Abstract
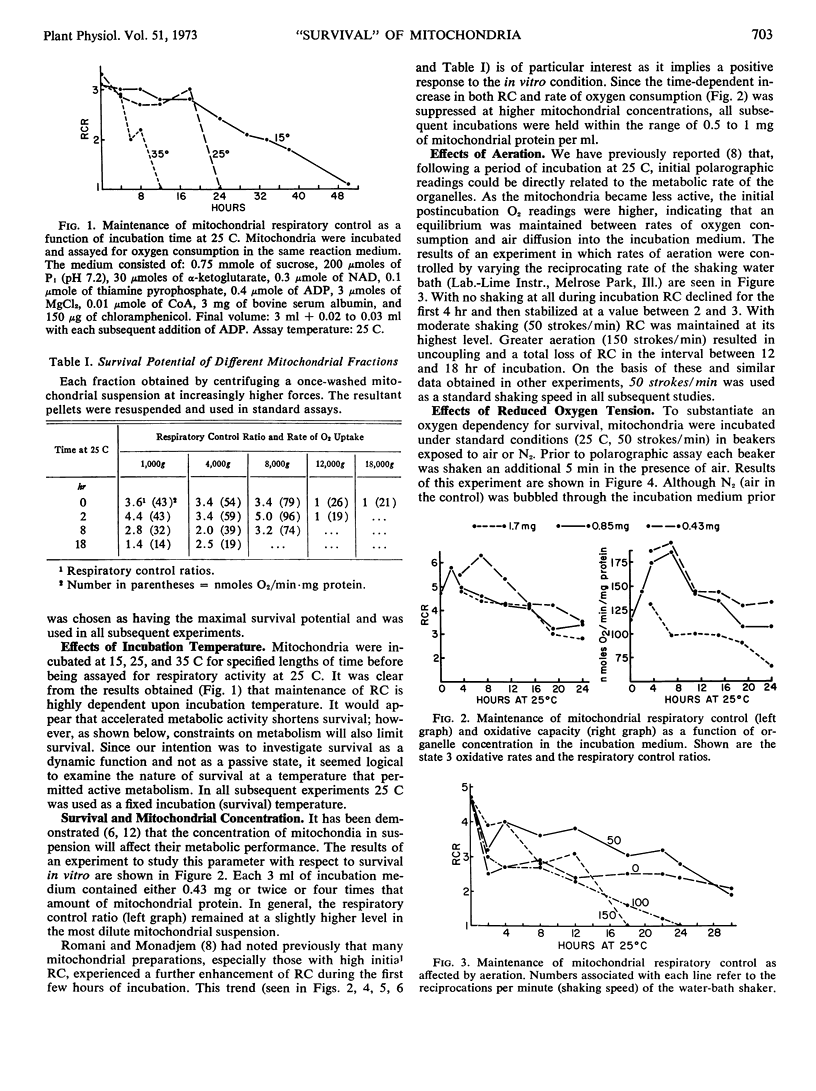
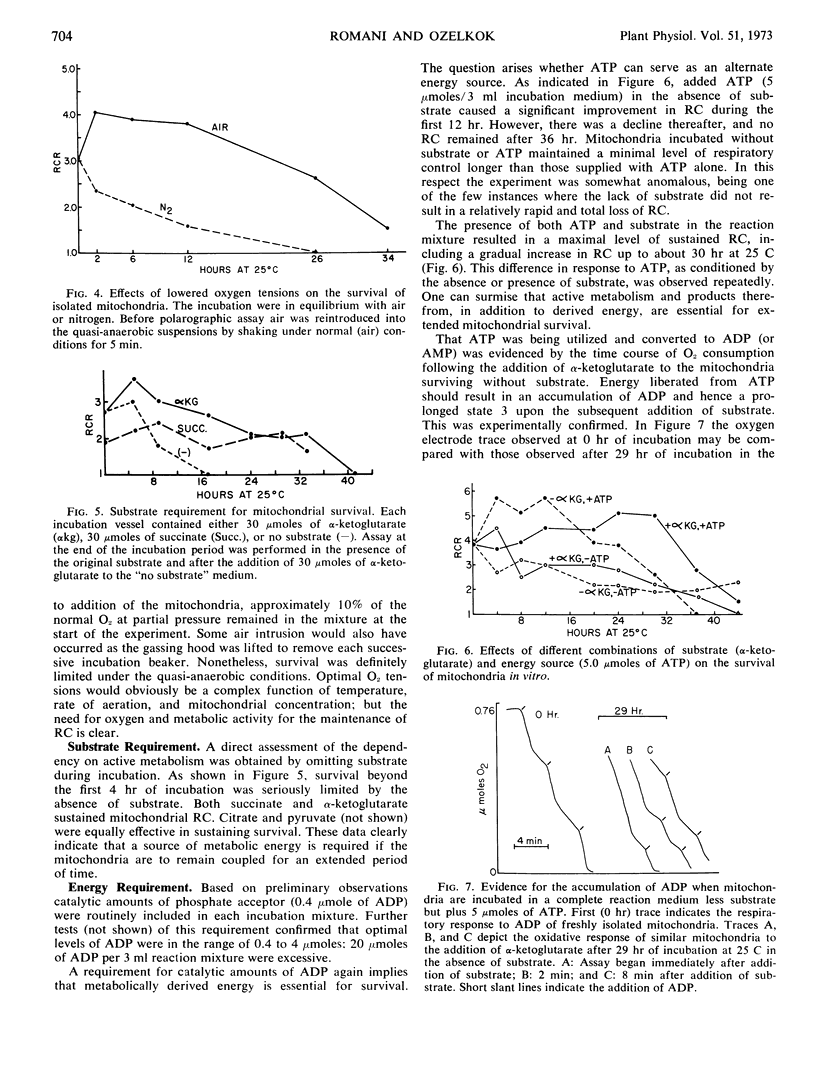
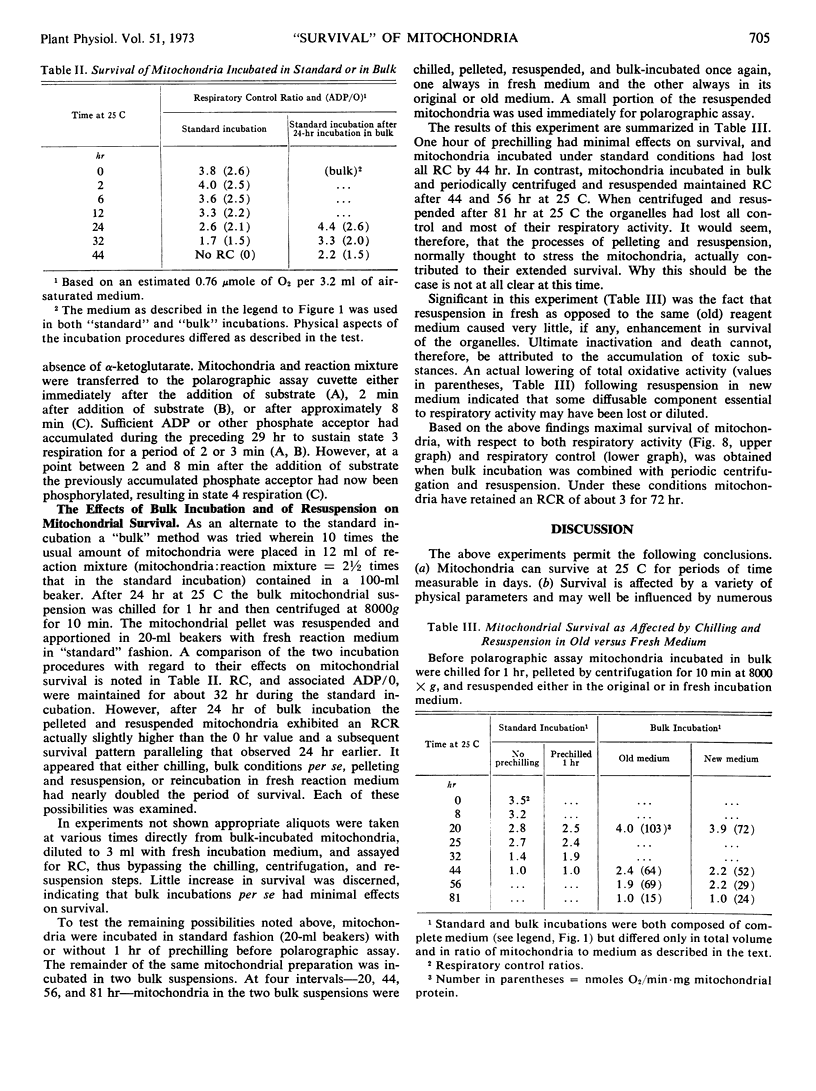
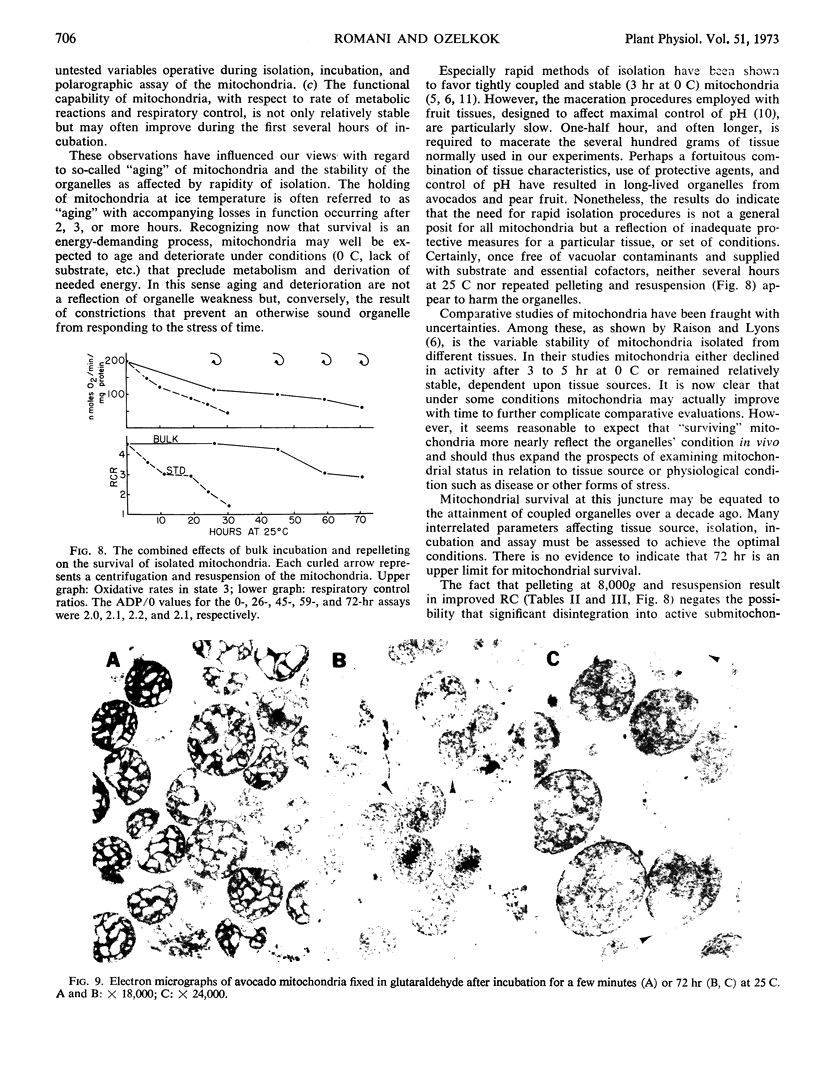
Isolated mitochondria have been maintained active and coupled for 72 hours at 25 C. Survival (retention of respiratory control) is a function of incubation temperature and dependent upon aeration and substrate. ATP does not entirely substitute for substrate, indicating a need for products of active metabolism other than energy. An improvement in respiratory control is often observed during the first several hours of incubation. Sedimentation and resuspension at 24-hour intervals prolonged survival. As revealed by electron microscopy, mitochondria maintained their basic structure during a 72-hour period at 25 C.
Survival is a dynamic, energy-requiring process and must be distinguished from so-called “aging” of organelles at ice temperatures. As a manifestation of partial autonomy, survival may prove useful in assessing aspects of mitochondrial function and the mitochondrial-cellular interrelationship.