Background: MAP3K8 (Cot/tpl2) activates MKK1/2-Erk1/2 upon stimulation of receptors from the Toll-like/interleukin-1 receptor superfamily.
Results: Cot/tpl2 plays an essential role in acetaminophen-induced liver injury by modulating the generation of inflammatory signals induced by necrotic cells.
Conclusion: Sterile inflammatory processes triggered by tissue damage are modulated by Cot/tpl2.
Significance: Cot/tpl2 contributes to the development of pathologies associated with inflammation triggered by damage-associated molecular patterns.
Keywords: Cytokine, Drug Action, ERK, Hepatocyte, Inflammation, Leukocyte, Liver Injury, Macrophages, Necrosis (Necrotic Death)
Abstract
Cot/tpl2 (MAP3K8) activates MKK1/2-Erk1/2 following stimulation of the Toll-like/IL-1 receptor superfamily. Here, we investigated the role of Cot/tpl2 in sterile inflammation and drug-induced liver toxicity. Cot/tpl2 KO mice exhibited reduced hepatic injury after acetaminophen challenge, as evidenced by decreased serum levels of both alanine and aspartate aminotransferases, decreased hepatic necrosis, and increased survival relative to Wt mice. Serum levels of both alanine and aspartate aminotransferases were also lower after intraperitoneal injection of acetaminophen in mice expressing an inactive form of Cot/tpl2 compared with Wt mice, suggesting that Cot/tpl2 activity contributes to acetaminophen-induced liver injury. Furthermore, Cot/tpl2 deficiency reduced neutrophil and macrophage infiltration in the liver of mice treated with acetaminophen, as well as their hepatic and systemic levels of IL-1α. Intraperitoneal injection of damage-associated molecular patterns from necrotic hepatocytes also impaired the recruitment of leukocytes and decreased the levels of several cytokines in the peritoneal cavity in Cot/tpl2 KO mice compared with Wt counterparts. Moreover, similar activation profiles of intracellular pathways were observed in Wt macrophages stimulated with Wt or Cot/tpl2 KO damage-associated molecular patterns. However, upon stimulation with damage-associated molecular patterns, the activation of Erk1/2 and JNK was deficient in Cot/tpl2 KO macrophages compared with their Wt counterparts; an effect accompanied by weaker release of several cytokines, including IL-1α, an important component in the development of sterile inflammation. Taken together, these findings indicate that Cot/tpl2 contributes to acetaminophen-induced liver injury, providing some insight into the underlying molecular mechanisms.
Introduction
The liver is a crucial metabolic organ and is highly susceptible to drug toxicity. Acetaminophen (APAP)4 is one of the best-selling analgesics and antipyretics on the market in the United States and Western Europe, although it also accounts for ∼50% of all cases of acute liver failure (1–3). APAP overdose generates N-acetyl-p-benzoquinone imine, which depletes hepatic glutathione and promotes oxidative stress, leading finally to hepatocyte necrosis (reviewed in Refs. 4, 5). Necrosis occurs when cells die rapidly in response to acute injury, ultimately provoking the release of intracellular constituents into the surrounding milieu (6). Some of these components released belong to a group of molecules known as damage-associated molecular patterns (DAMPs). Outside of their physiological environment, these molecules behave as “danger sensors” and are capable of triggering an inflammatory response in a similar way as previously described for the pathogen-associated molecular patterns (PAMPs) (reviewed in Refs. 7, 8). Similar to pathogen-induced inflammation, sterile inflammation initiated by necrotic cells alerts the host tissue to host damage by activating the innate immune system (9, 10). Initially, this process is manifested by the production of pro-inflammatory mediators and triggering the recruitment of leukocytes to the damaged tissue (7, 11–13). However, when excessive, this inflammatory response can contribute to severe organ damage and dysfunction (7, 11, 14). DAMPs include a wide variety of molecules, and the number of known DAMPs is growing continually (7, 15–18).
DAMPs activate a variety of receptor types in different cells, among which are the members of the pattern-recognition receptor (PRR) family, including the receptors of the Toll-like receptor (TLR) family (7, 10, 16, 18, 19). Upon activation, all TLRs (except TLR3) recruit the MyD88 adaptor protein, which mediates activation of the p38α and JNK MAP pathways, and the canonical IKKs, IKKα, and IKKβ (20, 21). Activated IKKβ phosphorylates p105 NFκB, marking it for partial proteolysis. In resting cells, Cot/tpl2 (MAP3K8) forms an inactive complex with p105 NFκB and ABIN2, from which Cot/tpl2 is released following proteolysis of p105 NFκB (reviewed in Refs. 22, 23). The dissociated and activated Cot/tpl2 stimulates MKK1/2 and consequently Erk1/2 (24–26), and it is subsequently rapidly degraded through the proteasome pathway (27, 28). Cot/tpl2 is the only MAP3K to activate the Erk1/2 pathway in response to both TLR activation and IL-1 or TNFα stimulation (29, 30) in different cell types, including macrophages, epithelial, and stellate cells (25, 29, 31). Moreover, Cot/tpl2 can also activate the MAP kinases JNK and p38α in certain conditions, in a cell type- and stimulus-specific manner (22, 30). Thus, Cot/tpl2 fulfills a role in TLR, IL-1, and TNFα intracellular signaling that cannot be substituted by any other protein (22). Accordingly, Cot/tpl2 represents an interesting anti-inflammatory target, given that it does not modulate Erk1/2 phosphorylation via the activation of one or more Raf isoforms (32, 33). Cot/tpl2 contributes to generation of inflammatory nociception and neutrophil recruitment in mice hindpaw upon zymosan injection (34), promotes the Crohn-like inflammatory bowel disease (35, 36), and plays a role in the development of acute pancreatitis (37). However, the double APC KO Cot/tpl2 KO mice show increased deficiency intestinal inflammation and tumorigenesis compared with APC KO Cot/tpl2 Wt and Cot/tpl2 deficiency also increases the ovalbumin-induced airway allergic response (38).
Here, we show that Cot/tpl2 mediates, both in vitro and in vivo, DAMP-induced IL-1α production. Cot/tpl2 participates in the recruitment of leukocytes to damaged tissue in mice injected intraperitoneally with DAMPs or with a toxic dose of APAP. Furthermore, APAP-induced liver injury is markedly reduced in Cot/tpl2-deficient mice. Taken together, these data indicate that Cot/tpl2 contributes to drug-induced toxicity associated with cell necrosis.
