neurosci. 2003;116:1–6. doi: 10.1016/s0306-4522(02)00670-x. [DOI] [PubMed] [Google Scholar]
- 159.Zhao Z, et al. Connective tissue growth factor (CTGF) expression in the brain is a downstream effector of insulin resistance- associated promotion of Alzheimer’s disease beta-amyloid neuropathology. FASEB J. 2005;19:2081–2082. doi: 10.1096/fj.05-4359fje. [DOI] [PubMed] [Google Scholar]
- 160.Hurvitz JR, et al. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat. Genet. 1999;23:94–98. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- 161.Nakamura Y, et al. The CCN family member Wisp3, mutant in progressive pseudorheumatoid dysplasia, modulates BMP and Wnt signaling. J. Clin. Invest. 2007;117:3075–3086. doi: 10.1172/JCI32001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fonseca C, et al. A polymorphism in the CTGF promoter region associated with systemic sclerosis. N. Engl. J. Med. 2007;357:1210–1220. doi: 10.1056/NEJMoa067655. [DOI] [PubMed] [Google Scholar]
- 163.Kawaguchi Y, et al. Association study of a polymorphism of the CTGF gene and susceptibility to systemic sclerosis in the Japanese population. Ann. Rheum. Dis. 2009;68:1921–1924. doi: 10.1136/ard.2008.100586. [DOI] [PubMed] [Google Scholar]
- 164.Granel B, et al. Association between a CTGF gene polymorphism and systemic sclerosis in a French population. J. Rheumatol. 2010;37:351–358. doi: 10.3899/jrheum.090290. [DOI] [PubMed] [Google Scholar]
- 165.Rueda B, et al. A large multicentre analysis of CTGF −945 promoter polymorphism does not confirm association with systemic sclerosis susceptibility or phenotype. Ann. Rheum. Dis. 2009;68:1618–1620. doi: 10.1136/ard.2008.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brigstock DR. Strategies for blocking the fibrogenic actions of connective tissue growth factor (CCN2): From pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J. Cell Commun. Signal. 2009;3:5–18. doi: 10.1007/s12079-009-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vial C, Gutierrez J, Santander C, Cabrera D, Brandan E. Decorin Interacts with Connective Tissue Growth Factor (CTGF)/CCN2 by LRR12 Inhibiting Its Biological Activity. J. Biol Chem. 2011;286:24242–24252. doi: 10.1074/jbc.M110.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol. Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- 169.Leu S-J, Lam SCT, Lau LF. Proangiogenic activities of CYR61 (CCN1) mediated through integrins αvβ3 and α6β1 in human umbilical vein endothelial cells. J. Biol. Chem. 2002;277:46248–46255. doi: 10.1074/jbc.M209288200. [DOI] [PubMed] [Google Scholar]
- 170.Leu S-J, et al. Identification of a novel integrin α6β1 binding site in the angiogenic Inducer CCN1 (CYR61) J. Biol. Chem. 2003;278:33801–33808. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- 171.Leu S-J, et al. Targeted mutagenesis of the matricellular protein CCN1 (CYR61): selective inactivation of integrin α6β1-heparan sulfate proteoglycan coreceptor-mediated cellular activities. J. Biol. Chem. 2004;279:44177–44187. doi: 10.1074/jbc.M407850200. [DOI] [PubMed] [Google Scholar]
- 172.Wang Q, et al. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis. Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mageto Y, Flaherty K, Brown A, Raghu G. Safety and Tolerability of Human Monoclonal Antibody FG-3019, Anti-Connective Tissue Growth Factor, in Patients with Idiopathic Pulmonary Fibrosis. Chest. 2004;126:773S. [Google Scholar]
- 174.Zhang Y, et al. MicroRNA-155 contributes to preeclampsia by down-regulating CYR61. Am. J. Obstet. Gynecol. 2010;202:466–467. doi: 10.1016/j.ajog.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 175.Dendooven A, Gerritsen KG, Nguyen TQ, Kok RJ, Goldschmeding R. Connective tissue growth factor (CTGF/CCN2) ELISA: a novel tool for monitoring fibrosis. Biomarkers. 2011;16:289–301. doi: 10.3109/1354750X.2011.561366. [DOI] [PubMed] [Google Scholar]
- 176.D’Antonio KB, et al. Decreased expression of Cyr61 is associated with prostate cancer recurrence after surgical treatment. Clin. Cancer Res. 2010;16:5908–5913. doi: 10.1158/1078-0432.CCR-10-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Walsh CT, Stupack D, Brown JH. G protein-coupled receptors go extracellular: RhoA integrates the integrins. Mol. Interv. 2008;8:165–173. doi: 10.1124/mi.8.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Su JL, et al. CYR61 regulates BMP-2-dependent osteoblast differentiation through the αvβ3 integrin/integrin-linked kinase/ERK pathway. J. Biol Chem. 2010;285:31325–31336. doi: 10.1074/jbc.M109.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu SH, Lu C, Dong L, Chen ZQ. Signal transduction involved in CTGF-induced production of chemokines in mesangial cells. Growth Factors. 2008;26:192–200. doi: 10.1080/08977190802227828. [DOI] [PubMed] [Google Scholar]
