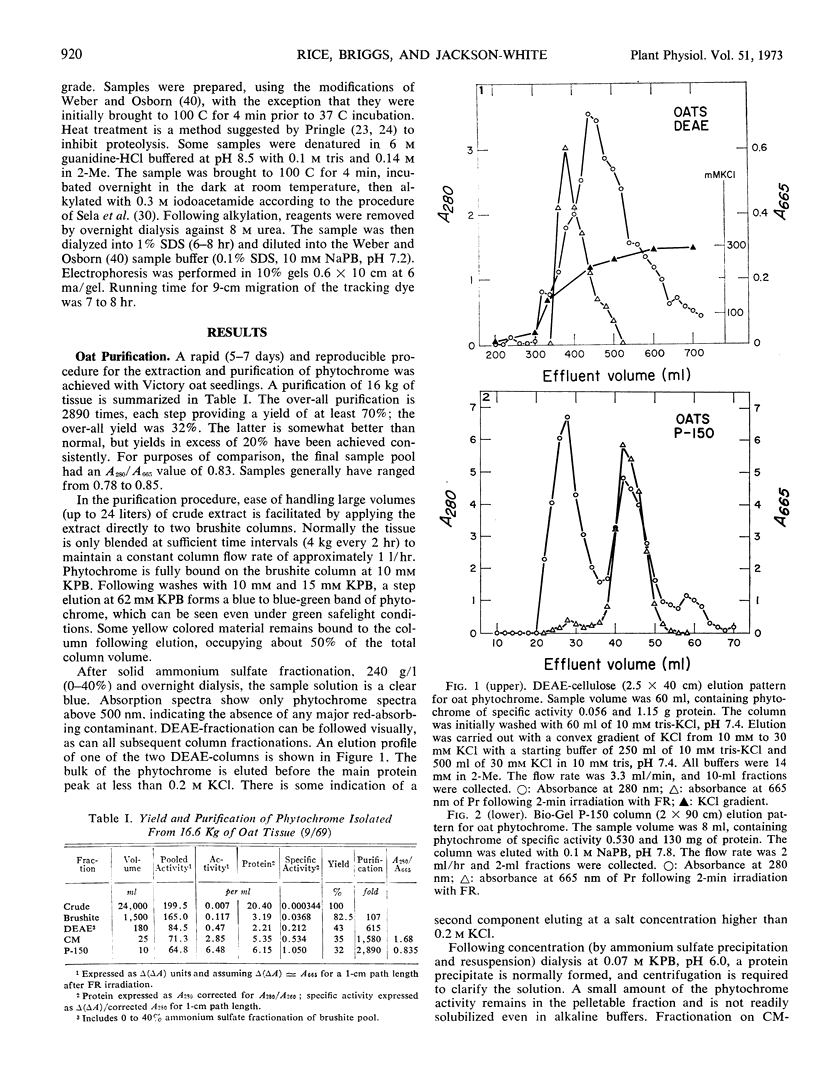
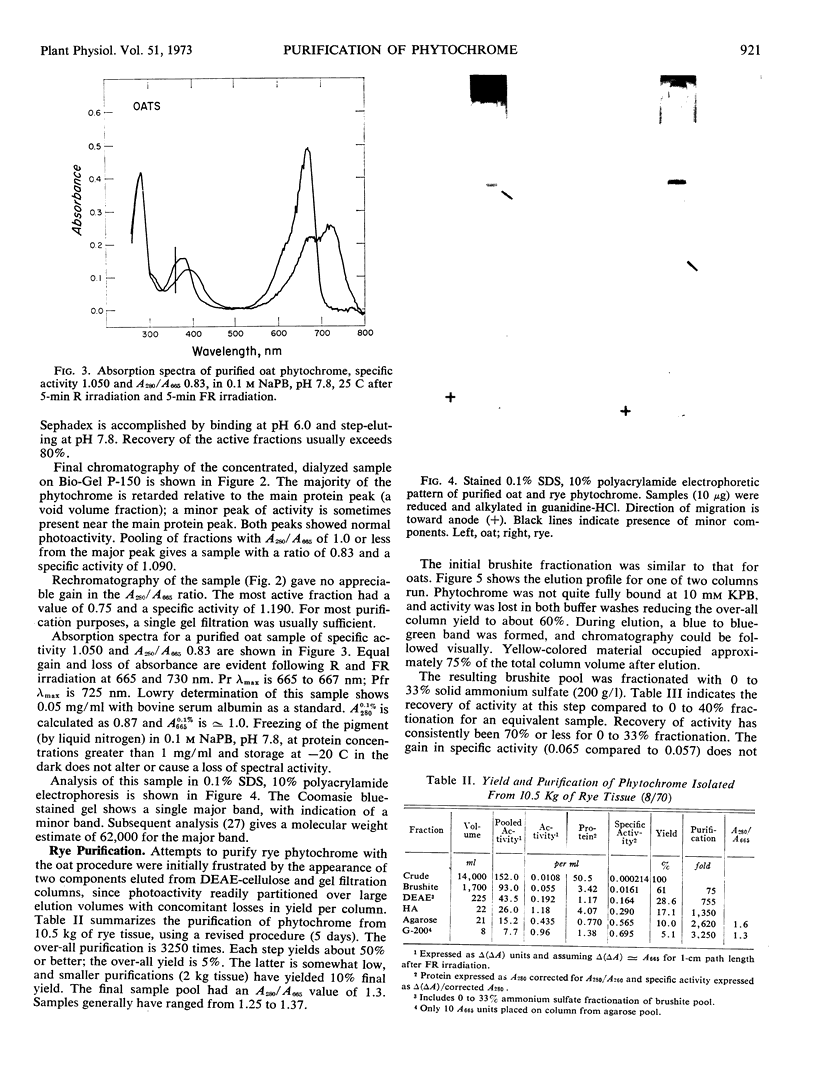
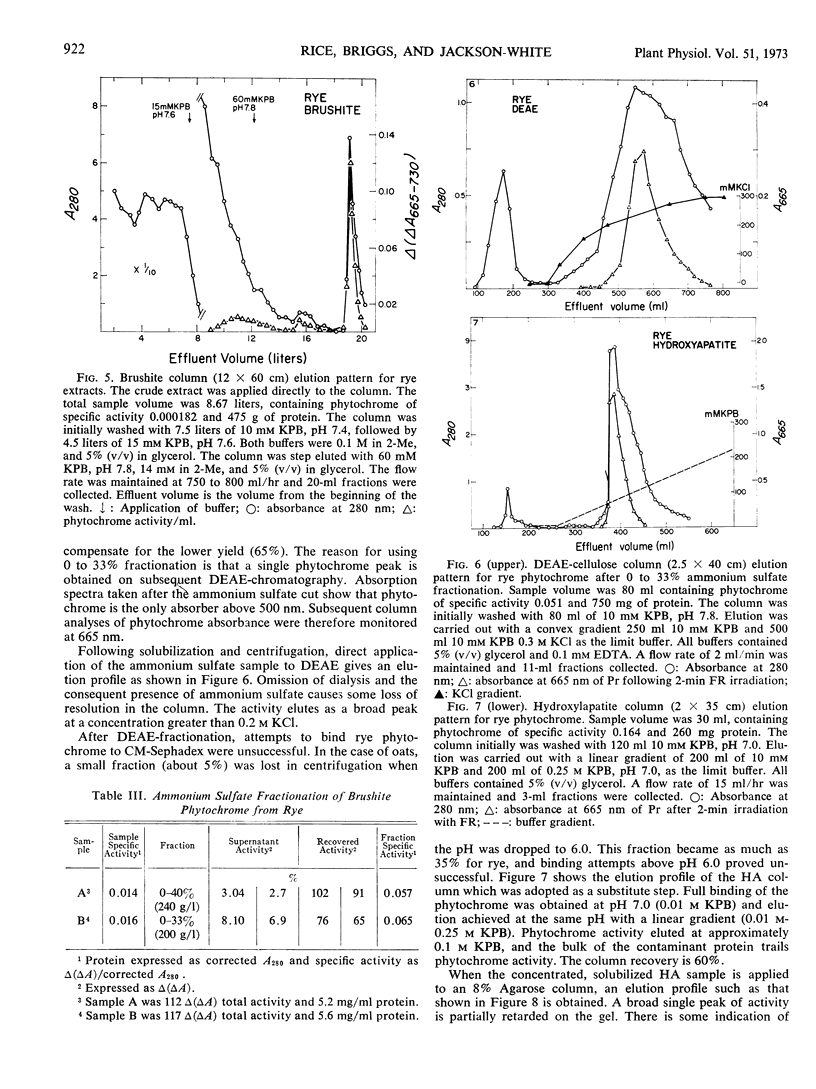
Abstract
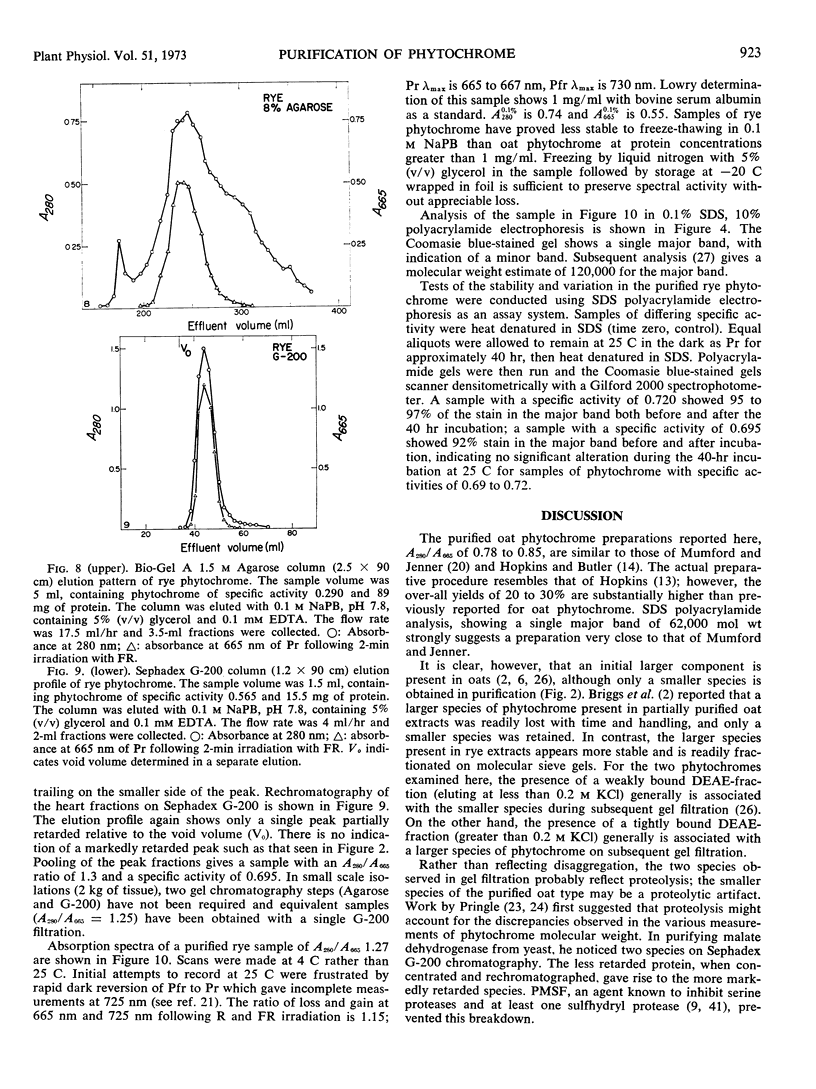
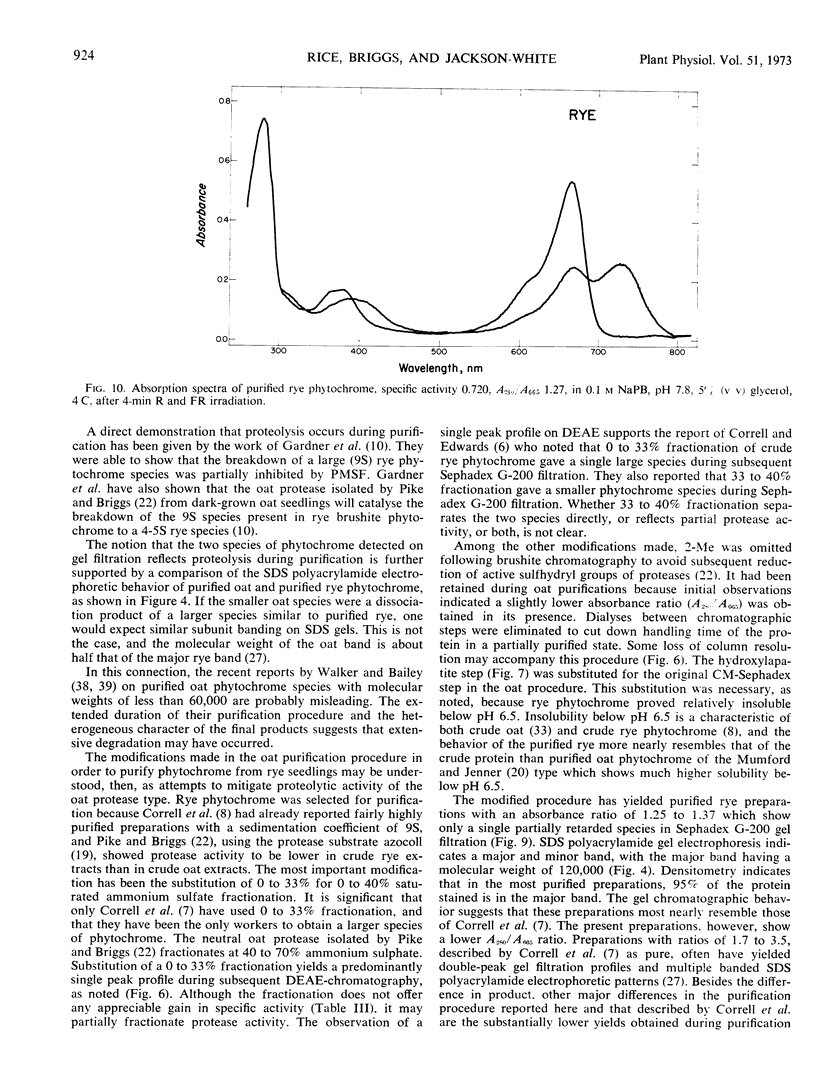
A purification procedure employing normal chromatographic techniques is outlined for isolating phytochrome from etiolated oat (Avena sativa L.) seedlings. Yields in excess of 20% (25 milligrams or more) of phytochrome in crude extract were obtained from 10- to 15-kilograms lots. The purified oat phytochrome had an absorbance ratio (A280 nm/A665 nm) of 0.78 to 0.85, comparable to reported values, and gave a single major band with an estimated molecular weight of 62,000 on electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. A modification of the oat isolation procedure was used to isolate phytochrome from etiolated rye Secale cereale cv. Balbo) seedlings. During isolation rye phytochrome exhibited chromatographic profiles differing from oat phytochrome on diethylaminoethyl cellulose and on molecular sieve gels. It eluted at a higher salt concentration on diethylaminoethyl cellulose and nearer the void volume on molecular sieve gels. Yields of 5 to 10% (7.5-10 milligrams) of phytochrome in crude extract were obtained from 10- to 12-kilogram seedling lots. The purified rye phytochrome had an absorbance ratio of 1.25 to 1.37, significantly lower than values in the literature and gave a single major band with an estimated molecular weight