EXPERIMENTAL PROCEDURES
Cells and Stimuli
Immortalized Wt and Cot/tpl2 KO hepatocytes were generated from pools of 4–6 livers obtained from Wt or Cot/tpl2 KO neonatal mice (3.5–4-day-old) that were digested with collagenase and then cultured. The cells were subsequently immortalized with the SV40 Large T antigen, as described previously (39). Hepatocytes were incubated for 18 h with APAP (Sigma-Aldrich) and/or TNFα (Peprotech), and their viability was then assayed using the MTT kit (Roche), according to the manufacturer's instructions. DAMPs were generated from Wt and Cot/tpl2 KO hepatocytes as described previously (12, 13) with minor modifications: prior to 1-h heatshock at 60 °C, the hepatocytes were washed five times with PBS and resuspended in DMEM-HEPES. Bone marrow-derived macrophages (BMDM) or peritoneal macrophages were generated as described previously (26) and then stimulated with DAMPs (300 μg/ml), LPS (300 ng/ml, Sigma-Aldrich), or PMA (10 μm, Sigma-Aldrich). When necessary, the IKKβ inhibitor B1605906 (10 μm), the JNK inhibitor SP 600125 (12 μm), or the Erk1/2 inhibitor PD 0325901 (0.5 μm), gifts from Sir Philip Cohen (Dundee), were added prior to stimulation.
Animals and Animal Treatments
C57BL/6 Wt, C57BL/6 KO, and C57BL/6 Cot/tpl2 KD littermates were generated from the crossing of heterozygous mice (34), and they were used for experiments at 10–12 weeks of age. All animals were handled in accordance with institutional guidelines for the care and use of laboratory animals in research. The mice were fasted overnight before treatment, and the APAP solution administered (10 mg/ml) was prepared freshly in warmed (55 °C) PBS that was cooled to 35 °C before it was injected. For survival studies, 600 g/kg APAP (intraperitoneal) was administered to mice. A comprehensive laboratory animal monitoring system (TSE) was used to analyze metabolic behavior 4 h after injection, measuring O2 consumption and CO2 production every 10 min for 48 h. The time of death was determined as the point at which the respiratory exchange ratio (RER; O2/CO2) became zero or negative. To evaluate hepatotoxicity the animals were administered intraperitoneally (i.p.) either with PBS (control) or with APAP (450 g/kg), food was withheld for 4 h, and at the times indicated, the mice were sacrificed. Liver tissue was taken from each mouse, immediately ground into small fragments, frozen in liquid nitrogen, and stored at −80 °C. Blood was collected, and the serum was stored at −80 °C until use. The ALT, AST, and lactate dehydrogenase (LDH) levels were determined (at the UCM, Madrid), and cytokine levels were assessed by Luminex analysis (at the CNB, Madrid). Peritoneal leukocyte recruitment in mice was measured 18 h after injection of liver homogenate (32 mg, intraperitoneal) or of DAMPs generated from hepatocytes (1.7 mg, 35 × 106 cells). Peritoneal cells were collected as described previously (12, 13, 34). Hepatic non-parenchymal cells were isolated as described previously (40). To analyze liver damage, tissue samples were rinsed with saline and fixed by immersion in 4% formalin for 24 h and treated with hematoxylin and eosin (H&E) staining as described previously (34). The analysis of the necrotic areas as well as the quantification (Image J) of the liver areas with congestive changes occupied by erythrocytes (including the intravascular and hemorrhagic areas) were performed in seven randomly selected microscopic fields from each sample using a ×10 objective.
Flow Cytometry
Cells (0.3–0.5 × 106 cells/test) were incubated with CD16/32 (2.4G2, Cultek) for 20 min at room temperature, and they were subsequently stained for 1 h at 4 °C in the dark with the following antibodies (5 μg/ml): CD3-PECy7 (Hamster IgG, eBioscience), CD11a-PECy7 (rat IgG2ak, Pharmingen), CD11b-(Mac1)-PECy7 (rat IgG2bk anti-mouse, eBioscience), CD45-FITC (rat IgG, Beckman), F4/80-APC (rat IgG2ak, eBioscience), Ly6G-PE (rat IgG2ak, Pharmingen), and NK1.1-APC (mice IgG2ak anti-mouse, Pharmingen), or their corresponding isotype controls (Pharmingen, eBioscience). After three washes, Perfect-Count microspheres (Cytognos) were added to quantify the exact number of cells. Flow cytometry analysis was performed using the CXP program. To determine the oxidative burst of peritoneal leukocytes, 3 × 105 cells were resuspended in PBS, incubated in the dark with 10 μm 2′,7′-dichlorofluorescin diacetate (Invitrogen), and subsequently incubated in the presence or absence of 10 μm PMA for 15 min at 37 °C. After several washes, the cells were analyzed by flow cytometry.
Western Blot and RT-PCR Analysis
Homogenized liver extracts and cell extracts were analyzed in Western blots (41), probed with primary antibodies raised against the following proteins: Cot/tpl2, Erk2, p38α, and p52 JNK2 (Santa Cruz Biotechnology); P-S933 p105 NFκB, P-T202/Y204 Erk1/2, and P-T180/Y182 p38α (Cell Signaling); and P-T183/Y185 p48/p52 JNK (Invitrogen). Secondary antibodies raised against rabbit (Cell Signaling), goat (DAKO), and mouse (Amersham Biosciences) were used to detect the primary antibodies. RNA extraction and RT-PCR analysis were performed as described previously (42). The specific TAQMAN primers (Applied Biosystems) IL-1α, IL-1β, IL-6, IL-10, TNFα, and β-actin were used.
Statistical Analysis
Data are presented as the mean ± S.D., and they were analyzed using the Student's t test. Values were considered statistically significant at p < 0.05: *, p < 0.05, **, p < 0.01, ***, p < 0.001.
RESULTS
Cot/tpl2 Participates in APAP-induced Liver Damage
Following APAP administration (600 g/kg, intraperitoneal), 12% survival was observed in Wt mice in the 15 to 30 h following injection, whereas all the Cot/tpl2 KO mice survived for the duration of the experiment (Fig. 1A). The serum levels of ALT, AST, and LDH were all lower in APAP-treated (450 g/kg, intraperitoneal) Cot/tpl2 KO mice than in their Wt counterparts (Fig. 1B and supplemental Fig. S1). Increased hepatic P-JNK levels, as a marker of liver damage, were observed 6 h after APAP-induced liver injury (4, 43), and Cot/tpl2 deficiency decreased JNK phosphorylation in APAP-injected mice (supplemental Fig. S1). Moreover, the liver of Cot/tpl2 KO mice revealed a marked histological attenuation of liver injury compared with the severe centrilobular necrosis and hemorrhage observed in Wt animals (Fig. 1C), indicating that Cot/tpl2 deficiency protected against APAP-induced hepatotoxicity. In Cot/tpl2 KD mice that express an inactive version of Cot/tpl2 (42), the levels of AST and ALT after APAP treatment were also diminished compared with those in Wt mice (Fig. 1D). These data indicate that APAP-induced liver injury is modulated by Cot/tpl2 activity rather than by modifications in the Cot/tpl2-ABIN2-p105 NFκB complex, because of Cot/tpl2 knockdown.
FIGURE 1.