- 180.Canalis E, Zanotti S, Beamer WG, Economides AN, Smerdel-Ramoya A. Connective tissue growth factor is required for skeletal development and postnatal skeletal homeostasis in male mice. Endocrinology. 2010;151:3490–3501. doi: 10.1210/en.2010-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Doherty HE, Kim HS, Hiller S, Sulik KK, Maeda N. A mouse strain where basal connective tissue growth factor gene expression can be switched from low to high. PLoS. ONE. 2010;5:e12909. doi: 10.1371/journal.pone.0012909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tong Z, et al. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50:939–947. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Watari H, Xiong Y, Hassan MK, Sakuragi N. Cyr61, a member of ccn (connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed) family, predicts survival of patients with endometrial cancer of endometrioid subtype. Gynecol. Oncol. 2009;112:229–234. doi: 10.1016/j.ygyno.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 184.Chien W, et al. Cyr61 suppresses growth of human endometrial cancer cells. J Biol. Chem. 2004;279:53087–53096. doi: 10.1074/jbc.M410254200. [DOI] [PubMed] [Google Scholar]
- 185.Kikuchi R, et al. Promoter hypermethylation contributes to frequent inactivation of a putative conditional tumor suppressor gene connective tissue growth factor in ovarian cancer. Cancer Res. 2007;67:7095–7105. doi: 10.1158/0008-5472.CAN-06-4567. [DOI] [PubMed] [Google Scholar]
- 186.Tong X, et al. Cyr61, a member of CCN family, is a tumor suppressor in non-small cell lung cancer. J Biol. Chem. 2001;276:47709–47714. doi: 10.1074/jbc.M107878200. [DOI] [PubMed] [Google Scholar]
- 187.Dhar G, et al. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett. 2007;254:63–70. doi: 10.1016/j.canlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 188.Ladwa R, Pringle H, Kumar R, West K. Expression of CTGF and Cyr61 in colorectal cancer. J. Clin. Pathol. 2011;64:58–64. doi: 10.1136/jcp.2010.082768. [DOI] [PubMed] [Google Scholar]
- 189.Croci S, et al. Inhibition of connective tissue growth factor (CTGF/CCN2) expression decreases the survival and myogenic differentiation of human rhabdomyosarcoma cells. Cancer Res. 2004;64:1730–1736. doi: 10.1158/0008-5472.can-3502-02. [DOI] [PubMed] [Google Scholar]
- 190.Boag JM, et al. High expression of connective tissue growth factor in pre-B acute lymphoblastic leukaemia. Br. J. Haematol. 2007;138:740–748. doi: 10.1111/j.1365-2141.2007.06739.x. [DOI] [PubMed] [Google Scholar]
- 191.Bouchard L, et al. CYR61 polymorphisms are associated with plasma HDL-cholesterol levels in obese individuals. Clin. Genet. 2007;72:224–229. doi: 10.1111/j.1399-0004.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 192.Fernandez-Rozadilla C, et al. Colorectal cancer susceptibility quantitative trait loci in mice as a novel approach to detect low-penetrance variants in humans: a two-stage case-control study. Cancer Epidemiol. Biomarkers Prev. 2010;19:619–623. doi: 10.1158/1055-9965.EPI-09-1175. [DOI] [PubMed] [Google Scholar]
- 193.Dessein A, et al. Variants of CTGF are associated with hepatic fibrosis in Chinese, Sudanese, and Brazilians infected with schistosomes. J. Exp. Med. 2009;206:2321–2328. doi: 10.1084/jem.20090383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cozzolino M, et al. CCN2 (CTGF) gene polymorphism is a novel prognostic risk factor for cardiovascular outcomes in hemodialysis patients. Blood Purif. 2010;30:272–276. doi: 10.1159/000320706. [DOI] [PubMed] [Google Scholar]
- 195.Ortlepp JR, et al. The amount of calcium-deficient hexagonal hydroxyapatite in aortic valves is influenced by gender and associated with genetic polymorphisms in patients with severe calcific aortic stenosis. Eur. Heart J. 2004;25:514–522. doi: 10.1016/j.ehj.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 196.Wang B, et al. Genetic variant in the promoter of connective tissue growth factor gene confers susceptibility to nephropathy in type 1 diabetes. J. Med. Genet. 2010;47:391–397. doi: 10.1136/jmg.2009.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yamada Y, Ando F, Shimokata H. Association of polymorphisms of SORBS1, GCK and WISP1 with hypertension in community-dwelling Japanese individuals. Hypertens. Res. 2009;32:325–331. doi: 10.1038/hr.2009.23. [DOI] [PubMed] [Google Scholar]
