Abstract
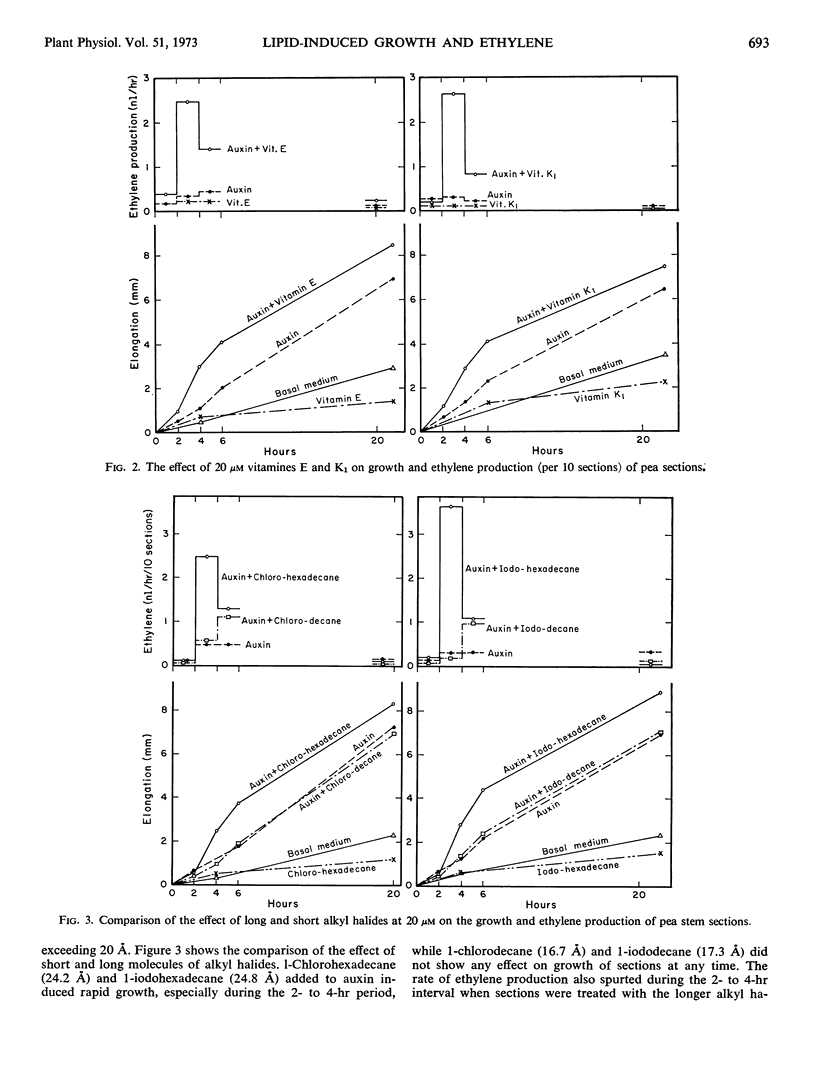
Lipids which are active oleanimins, i.e., those which stimulate respiration and auxin-induced cell elongation of pea stem sections, also initiate a period of ethylene formation in them after a lag period of at least 1 hour. Production of ethylene requires auxin, is inhibited by cycloheximide and dinitrophenol applied during or before the lag period, and is greatly stimulated by lipids longer than 20 Ångstroms in length such as heptadecyl-benzene, chloro- or iodohexadecane, triolein, and vitamins E and K1, but not by the shorter chloro- and iododecane. β-Stigmasterol at 10 to 40 μm concentrations depresses both oleanimin-induced growth and ethylene formation.
The effect of oleanimins on the growth rate steadily declines and disappears after 6 hours, whereas oleanimin induction of ethylene stays at a high level until it rapidly disappears after 6 hours. Nongrowing second internode sections also produce ethylene on oleanimin treatment, so ethylene formation is not dependent on cell elongation even though it requires auxin. Preincubation with heptadecylbenzene or auxin does not change the delay of an hour or more before ethylene is produced, whereas increases in growth are noted at the earliest measurements. Oleanimins stimulate growth at less than optimal auxin concentration, even as low as 20 nm, where a proportional ethylene formation is not noted. It is concluded that ethylene formation is not causally related to growth in these tissues.
The decline in oleanimin-induced ethylene formation is not changed by renewal of the incubation medium, and sucrose which is required to maintain growth for 20 hours does not influence growth or ethylene formation up to 6 hours. l-Methionine increases ethylene formed after heptadecylbenzene treatment, but unexpectedly, malonate is much more effective.