APAP-induced liver injury in Wt and Cot/tpl2-deficient mice. A, survival of Wt (n = 8) and Cot/tpl2 KO mice (n = 8) after APAP administration (600 g/kg, intraperitoneal). B, Cot/tpl2 KO mice were injected with APAP (450 g/kg, intraperitoneal), and serum ALT and AST levels were measured at various times. Data represent the mean ± S.D. from four experiments performed in quadruplicate. C, Wt and Cot/tpl2 KO mice were injected (450 g/kg, intraperitoneal) with APAP or with PBS for 24 h. H&E staining of liver sections. Images from PBS-injected mice are representative of three mice, and those from mice that received APAP are representative of seven animals from which 7 randomly selected fields were analyzed. Scale bars, 100 μm. Graph shows the mean ± S.E. from the % congestive area analyzed in 49 different fields obtained from 7 APAP-treated Wt, 7 APAP-treated Cot/tpl2 KO mice, and from 3 WT PBS-injected and 3 Cot/tpl2 KO PBS-injected mice. D, Wt and Cot/tpl2 KD mice were injected with APAP (450 g/kg, intraperitoneal), serum ALT and AST levels were determined 18 h later. Graphs represent the mean ± S.D. of three independent experiments performed in quadruplicate.
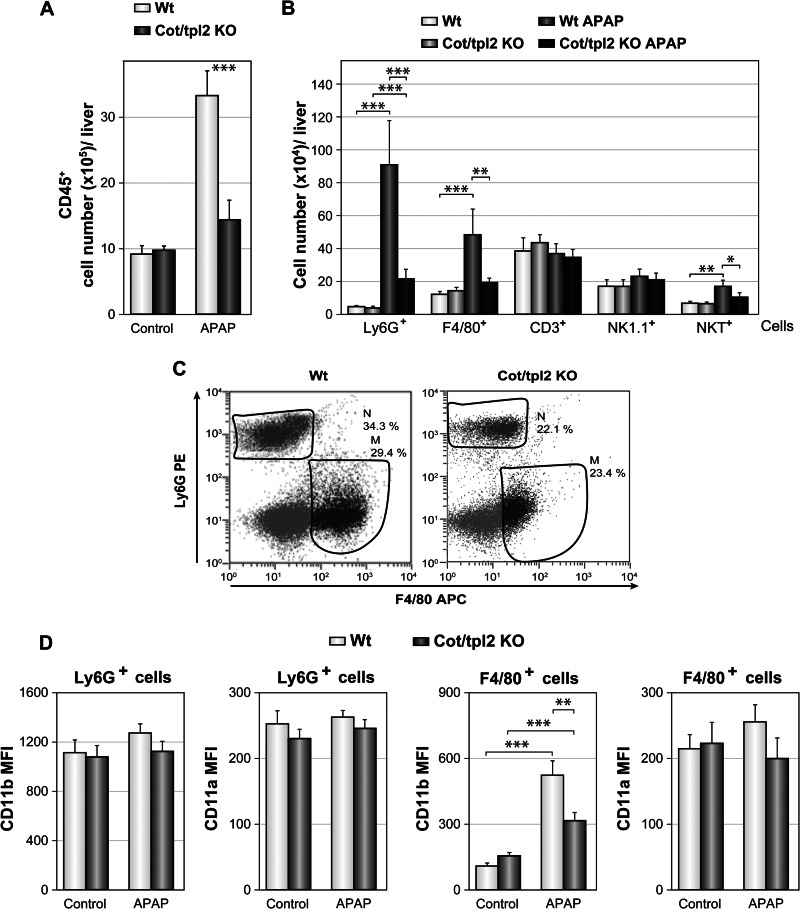
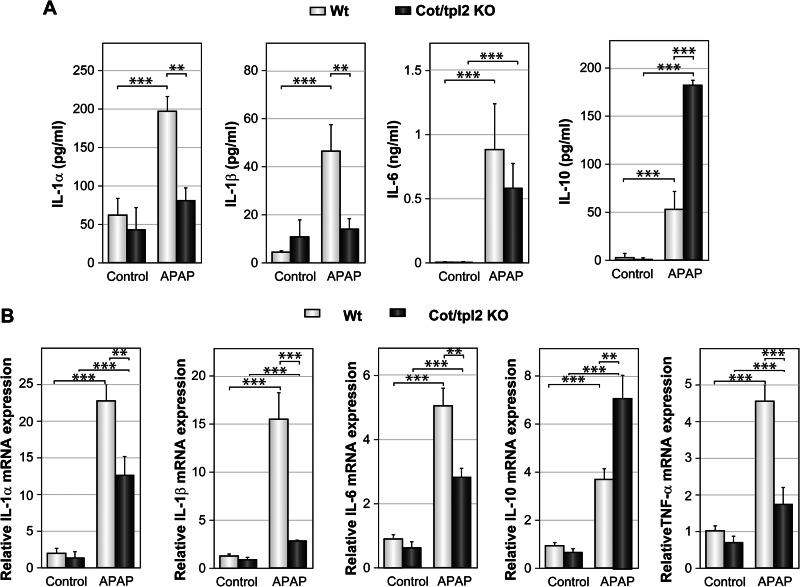
APAP-induced liver injury triggers the recruitment of leukocytes to the liver (8, 44, 45). Upon intraperitoneal injection of PBS, similar numbers of hepatic leukocytes were detected in Wt and Cot/tpl2 KO mice; yet APAP injection produced a 3-fold increase in the number of hepatic leukocytes (CD45+) in Wt mice, whereas only a 1.5-fold increase was observed in APAP-treated Cot/tpl2 KO mice (Fig. 2A). Analysis of the leukocytes recruited revealed that Cot/tpl2 deficiency mainly decreased the number of infiltrated neutrophils (Ly6G+) and macrophages (F4/80+: Fig. 2, B and C). In the neutrophils, neither Cot/tpl2 deficiency nor APAP treatment altered the mean fluorescence intensity (MFI) of the CD11a and CD11b activation markers. However, the macrophages recruited to the liver following APAP administration had a higher CD11b MFI (44), an effect that was less pronounced in Cot/tpl2 KO macrophages (Fig. 2D). Following APAP challenge, Cot/tpl2 also triggered the recruitment of NKT (CD3+NK1.1+) cells to the liver (Fig. 2B). Moreover, the serum IL-1α, IL-1β, and IL-6 levels were lower in APAP-treated Cot/tpl2 KO mice than in Wt mice, while Cot/tpl2 deficiency promoted an increase in IL-10 levels (Fig. 3A). Similarly, 6 h upon APAP challenge, the liver of Cot/tpl2 KO mice showed decreased mRNA levels of IL-1α, IL-1β, IL-6, and TNFα, but higher IL-10 levels compared with Wt-treated mice (Fig. 3B).
FIGURE 2.