of 120,000 on electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. It is suggested that the absorbance ratio and electrophoretic behavior of rye phytochrome are indices of purified native phytochrome, and that oat phytochrome as it has been described is an artifact which arises as a result of endogenous proteolysis during isolation. A rationale is provided for further modifications of the purification procedure to alleviate presumed protease contaminants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Briggs W. R., Zollinger W. D., Platz B. B. Some Properties of Phytochrome Isolated From Dark-grown Oat Seedlings (Avena sativa L.). Plant Physiol. 1968 Aug;43(8):1239–1243. doi: 10.1104/pp.43.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Norris K. H., Siegelman H. W., Hendricks S. B. DETECTION, ASSAY, AND PRELIMINARY PURIFICATION OF THE PIGMENT CONTROLLING PHOTORESPONSIVE DEVELOPMENT OF PLANTS. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1703–1708. doi: 10.1073/pnas.45.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll D. L., Edwards J. L., Klein W. H., Shropshire W., Jr Phytochrome in etiolated annual rye. 3. Isolation of photoreversible phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):36–45. doi: 10.1016/0005-2795(68)90231-6. [DOI] [PubMed] [Google Scholar]
- Correll D. L., Edwards J. L. The aggregation States of phytochrome from etiolated rye and oat seedings. Plant Physiol. 1970 Jan;45(1):81–85. doi: 10.1104/pp.45.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll D. L., Steers E., Jr, Towe K. M., Shropshire W., Jr Phytochrome in etiolated annual rye. IV. Physical and chemical characterization of phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):46–57. doi: 10.1016/0005-2795(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D. W., Butler W. L. Immunochemical and spectroscopic evidence for protein conformational changes in phytochrome transformations. Plant Physiol. 1970 May;45(5):567–570. doi: 10.1104/pp.45.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes H. H., van Rooijen A., Geers J. M., Greuell E. H. Large-scale isolation of phytochrome from oat seedlings. Biochim Biophys Acta. 1969 Mar;175(2):409–413. doi: 10.1016/0005-2795(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Moore G. L. Use of azo-dye-bound collagen to measure reaction velocities of proteolytic enzymes. Anal Biochem. 1969 Oct 15;32(1):122–127. doi: 10.1016/0003-2697(69)90111-0. [DOI] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Purification and characterization of phytochrome from oat seedlings. Biochemistry. 1966 Nov;5(11):3657–3662. doi: 10.1021/bi00875a039. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. Partial Purification and Characterization of a Phytochrome-degrading Neutral Protease from Etiolated Oat Shoots. Plant Physiol. 1972 Apr;49(4):521–530. doi: 10.1104/pp.49.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. The dark reactions of rye phytochrome in vivo and in vitro. Plant Physiol. 