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- Giles K. L., Sarafis V. Chloroplast survival and division in vitro. Nat New Biol. 1972 Mar 15;236(63):56–58. doi: 10.1038/newbio236056a0. [DOI] [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. VII. Oxidative and phosphorylative activities throughout the climacteric cycle. Plant Physiol. 1965 Nov;40(6):1116–1123. doi: 10.1104/pp.40.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M. The influence of mitochondrial concentration and storage on the respiratory control of isolated plant mitochondria. Plant Physiol. 1970 Apr;45(4):382–385. doi: 10.1104/pp.45.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. M., Leech R. M. Division of chloroplasts in an artificial environment. Nature. 1970 Aug 1;227(5257):463–465. doi: 10.1038/227463a0. [DOI] [PubMed] [Google Scholar]
- Riesz P., White F. H., Jr Determination of free radicals in gamma irradiated proteins. Nature. 1967 Dec 23;216(5121):1208–1210. doi: 10.1038/2161208b0. [DOI] [PubMed] [Google Scholar]
- Romani R. J., Yu I. K., Fisher L. K. Isolation of tightly coupled mitochondria from acidic plant tissues. Plant Physiol. 1969 Feb;44(2):311–312. doi: 10.1104/pp.44.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R., Monadjem A. Mitochondrial longevity in vitro: the retention of respiratory control. Proc Natl Acad Sci U S A. 1970 Jul;66(3):869–873. doi: 10.1073/pnas.66.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkissian I. V., Srivastava H. K. On methods of isolation of active, tightly coupled mitochondria of wheat seedlings. Plant Physiol. 1968 Sep;43(9):1406–1410. doi: 10.1104/pp.43.9.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarjan E. M., Von Korff R. W. Factors affecting the respiratory control ratio of rabbit heart mitochondria. J Biol Chem. 1967 Jan 25;242(2):318–324. [PubMed] [Google Scholar]
- Wilson S. B., Bonner W. D. Preparation and some properties of submitochondrial particles from tightly coupled mung bean mitochondria. Plant Physiol. 1970 Jul;46(1):25–30. doi: 10.1104/pp.46.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]