Leukocyte recruitment in the liver of Wt and Cot/tpl2 KO mice following APAP injection. A, mice were injected intraperitoneally with PBS (Control) or APAP (450 g/kg), and CD45+ liver cells were isolated 18 h later and analyzed by flow cytometry. B, analysis of Ly6G+, F4/80+, CD3+ (CD3+NK1.1−), NK1.1+ (NK1.1+CD3−), and NKT (CD3+NK1.1+) cells from the CD45+-gated cells in A. C, representative Ly6G+ versus F4/80+ staining FACS profile from the CD45+-gated cells obtained in A. D, CD11b and CD11a MFI of F4/80+ and Ly6G+ cells isolated from the livers of Wt and Cot/tpl2 KO mice 18 h after intraperitoneal injection with PBS (Control) or APAP (450 mg/kg). Graphs represent the mean ± S.D. of one experiment performed in quadruplicate and similar data were obtained in three additional experiments. Data in A and B represent the mean ± S.D. of four independent experiments performed in quadruplicate.
FIGURE 3.
Cot/tpl2 modulates cytokine production associated with APAP-induced liver injury. Wt and Cot/tpl2 KO mice were challenged with APAP (450 g/kg, intraperitoneal), and cytokines levels were determined. A, serum levels of il-1α, IL-1β, IL-6, and IL-10 were determined 18 h later using a Luminex assay. TNFα could not be detected. B, IL-1α, IL-1β, IL-6, IL-10, and TNFα mRNA expression levels upon 6 h of APAP challenge were determined by RT-PCR analysis. A and B, data represent the mean ± S.D. of four independent experiments performed in quadruplicate.
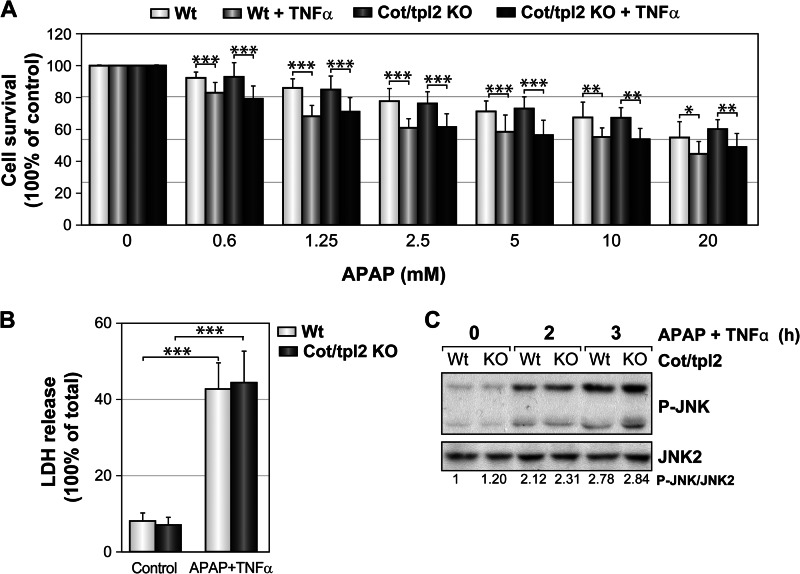
To determine whether Cot/tpl2 also modulates APAP-induced hepatotoxicity in isolated hepatocytes, immortalized Wt and Cot/tpl2 KO hepatocytes were incubated with different concentrations of APAP for 18 h. Similar survival rates were observed for both Wt and Cot/tpl2 KO cells in the presence of APAP (supplemental Fig. S2), and although the presence of TNFα along with APAP further increased cell toxicity, Cot/tpl2 did not appear to modulate hepatocyte cell death in either of these conditions (Fig. 4A). Accordingly, similar LDH levels, as a consequence of cell necrosis, were detected in the supernatant of both Wt and Cot/tpl2 KO hepatocytes following incubation for 18 h with APAP plus TNFα (Fig. 4B). Furthermore, in APAP plus TNFα-treated hepatocytes Cot/tpl2 deficiency did not modulate the activity of JNK (Fig. 4C).
FIGURE 4.
Toxicity induced by APAP plus TNFα in Wt and Cot/tpl2 KO hepatocytes. A, Wt and Cot/tpl2 KO hepatocytes were incubated with 10 ng/ml of TNFα in combination with different concentrations of APAP, and their viability was assessed 18 h later through a MTT assay. The value obtained for Wt and Cot/tpl2 KO hepatocytes incubated in the absence of APAP was considered as 100%. B, LDH was determined in the cell supernatant of Wt and Cot/tpl2 KO hepatocytes incubated with 10 ng/ml of TNFα plus 20 mm of APAP for 18 h. The 100% value is given to the one obtained after Triton X-100 permeabilization. C, hepatocytes were incubated with 20 mm APAP plus 20 ng/ml TNFα for 0, 2, and 3 h, and P-JNK and JNK2 levels were measured by Western blot. The relative relation of P-JNK levels/JNK2 levels was determined by densitometric quantification of the radiographs, given the value of 1 to the one obtained with Wt hepatocytes at time 0. Similar data have been obtained in three independent experiments. A and B, graphs show the mean ± S.D. of three independent experiments performed in triplicate.