1972 Apr;49(4):514–520. doi: 10.1104/pp.49.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. R. The molecular weight of the undegraded polypeptide chain of yeast hexokinase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):46–52. doi: 10.1016/0006-291x(70)90755-2. [DOI] [PubMed] [Google Scholar]
- Purves W. K., Briggs W. R. Kinetically distinguishable populations of phytochrome. Plant Physiol. 1968 Aug;43(8):1259–1263. doi: 10.1104/pp.43.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S. J. Chemical evidence for conformational differences between the red- and far-red-absorbing forms of oat phytochrome. Biochemistry. 1972 May 9;11(10):1930–1936. doi: 10.1021/bi00760a030. [DOI] [PubMed] [Google Scholar]
- Roux S. J., Hillman W. S. The effect of glutaraldehyde and two monoaldehydes on phytochrome. Arch Biochem Biophys. 1969 May;131(2):423–429. doi: 10.1016/0003-9861(69)90414-7. [DOI] [PubMed] [Google Scholar]
- SELA M., WHITE F. H., Jr, ANFINSEN C. B. The reductive cleavage of disulfide bonds and its application to problems of protein structure. Biochim Biophys Acta. 1959 Feb;31(2):417–426. doi: 10.1016/0006-3002(59)90016-2. [DOI] [PubMed] [Google Scholar]
- SIEGELMAN H. W., FIRER E. M. PURIFICATION OF PHYTOCHROME FROM OAT SEEDLINGS. Biochemistry. 1964 Mar;3:418–423. doi: 10.1021/bi00891a019. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Wieczorek G. A., Turner B. C. Preparation of calcium phosphate for protein chromatography. Anal Biochem. 1965 Dec;13(3):402–404. doi: 10.1016/0003-2697(65)90332-5. [DOI] [PubMed] [Google Scholar]