- 198.Urano T, et al. Association of a single nucleotide polymorphism in the WISP1 gene with spinal osteoarthritis in postmenopausal Japanese women. J. Bone Miner. Metab. 2007;25:253–258. doi: 10.1007/s00774-007-0757-9. [DOI] [PubMed] [Google Scholar]
- 199.Thorstensen L, et al. WNT1 inducible signaling pathway protein 3, WISP-3, a novel target gene in colorectal carcinomas with microsatellite instability. Gastroenterology. 2001;121:1275–1280. doi: 10.1053/gast.2001.29570. [DOI] [PubMed] [Google Scholar]
- 200.Lamb R, Thomson W, Ogilvie E, Donn R. Wnt-1-inducible signaling pathway protein 3 and susceptibility to juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:3548–3553. doi: 10.1002/art.21392. [DOI] [PubMed] [Google Scholar]
- 201.Katsube K, Sakamoto K, Tamamura Y, Yamaguchi A. Role of CCN, a vertebrate specific gene family, in development. Dev. Growth Differ. 2009;51:55–67. doi: 10.1111/j.1440-169X.2009.01077.x. [DOI] [PubMed] [Google Scholar]
- 202.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 203.Duisters RF, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009;104:170–178. 6p. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]
- 204.Ohgawara T, et al. Regulation of chondrocytic phenotype by micro RNA 18a: involvement of Ccn2/Ctgf as a major target gene. FEBS Lett. 2009;583:1006–1010. doi: 10.1016/j.febslet.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 205.Johannes G, Carter MS, Eisen MB, Brown PO, Sarnow P. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13118–13123. doi: 10.1073/pnas.96.23.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mukudai Y, et al. A coding RNA segment that enhances the ribosomal recruitment of chicken ccn1 mRNA. J. Cell Biochem. 2010;111:1607–1618. doi: 10.1002/jcb.22894. [DOI] [PubMed] [Google Scholar]
- 207.Perbal B. Alternative splicing of CCN mRNAs …it has been upon us. J. Cell Commun. Signal. 2009;3:153–157. doi: 10.1007/s12079-009-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hirschfeld M, et al. Expression of tumor-promoting Cyr61 is regulated by hTRA2-{beta}1 and acidosis. Hum. Mol. Genet. 2011;20:2356–2365. doi: 10.1093/hmg/ddr128. [DOI] [PubMed] [Google Scholar]
- 209.Guillon-Munos A, et al. Kallikrein-related peptidase 12 hydrolyzes matricellular proteins of the CCN family and modifies interactions of CCN1 and CCN5 with growth factors. J. Biol Chem. 2011;286:25505–25518. doi: 10.1074/jbc.M110.213231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang W, et al. Impact of CCN3 (NOV) glycosylation on migration/invasion properties and cell growth of the choriocarcinoma cell line Jeg3. Hum. Reprod. 2011;26:2850–2860. doi: 10.1093/humrep/der239. [DOI] [PubMed] [Google Scholar]
- 211.Leng E, Malcolm T, Tai G, Estable M, Sadowski I. Organization and expression of the cyr61 gene in normal human fibroblasts. J Biomed. Sci. 2002;9:59–67. doi: 10.1007/BF02256579. [DOI] [PubMed] [Google Scholar]
- 212.Sabbah M, et al. CCN5, a novel transcriptional repressor of transforming growth factor-β signaling pathway. Mol. Cell Biol. 2011;31:1459–1469. doi: 10.1128/MCB.01316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hoshijima M, et al. CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin alpha5beta1. FEBS Lett. 2006;580:1376–1382. doi: 10.1016/j.febslet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 214.Nishida T, et al. CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J. Cell Physiol. 2003;196:265–275. doi: 10.1002/jcp.10277. [DOI] [PubMed] [Google Scholar]
- 215.Francischetti IM, Kotsyfakis M, Andersen JF, Lukszo J. Cyr61/CCN1 displays high-affinity binding to the somatomedin B(1–44) domain of vitronectin. PLoS. ONE. 2010;5:e9356. doi: 10.1371/journal.pone.0009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nishida T, et al. Effect of CCN2 on FGF2-Induced Proliferation and MMP9 and MMP13 Productions by Chondrocytes. Endocrinology. 2011 Sep 13; doi: 10.1210/en.2011-0234. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 217.Gellhaus A, et al. Connexin43 interacts with NOV: a possible mechanism for negative regulation of cell growth in choriocarcinoma cells. J Biol. Chem. 2004;279:36931–36942. doi: 10.1074/jbc.M404073200. [DOI] [PubMed] [Google Scholar]
- 218.Fu CT, Bechberger JF, Ozog MA, Perbal B, Naus CC. CCN3 (NOV) interacts with connexin43 in C6 glioma cells: Possible mechanism of connexin-mediated growth suppression. J Biol. Chem. 2004;279:36943–36950. doi: 10.1074/jbc.M403952200. [DOI] [PubMed] [Google Scholar]
- 219.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 220.Chang L, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]