Involvement of Cot/tpl2 in Macrophage Activation by DAMPs
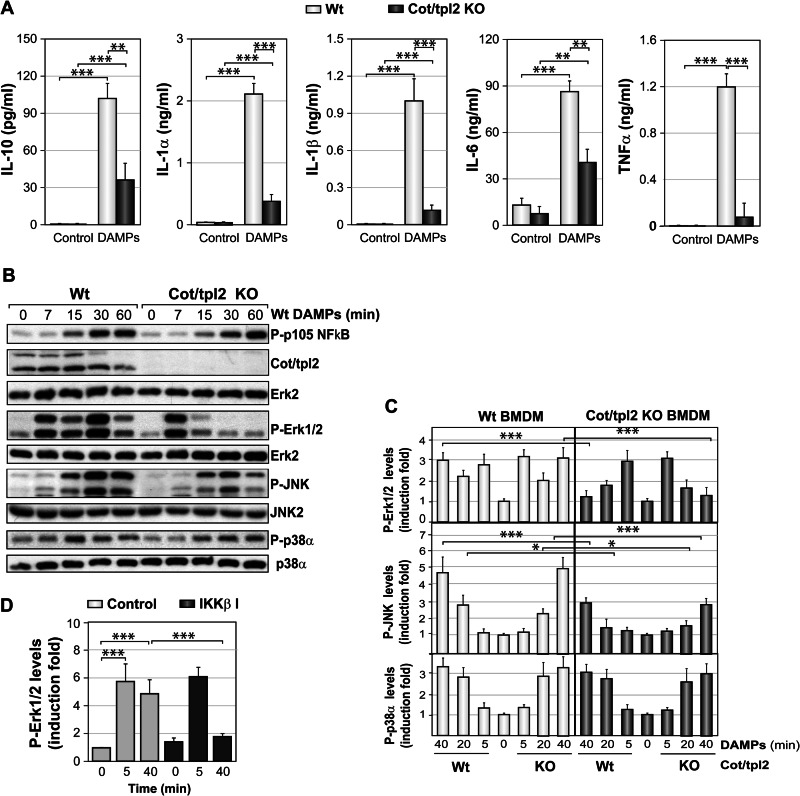
We evaluated the role of Cot/tpl2 in the activation of thioglycolate-elicited peritoneal macrophages by DAMPs obtained from Wt necrotic hepatocytes. After DAMPs stimulation, a decrease in IL-10, IL-1α, IL-1β, IL-6, and TNFα was detected in the supernatant of Cot/tpl2 KO with respect to Wt peritoneal macrophages (Fig. 5A). When the activation state of different intracellular pathways was analyzed in thioglycolate-elicited peritoneal macrophages stimulated with DAMPs, p105 NFκB phosphorylation, an upstream effector of Cot/tpl2, was similarly increased in both Wt and Cot/tpl2 KO macrophages (Fig. 5B). Moreover, in Wt macrophages, we observed the degradation of both the high and low molecular weight forms of Cot/tpl2, suggesting that it had dissociated from the p105 NFκB-ABIN2-Cot/tpl2 complex (26). Stimulation of both Wt and Cot/tpl2 KO macrophages resulted in an increase in Erk1/2 phosphorylation over 7 min that decreased rapidly in Cot/tpl2-deficient cells. In Wt peritoneal macrophages but not in Cot/tpl2 KO cells, a second increase of P-Erk1/2 levels was observed 30 min after stimulation (Fig. 5B). This early Erk1/2 activation peak following macrophage stimulation with DAMPs was not due to a possible FBS contamination contained in the DAMPs (supplemental Fig. S3). Activation of different TLRs in Wt and Cot/tpl2 KO peritoneal macrophages, as described previously in BMDM (46), triggered the phosphorylation of p105 NFκB by IKKβ, whereas Erk1/2 phosphorylation was observed only in Wt macrophages, evident as a single activation peak (supplemental Fig. S4). Cot/tpl2 deficiency decreased JNK activation, but not p38α, in peritoneal macrophages stimulated with Wt DAMPs (Fig. 5B). The loss of Cot/tpl2 expression in Cot/tpl2 KO hepatocytes did not affect the capacity of the obtained DAMPs to phosphorylate p105 NFκB or the different MAP kinases, as observed when BMDM were stimulated with Wt or Cot/tpl2 KO DAMPs (supplemental Fig. S5). As detected in peritoneal macrophages (Fig. 5B), the deficient expression of Cot/tpl2 in BMDM abolished the 30–40 min Erk1/2 activation and decreased JNK phosphorylation following the stimulation with Wt or Cot/tpl2 KO DAMPs (Fig. 5C and supplemental Fig. S5). Furthermore, in Wt BMDM stimulated with DAMPs, the IKKβ inhibitor B1605906 blocked the activation of Erk1/2 at 40 min but not that at 5 min (Fig. 5D). We previously demonstrated that Erk1/2 activation by LPS is entirely dependent on Cot/tpl2, whereas Erk1/2 activation by PMA is Cot/tpl2-independent (24). Indeed, IKKβ inhibition blocked LPS- but not PMA-induced Erk1/2 activation (supplemental Fig. S6). Together, these data indicate that two distinct intracellular signal pathways regulate Erk1/2 activation upon DAMPs stimulation of macrophages, one IKKβ-Cot/tpl2-dependent and another IKKβ-Cot/tpl2-independent. Furthermore, taking into account that Cot/tpl2 deficiency impaired both Erk1/2 and JNK phosphorylation in DAMPs-stimulated macrophages, we next decided to investigate the role of both Erk1/2 and JNK MAP kinases in the production of different cytokines. To this end, Wt peritoneal macrophages were stimulated for 18 h with DAMPs in the presence of the JNK inhibitor SP 600125 or in the presence of the Erk1/2 inhibitor PD 0325901. The measurement of IL-10, IL-1α, IL-1β, IL-6, and TNFα levels in the different cell supernatants suggested that Cot/tpl2 mainly controls the production of the different cytokines by its capacity to mediate Erk1/2 activation in DAMPs-stimulated macrophages (supplemental Fig. S7).
FIGURE 5.
Activation of Wt and Cot/tpl2 KO macrophages by DAMPs. A, concentration of different cytokines in the supernatant of Wt and Cot/tpl2 KO peritoneal macrophages incubated for 18 h in the presence or absence of Wt DAMPs (300 μg/ml). The data represent the results of three independent experiments performed in triplicate. B, Wt and Cot/tpl2 KO peritoneal macrophages were treated as described in A, and at the times indicated, the levels of P-p105 NFκB, Cot/tpl2, P-Erk1/2, P-JNK, and P-p38α were measured in Western blots. The total Erk2, JNK2, and p38α levels were determined as protein loading controls, and one representative experiment of the three performed is shown. C, Wt and Cot/tpl2 KO BMDM were stimulated with Wt and Cot/tpl2 KO DAMPs (300 μg/ml), and at the times indicated, the cell extracts were analyzed in Western blots probed with the antibodies indicated in B. The P-p105 NFκB/Erk2, P-Erk1/2/Erk2, P-JNK/JNK2, and P-p38α/p38α values are expressed relative to the Wt time 0 point, and the data in the graphs represent the mean ± S.D. from four independent experiments. D, Wt BMDM were stimulated with Wt DAMPs (300 μg/ml) in the absence (Control) or presence of 10 μm of the IKKβ inhibitor (IKKβ I) B1605906 for the indicated times. Subsequently P-Erk1/2 and Erk2 levels were determined by Western blot analysis. The graphs represent the mean ± S.D. from three independent experiments of P-Erk1/2 fold induction relative to the Wt time 0 point, after normalizing values to, respectively, total Erk2.
Cot/tpl2 Modulates Sterile Inflammation in Vivo
Intraperitoneal injection of DAMPs induces the recruitment of leukocytes to the peritoneum (12, 13). Cot/tpl2 deficiency in mice significantly decreased the peritoneal recruitment of both macrophages and neutrophils 18 h after intraperitoneal injection of Wt or Cot/tpl2 KO DAMPs (Fig. 6, A and B). However, Cot/tpl2 deficiency did not alter the CD11a and Cd11b MFI values of the cells recruited (supplemental Fig. S8). Similarly, compared with Wt mice, Cot/tpl2 KD mice showed reduced peritoneal recruitment of both macrophages and neutrophils 18 h after intraperitoneal injection of Wt liver homogenate (supplemental Fig. S9). Oxidative burst analysis of the Wt and Cot/tpl2 KO peritoneal leukocytes recruited indicated that Cot/tpl2 deficiency slightly reduced their oxidative burst capacity, a difference that augmented in Cot/tpl2 KO leukocytes upon PMA stimulation (Fig. 6C). On the other hand, following intraperitoneal injection of Wt DAMPs, peritoneal levels of IL-1α, IL-1β, and IL-6 diminished in Cot/tpl2 KO mice compared with their Wt counterparts (Fig. 6D).