- Taylor A. O., Bonner B. A. Isolation of phytochrome from the alga mesotaenium and liverwort sphaerocarpos. Plant Physiol. 1967 Jun;42(6):762–766. doi: 10.1104/pp.42.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O. In vitro phytochrome dark reversion process. Plant Physiol. 1968 May;43(5):767–774. doi: 10.1104/pp.43.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Bailey J. L. Studies on phytochrome. Some properties of electrophoretically pure phytochrome. Biochem J. 1970 Dec;120(3):613–622. doi: 10.1042/bj1200613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Bailey J. L. Studies on phytochrome. Two photoreversible chromoproteins from etiolated oat seedlings. Biochem J. 1970 Dec;120(3):607–612. doi: 10.1042/bj1200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Bailey J. L. Two spectrally different forms of the phytochrome chromophore extracted from etiolated oat seedlings. Biochem J. 1968 Apr;107(4):603–605. doi: 10.1042/bj1070603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whitaker J. R., Perez-Villase ñor J. Chemical modification of papain. I. Reaction with the chloromethyl ketones of phenylalanine and lysine and with phenylmethyl-sulfonyl fluoride. Arch Biochem Biophys. 1968 Mar 20;124(1):70–78. doi: 10.1016/0003-9861(68)90304-4. [DOI] [PubMed] [Google Scholar]