Auxin concentrations supraoptimal for growth cause no growth rate reductions for the first 10 hours, but they greatly enhance oleanimin-induced ethylene formation even when heptadecylbenzene is added after 6 hours. Applied ethylene even at concentrations much above those produced by the tissue itself fails to stimulate or inhibit short term pea stem section growth. It is concluded that the effect of oleanmins on growth is not mediated by ethylene. The similarities in concentration and molecular dimensions of these structurally diverse lipids which simultaneously stimulate respiration, growth, and ethylene formation, suggest a single site of action located in a regulatory membrane.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Auxin stimulation of ethylene evolution. Plant Physiol. 1966 Apr;41(4):585–588. doi: 10.1104/pp.41.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae W. A., Venis M. A., Jursic F., Dumas T. Does ethylene mediate root growth inhibition by indole-3-acetic Acid? Plant Physiol. 1968 Sep;43(9):1375–1379. doi: 10.1104/pp.43.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURG S. P., BURG E. A. ETHYLENE ACTION AND THE RIPENING OF FRUITS. Science. 1965 May 28;148(3674):1190–1196. doi: 10.1126/science.148.3674.1190. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Ethylene formation in pea seedlings; its relation to the inhibition of bud growth caused by indole-3-acetic Acid. Plant Physiol. 1968 Jul;43(7):1069–1074. doi: 10.1104/pp.43.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci U S A. 1966 Feb;55(2):262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Clagett C. O. Conversion of methionine to ethylene in vegetative tissue and fruits. Biochem Biophys Res Commun. 1967 Apr 20;27(2):125–130. doi: 10.1016/s0006-291x(67)80050-0. [DOI] [PubMed] [Google Scholar]
- Chadwick A. V., Burg S. P. Regulation of root growth by auxin-ethylene interaction. Plant Physiol. 1970 Feb;45(2):192–200. doi: 10.1104/pp.45.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Lieberman M. Effects of Kinetin, IAA, and Gibberellin on Ethylene Production, and Their Interactions in Growth of Seedlings. Plant Physiol. 1968 Dec;43(12):2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L. J., Goodwin T. W. The biosynthesis of sterols in higher plants. Biochem J. 1966 Jun;99(3):735–746. doi: 10.1042/bj0990735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl J. D., Pratt H. K., Bonner B. A. An effect of light on the production of ethylene and the growth of the plumular portion of etiolated pea seedlings. Plant Physiol. 1967 Aug;42(8):1077–1080. doi: 10.1104/pp.42.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaseki H. Induction of peroxidase activity by ethylene in sweet potato. Plant Physiol. 1970 Jul;46(1):172–174. doi: 10.1104/pp.46.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Newcomb W., Burg S. P. Mechanism of Auxin-induced Ethylene Production. Plant Physiol. 1971 Apr;47(4):504–509. doi: 10.1104/pp.47.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku H. S., Pratt H. K. Active mitochondria do not produce ethylene. Plant Physiol. 1968 Jun;43(6):999–1001. doi: 10.1104/pp.43.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku H. S., Yang S. F., Pratt H. K. Enzymic evolution of ethylene from methional by a pea seedling extract. Arch Biochem Biophys. 1967 Mar 20;118(3):756–758. doi: 10.1016/0003-9861(67)90413-4. [DOI] [PubMed] [Google Scholar]
- Larue T. A., Gamborg O. L. Ethylene Production by Plant Cell Cultures: Variations in Production during Growing Cycle and in Different Plant Species. Plant Physiol. 1971 Oct;48(4):394–398. doi: 10.1104/pp.48.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marei N., Crane J. C. Growth and Respiratory Response of Fig (Ficus carica L. cv. Mission) Fruits to Ethylene. Plant Physiol. 1971 Sep;48(3):249–254. doi: 10.1104/pp.48.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxie E. C., Eaks I. L., Sommer N. F., Rae H. L., El-Batal S. Effect of Gamma Radiation on Rate of Ethylene and Carbon Dioxide Evolution by Lemon Fruit. Plant Physiol. 1965 May;40(3):407–409. doi: 10.1104/pp.40.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlasson W. B., Pratt H. K. Effects of Wounding on Respiration and Ethylene Production by Cantaloupe Fruit Tissue. Plant Physiol. 1964 Jan;39(1):128–132. doi: 10.1104/pp.39.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny D., Stowe B. B. Relationship between growth and respiration induced by lipids in pea stem sections. Plant Physiol. 1965 Nov;40(6):1140–1145. doi: 10.1104/pp.40.6.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny D., Stowe B. B. Relationship of lipid metabolism to the respiration and growth of pea stem sections. Plant Physiol. 1966 Feb;41(2):360–365. doi: 10.1104/pp.41.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Pratt H. K. Effects of ethylene on potato tuber respiration. Plant Physiol. 1972 Feb;49(2):252–255. doi: 10.1104/pp.49.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe B. B., Dotts M. A. Probing a membrane matrix regulating hormone action: I. The molecular length of effective lipids. Plant Physiol. 1971 Nov;48(5):559–565. doi: 10.1104/pp.48.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe B. B. Growth Promotion in Pea Stem Sections. I. Stimulation of Auxin and Gibberellin Action by Alkyl Lipids. Plant Physiol. 1960 Mar;35(2):262–269. doi: 10.1104/pp.35.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe B. B., Obreiter J. B. Growth Promotion in Pea Stem Sections. II. By Natural Oils & Isoprenoid Vitamins. Plant Physiol. 1962 Mar;37(2):158–164. doi: 10.1104/pp.37.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]