FIGURE 6.
Leukocytes recruitment following DAMPs-induced peritonitis in Wt and Cot/tpl2 KO mice. Animals were injected (intraperitoneal) with Wt or Cot/tpl2 KO DAMPs (1.7 mg) or with PBS (Control), and peritoneal cells were isolated 18 h later. A, number of isolated peritoneal macrophages (F4/80+) and neutrophils (LY6G+) recovered after injection of Wt or Cot/tpl2 KO DAMPs in Wt and Cot/tpl2 KO mice. B, one representative Ly6G+ versus F4/80+ staining FACS profile of Wt and Cot/tpl2 KO peritoneal cells isolated 18 h after injection of Wt DAMPs in Wt and Cot/tpl2 KO mice. C, oxidative burst activity of isolated Wt and Cot/tpl2 KO peritoneal cells incubated in the presence or absence of PMA (10 μm) in vitro. The relative induction is expressed in terms of that obtained in unstimulated Wt cells. D, levels of the indicated cytokines in the peritoneal cavity of Wt and Cot/tpl2 KO mice treated as described in B. A, C, and D, graphs represent the mean ± S.D. of three independent experiments performed in triplicate.
DISCUSSION
APAP overdoses are the number 1 cause of acute liver failure in the Western world, accounting for 50% of them (1–3). The present study demonstrates the fundamental role of Cot/tpl2 activity in APAP-induced liver damage. APAP-induced liver injury is associated with sterile inflammation (8, 44, 45), and Cot/tpl2 participates in its development by modulating the production of cytokines and the liver recruitment of leukocytes. In this context, it has been previously shown that TLR9 KO mice, with reduced levels of inflammatory cytokines, show resistance to acetaminophen toxicity (47). While some controversy surrounds the role of macrophages in the pathogenesis of APAP-mediated liver damage, neutrophil recruitment has been implicated in this process (40, 48–50). Indeed, neutrophil infiltration is associated with increased tissue necrosis because of the release of cytotoxic agents such as reactive oxygen species (51).
APAP overdose generates in hepatocytes the N-acetyl-p-benzoquinone imine that depletes glutathione and then generates oxidative stress reactions, leading to hepatocyte necrosis (4). DAMPs, once released into the surrounding tissue as a consequence of cell necrosis, orchestrate an inflammatory response, which in many cases can cause a second wave of destruction contributing to the pathogenesis of many damaging conditions (7, 11, 14). DAMPs are recognized by different receptors of the PPR family, including different TLRs (7, 10, 16). However, the i.p. recruitment of inflammatory cells upon intraperitoneal injection of DAMPs is not affected by the loss of a single TLR (12), although the inflammatory response to DAMPs is significantly attenuated in IL-1R and MyD88 KO mice (12). Here, we show that Cot/tpl2 participates in the i.p. recruitment of leukocytes in response to stimulation with DAMPs. IL-1R and virtually all TLRs (except TLR3) signal via MyD88 (20). Indeed, Cot/tpl2, activated via MyD88 or Trif, triggers the MKK1-Erk1/2 pathway following stimulation of IL-1R and TNFαR and also of all the different TLRs, including TLR3 (29, 30, 46).
Here we show that Cot/tpl2 once activated mediates, both in vivo and in vitro, IL-1α and IL-1β production. Sterile inflammation is largely dependent on IL-1α. Ιndeed, sterile inflammation requires this cytokine to a greater extent than pathogen-induced inflammation and its role in sterile inflammation cannot be substituted by IL-1β or any other cytokine (52–55). The peritoneal recruitment of neutrophils in response to DAMPs is largely dependent on IL-1α (13), and it has been recently reported that IL-1α and IL-1β trigger the recruitment of different myeloid cells (53).
In LPS-stimulated macrophages Cot/tpl2 mediates the production of the pro-inflammatory cytokine TNFα, but also of the anti-inflammatory cytokine IL-10 (25, 42, 56), and similar effects were observed here upon stimulation of macrophages with DAMPs. However, the contribution of Cot/tpl2 to the expression of these cytokines in vivo is not as straightforward as that observed in individual isolated cells. Cot/tpl2 controls TNFα synthesis upon i.p. injection of LPS/d-galactosamine in mice (25) but not following zymosan-induced intraplantar inflammation or following an infection with Listeria monocytogenes (34, 57). Conversely, Cot/tpl2 deficiency in APC KO mice results in a decrease in serum IL-10 (58), while in LPS and CpG-DNA-treated mice, Cot/tpl2 blocks IL-10 production (59). Our findings demonstrate that Cot/tpl2 only mildly increases peritoneal IL-10 levels upon injection with DAMPs, and it decreases serum IL-10 levels in response to APAP-induced liver injury. Although IL-10 protects against APAP-induced liver injury (60), it remains to be determined whether the increased levels of IL-10 in Cot/tpl2 KO mice at least in part attenuate liver injury.
Tissue damage is recognized at the cell level. Different DAMPs are recognized by the different receptors of the PPR family. The failure of DAMPs from necrotic cells to express specific molecules modifies their capacity to develop sterile inflammation (7, 15–18). However, dampened Cot/tpl2 expression in hepatocytes does not affect the capacity of their obtained DAMPs to activate macrophages in vitro or to initiate the sterile inflammation response in vivo.
Sterile inflammation is initiated by DAMPs that upon stimulation of different cell types including macrophages (12), produce IL-1α a major regulator of sterile inflammation (52–55). In macrophages, DAMPs activate Erk1/2, and our data indicate that Cot/tpl2 mediates the activation of Erk1/2 after 30 min but not after 5 min following stimulation. This rapid increase in Erk1/2 phosphorylation is probably mediated by the activation of another MAP3K, most likely one of the RAF proteins. Cot/tpl2 is the only MAP3K to activate the Erk1/2 pathway in response to both TLR activation and IL-1 or TNFα stimulation (29, 30). However, cell stimulation with DAMPs results in activation of a variety of receptors (7), thus the two different intracellular pathways involved in Erk1/2 phosphorylation under this cellular condition. Cot/tpl2 can also activate JNK (22, 30), and here we show that Cot/tpl2 partially mediates JNK phosphorylation upon macrophage activation with DAMPs. However, the decreased production of cytokines in the macrophages from Cot/tpl2 KO mice is due to impaired activation of the Erk1/2 intracellular signal pathway. In conclusion, our data show that Cot/tpl2 participates in sterile inflammatory pathways triggered by damaged tissue and plays an essential role in APAP-induced liver injury.
Supplementary Material
Acknowledgments
B1605906, SP 600125, and PD 0325901 were gifts from Sir Philip Cohen (Dundee).
This work was supported by Grants SAF 2011-24481 (to S. A.) and SAF 2012-33283 (to A. M. V.) from MINECO (Spain) Comunidad de Madrid S2010/BMD-2423, EFSD/Amylin Programme 2011 (to A. M. V.) and a grant from the Mutua Madrileña (to S. A.).

This article contains supplemental Figs. S1–S9.
- APAP
- acetaminophen
- BMDM
- bone marrow-derived macrophages
- DAMP
- damage-associated molecular pattern
- PAMP
- pathogen-associated molecular pattern
- TLR
- Toll-like receptor
- LPS
- lipopolysaccharide
- i.p.
- intraperitoneal
- LDH
- lactate dehydrogenase
- MFI
- mean fluorescence intensity.
REFERENCES
- 1. Kaufman D. W., Kelly J. P., Rosenberg L., Anderson T. E., Mitchell A. A. (2002) Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 287, 337–344 [DOI] [PubMed] [Google Scholar]
- 2. Blazer D. G., Wu L. T. (2009) Nonprescription use of pain relievers by middle-aged and elderly community-living adults: National Survey on Drug Use and Health. J. Am. Geriatr. Soc. 57, 1252–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilcox C. M., Cryer B., Triadafilopoulos G. (2005) Patterns of use and public perception of over-the-counter pain relievers: focus on nonsteroidal antiinflammatory drugs. J. Rheumatol. 32, 2218–2224 [PubMed] [Google Scholar]
- 4. Hinson J. A., Roberts D. W., James L. P. (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 196, 369–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones D. P., Lemasters J. J., Han D., Boelsterli U. A., Kaplowitz N. (2010) Mechanisms of pathogenesis in drug hepatotoxicity putting the stress on mitochondria. Mol. Interv. 10, 98–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCall K. (2010) Genetic control of necrosis - another type of programmed cell death. Curr. Opin. Cell Biol. 22, 882–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen G. Y., Nunez G. (2010) Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams D. H., Ju C., Ramaiah S. K., Uetrecht J., Jaeschke H. (2010) Mechanisms of immune-mediated liver injury. Toxicol. Sci. 115, 307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rock K. L., Latz E., Ontiveros F., Kono H. (2010) The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeuchi O., Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140, 805–820 [DOI] [PubMed] [Google Scholar]
- 11. Maher J. J. (2009) DAMPs ramp up drug toxicity. J. Clin. Invest. 119, 246–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kono H., Karmarkar D., Iwakura Y., Rock K. L. (2010) Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J. Immunol. 184, 4470–4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C. J., Kono H., Golenbock D., Reed G., Akira S., Rock K. L. (2007) Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13, 851–856 [DOI] [PubMed] [Google Scholar]
- 14. Stirnimann G., Kessebohm K., Lauterburg B. (2010) Liver injury caused by drugs: an update. Swiss Med. Wkly 140, w13080. [DOI] [PubMed] [Google Scholar]
- 15. Kono H., Rock K. L. (2008) How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piccinini A. M., Midwood K. S. (2010) DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bianchi M. E. (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of Leukocyte Biology 81, 1–5 [DOI] [PubMed] [Google Scholar]
- 18. Sirisinha S. (2011) Insight into the mechanisms regulating immune homeostasis in health and disease. Asian Pac. J. Allergy Immunol. 29, 1–14 [PubMed] [Google Scholar]
- 19. Schwabe R. F., Seki E., Brenner D. A. (2006) Toll-like receptor signaling in the liver. Gastroenterology 130, 1886–1900 [DOI] [PubMed] [Google Scholar]
- 20. O'Neill L. A., Bowie A. G. (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat. Rev. Immunol. 7, 353–364 [DOI] [PubMed] [Google Scholar]
- 21. Akira S., Takeda K. (2004) Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 [DOI] [PubMed] [Google Scholar]
- 22. Gantke T., Sriskantharajah S., Sadowski M., Ley S. C. (2012) IκB kinase regulation of the TPL-2/ERK MAPK pathway. Immunol. Rev. 246, 168–182 [DOI] [PubMed] [Google Scholar]
- 23. Vougioukalaki M., Kanellis D. C., Gkouskou K., Eliopoulos A. G. (2011) Tpl2 kinase signal transduction in inflammation and cancer. Cancer Lett. 304, 80–89 [DOI] [PubMed] [Google Scholar]
- 24. Caivano M., Rodriguez C., Cohen P., Alemany S. (2003) 15-Deoxy-Δ12,14-prostaglandin J2 regulates endogenous Cot MAPK kinase kinase 1 activity induced by lipopolysaccharide. J. Biol. Chem. 278, 52124–52130 [DOI] [PubMed] [Google Scholar]
- 25. Dumitru C. D., Ceci J. D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J. H., Patriotis C., Jenkins N. A., Copeland N. G., Kollias G., Tsichlis P. N. (2000) TNF-α induction by LPS is regulated post-transcriptionally via a Tpl2/ERK-dependent pathway. Cell 103, 1071–1083 [DOI] [PubMed] [Google Scholar]
- 26. López-Peláez M., Soria-Castro I., Boscá L., Fernández M., Alemany S. (2011) Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: Implications for NO synthase 2 expression. Eur. J. Immunol. 41, 1733–1741 [DOI] [PubMed] [Google Scholar]
- 27. Waterfield M. R., Zhang M., Norman L. P., Sun S. C. (2003) NF-κB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol. Cell 11, 685–694 [DOI] [PubMed] [Google Scholar]
- 28. Gándara M. L., López P., Hernando R., Castaño J. G., Alemany S. (2003) The COOH-terminal domain of wild-type Cot regulates its stability and kinase specific activity. Mol. Cell Biol. 23, 7377–7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodríguez C., Pozo M., Nieto E., Fernández M., Alemany S. (2006) TRAF6 and Src kinase activity regulates Cot activation by IL-1. Cell Signal 18, 1376–1385 [DOI] [PubMed] [Google Scholar]
- 30. Das S., Cho J., Lambertz I., Kelliher M. A., Eliopoulos A. G., Du K., Tsichlis P. N. (2005) Tpl2/cot signals activate ERK, JNK, and NF-κB in a cell-type and stimulus-specific manner. J. Biol. Chem. 280, 23748–23757 [DOI] [PubMed] [Google Scholar]
- 31. Perugorria M. J., Murphy L. B., Fullard N., Chakraborty J. B., Vyrla D., Wilson C. L., Oakley F., Mann J., Mann D. A. (2013) Tumor progression locus 2/Cot is required for activation of extracellular regulated kinase in liver injury and toll-like receptor-induced TIMP-1 gene transcription in hepatic stellate cells in mice. Hepatology 57, 1238–1349 [DOI] [PubMed] [Google Scholar]
- 32. Cohen P. (2009) Targeting protein kinases for the development of anti-inflammatory drugs. Curr. Opin. Cell Biol. 21, 317–324 [DOI] [PubMed] [Google Scholar]
- 33. Gaestel M., Kotlyarov A., Kracht M. (2009) Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Discov. 8, 480–499 [DOI] [PubMed] [Google Scholar]
- 34. Soria-Castro I., Krzyzanowska A., Pelaéz M. L., Regadera J., Ferrer G., Montoliu L., Rodríguez-Ramos R., Fernández M., Alemany S. (2010) Cot/tpl2 (MAP3K8) mediates myeloperoxidase activity and hypernociception following peripheral inflammation. J. Biol. Chem. 285, 33805–33815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kontoyiannis D., Boulougouris G., Manoloukos M., Armaka M., Apostolaki M., Pizarro T., Kotlyarov A., Forster I., Flavell R., Gaestel M., Tsichlis P., Cominelli F., Kollias G. (2002) Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn's-like inflammatory bowel disease. J. Exp. Med. 196, 1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koliaraki V., Roulis M., Kollias G. (2012) Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigenesis. J. Clin. Invest. 122, 4231–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Acker G. J., Perides G., Weiss E. R., Das S., Tsichlis P. N., Steer M. L. (2007) Tumor progression locus-2 is a critical regulator of pancreatic and lung inflammation during acute pancreatitis. J. Biol. Chem. 282, 22140–22149 [DOI] [PubMed] [Google Scholar]
- 38. Watford W. T., Wang C. C., Tsatsanis C., Mielke L. A., Eliopoulos A. G., Daskalakis C., Charles N., Odom S., Rivera J., O'Shea J., Tsichlis P. N. (2010) Ablation of tumor progression locus 2 promotes a type 2 Th cell response in Ovalbumin-immunized mice. J. Immunol. 184, 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. González-Rodriguez A., Escribano O., Alba J., Rondinone C. M., Benito M., Valverde A. M. (2007) Levels of protein tyrosine phosphatase 1B determine susceptibility to apoptosis in serum-deprived hepatocytes. J. Cell Physiol. 212, 76–88 [DOI] [PubMed] [Google Scholar]
- 40. Liu Z. X., Han D., Gunawan B., Kaplowitz N. (2006) Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 43, 1220–1230 [DOI] [PubMed] [Google Scholar]
- 41. Rodríguez C., López P., Pozo M., Duce A. M., López-Pelaéz M., Fernández M., Alemany S. (2008) COX2 expression and Erk1/Erk2 activity mediate Cot-induced cell migration. Cell Signal 20, 1625–1631 [DOI] [PubMed] [Google Scholar]
- 42. López-Pelaéz M., Fumagalli S., Sanz C., Herrero C., Guerra S., Fernandez M., Alemany S. (2012) Cot/tpl2-MKK1/2-Erk1/2 controls mTORC1-mediated mRNA translation in Toll-like receptor-activated macrophages. Mol. Biol. Cell 23, 2982–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gunawan B. K., Liu Z. X., Han D., Hanawa N., Gaarde W. A., Kaplowitz N. (2006) c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 131, 165–178 [DOI] [PubMed] [Google Scholar]
- 44. Holt M. P., Cheng L., Ju C. (2008) Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc Biol. 84, 1410–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Antoniades C. G., Quaglia A., Taams L. S., Mitry R. R., Hussain M., Abeles R., Possamai L. A., Bruce M., McPhail M., Starling C., Wagner B., Barnardo A., Pomplun S., Auzinger G., Bernal W., Heaton N., Vergani D., Thursz M. R., Wendon J. (2012) Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 56, 735–746 [DOI] [PubMed] [Google Scholar]
- 46. Banerjee A., Gugasyan R., McMahon M., Gerondakis S. (2006) Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 103, 3274–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Imaeda A. B., Watanabe A., Sohail M. A., Mahmood S., Mohamadnejad M., Sutterwala F. S., Flavell R. A., Mehal W. Z. (2009) Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 119, 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X., Sun R., Wei H., Tian Z. (2013) High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of γδ T cells with macrophages. Hepatology 57, 373–384 [DOI] [PubMed] [Google Scholar]
- 49. Marques P. E., Amaral S. S., Pires D. A., Nogueira L. L., Soriani F. M., Freire Lima B. H., Oliveira Lopes G. A., Russo R. C., Avila T. V., Melgaço J. G., Oliveira A. G., Pinto M. A., Lima C. X., de Paula A. M., Cara D. C., Leite M. F., Teixeira M. M., Menezes G. B. (2012) Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56, 1971–1982 [DOI] [PubMed] [Google Scholar]
- 50. Jaeschke H., McGill M. R., Williams C. D. (2013) Pathophysiological relevance of neutrophils in acetaminophen hepatotoxicity. Hepatology 57, 419. [DOI] [PubMed] [Google Scholar]
- 51. Weiss S. J. (1989) Tissue destruction by neutrophils. N. Engl. J. Med. 320, 365–376 [DOI] [PubMed] [Google Scholar]
- 52. Berda-Haddad Y., Robert S., Salers P., Zekraoui L., Farnarier C., Dinarello C. A., Dignat-George F., Kaplanski G. (2011) Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proc. Natl. Acad. Sci. U.S.A. 108, 20684–20689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rider P., Carmi Y., Guttman O., Braiman A., Cohen I., Voronov E., White M. R., Dinarello C. A., Apte R. N. (2011) IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J. Immunol. 187, 4835–4843 [DOI] [PubMed] [Google Scholar]
- 54. Eigenbrod T., Park J. H., Harder J., Iwakura Y., Nuñez G. (2008) Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J. Immunol. 181, 8194–8198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Elkon K. B. (2007) IL-1α responds to necrotic cell death. Nat. Med. 13, 778–780 [DOI] [PubMed] [Google Scholar]
- 56. Kaiser F., Cook D., Papoutsopoulou S., Rajsbaum R., Wu X., Yang H. T., Grant S., Ricciardi-Castagnoli P., Tsichlis P. N., Ley S. C., O'Garra A. (2009) TPL-2 negatively regulates interferon-β production in macrophages and myeloid dendritic cells. J. Exp. Med. 206, 1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mielke L. A., Elkins K. L., Wei L., Starr R., Tsichlis P. N., O'Shea J. J., Watford W. T. (2009) Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1β production. J. Immunol. 183, 7984–7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Serebrennikova O. B., Tsatsanis C., Mao C., Gounaris E., Ren W., Siracusa L. D., Eliopoulos A. G., Khazaie K., Tsichlis P. N. (2012) Tpl2 ablation promotes intestinal inflammation and tumorigenesis in Apcmin mice by inhibiting IL-10 secretion and regulatory T-cell generation. Proc. Natl. Acad. Sci. U.S.A. 109, E1082–E1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sugimoto K., Ohata M., Miyoshi J., Ishizaki H., Tsuboi N., Masuda A., Yoshikai Y., Takamoto M., Sugane K., Matsuo S., Shimada Y., Matsuguchi T. (2004) A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J. Clin. Invest. 114, 857–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bourdi M., Masubuchi Y., Reilly T. P., Amouzadeh H. R., Martin J. L., George J. W., Shah A. G., Pohl L. R. (2002) Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 35, 289–298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.