Abstract
Purpose
The effects of astaxanthin (Ax) on the in vitro development of bovine embryos cultured under heat stress were investigated in combination with the assessment of its cellular accumulation and action on mitochondrial membrane potential (ΔΨm).
Methods
Bovine ≥8-cell embryos were collected on day 3 after in vitro fertilization and exposed to single (day 4) or repeated (day 4 and 5) heat stress (10 h/day at 40.5 °C). Ax was added into culture medium under the repeated heat stress and blastocyst development was evaluated. The cellular uptake of Ax in embryos was examined using bright-field and confocal laser-scanning microscopy, and high-performance liquid chromatography. The relationship between Ax and mitochondria localization was assessed using MitoTracker dye. The effects of Ax on ΔΨm were investigated using JC-1 dye.
Results
Blastocyst development in the repeated heat stress treatment decreased significantly (P < 0.05) compared with those in single heat stress or normal thermal treatment. The addition of Ax into culture medium did lead to a significant recovery in blastocyst development in the repeated heat-treated group. Ax was detected in cytoplasm of embryos and observed to colocalize with mitochondria. Ax recovered ΔΨm in embryos that was decreased by the heat treatment.
Conclusions
Ax ameliorated the heat stress-induced impairment of blastocyst development. Our results suggest that the direct action of Ax on mitochondrial activity via cellular uptake is a mechanism of the ameliorating effects.
Keywords: Preimplantation embryos, Heat stress, Astaxanthin, Cellular uptake, Mitochondria
Introduction
Mammalian fertility is largely affected by environmental factors such as pollutants, nutrition, and physical or psychological stresses [5, 23, 31]. Among these factors, heat stress impairs early postfertilization development in many in vivo and in vitro studies [7, 12, 40]. To circumvent the impairment of early development under heat stress, a few functional dietary substances have been investigated for their ameliorating effects on heat-induced developmental defects [21, 38, 41].
Astaxanthin (Ax) is a carotenoid that is abundant in fishery products and is reported to have various beneficial bioactive properties for human and animal health, including the prevention of cardiovascular disease, the promotion of immune responses, and anti-oxidative actions [14, 15]. These findings suggest a possible application of Ax in the enhancement of mammalian reproductive health. We previously reported that bovine preimplantation embryos exposed to heat stress in vitro had reduced rates of development and that Ax-containing oil added into the culture medium ameliorated the detrimental effects of heat stress [30]. However, unlike β-carotene, which is the most representative carotenoid investigated in terms of its implication in reproduction [11, 17], the mode of Ax action on mammalian preimplantation embryos remains largely unknown.
Recently, Ax has been shown to increase the mitochondrial membrane potential (ΔΨm) in a cultured cell line [54]. Mitochondria regulate cellular homeostasis, including the metabolism of respiratory substrates, oxidative phosphorylation (OXPHOS), ion homeostasis, reactive oxygen species (ROS) production, and apoptosis [13, 35]. The critical roles of mitochondria in mammalian oogenesis and early embryogenesis are emerging as a correlation to developmental outcome in oocyte maturation, fertilization, and postfertilization development [9, 49, 51]. Furthermore, the variability and sensitivity of mitochondrial activity in response to environmental insults have also been documented in oocytes and preimplantation embryos [9, 16, 52]. In the present study, we examined the effects of heat stress during day 4 and 5 postfertilization in combination with Ax treatment on the development of bovine embryos. The heat stress condition used was based on the daily change in rectal temperatures in cows that are exposed to heat stress in the hot season [37] to mimic a physiological situation. The cellular uptake and localization of Ax in preimplantation embryos as well as the effects of Ax on ΔΨm were also investigated.
Materials and Methods
Ethics statement
This study was carried out in accordance with the Regulation on Animal Experimentation at Kyoto University.
Chemicals
All chemicals used were purchased from Sigma-Aldrich (St. Louis, MO) or Wako Pure Chemical Industries (Osaka, Japan), unless otherwise specified. AstaREAL® (emulsified H. pluvialis extract containing 0.5 % [w/w] Ax) and an identical vehicle for emulsification (a mixture of maltosyl trehalose, water, medium chain triglycerides, polyglycerol esters of fatty acids, sucrose esters of fatty acids, and mix tocopherols) were provided by Fuji Chemical Industry Co., Ltd. (Toyama, Japan).
Culture media for in vitro production of bovine embryos
Culture media for in vitro maturation (IVM) of immature oocytes recovered from ovaries, in vitro fertilization (IVF) of matured oocytes, and in vitro culture (IVC) of zygotes up to the blastocyst stage were prepared following a previously published protocol based on synthetic oviduct fluid (SOF) containing amino acids [46] with some modifications: the concentration of sodium pyruvate was increased to 0.5 mM, and the media were modified for each of the applications according to a previous report [18]. In brief, the medium for IVM (IVMM) was supplemented with 5.6 mM glucose, 10 % (v/v) fetal calf serum, and 0.2 IU/ml follicular-stimulating hormone (Kyoritsu Seiyaku, Kawasaki, Japan); the medium for IVF (IVFM) was not supplemented with glucose, and the medium for IVC (IVCM) was supplemented with 1.5 mM glucose. Unless otherwise treated, all cultures were carried out at 38.5 °C, a normal body temperature for cows, under 5 % CO2, 5 % O2 and 90 % N2 with high humidity.
In vitro production of bovine embryos
Bovine oocytes were recovered from abattoir-derived ovaries by aspirating 2- to 8-mm follicles with a needle attached to a syringe. Cumulus-enclosed oocytes (CEOs) with compact dense cumulus cell layers were selected. Groups of 10 CEOs were matured in vitro in 50-μl drops of IVMM covered with mineral oil (Nacalai Tesque, Kyoto, Japan). Matured oocytes were fertilized in vitro with frozen-thawed bull sperm as described [39], except with regard to the medium used [18]. In brief, frozen-thawed bull semen was layered onto a discontinuous Percoll gradient solution (45 % and 90 % [v/v]) and centrifuged at 700 × g for 30 min. The pelleted spermatozoa were resuspended in IVFM and centrifuged again at 700 × g for 10 min. The spermatozoa in the pellet were again resuspended in IVFM at a concentration of 2 × 106 cells/ml. Immediately prior to insemination, the groups of 10 CEOs after IVM were transferred to 50-μl drops of IVFM supplemented with 3.6 U/ml heparin. Fifty microliters of the sperm suspension was added to each mineral oil-covered drop containing the CEOs. Thus, the final concentrations of spermatozoa and heparin in the drops were 1 × 106 cells/ml and 1.8 U/ml, respectively. The CEOs and spermatozoa were coincubated for 20 h.
After IVF, the resulting putative zygotes were freed from the cumulus cells by pipetting and subsequently cultured in 500 μl of IVCM covered with mineral oil. The day of IVF and the beginning of insemination were designated as day 0 and 0 h post-insemination (hpi), respectively.
Effects of single and repeated heat stress on the development of bovine embryos in vitro
On day 3 at 72 hpi, embryos that had developed to the 8-cell stage or beyond (≥8-cell) were transferred to 500 μl of IVCM supplemented with 0.045 % (v/v) of the corresponding vehicle used in the following experiments and were subsequently cultured up to day 8 (192 hpi). Embryos were cultured basically at 38.5 °C (Normal). Embryos that were exposed once (91–101 hpi on day 4) or twice (91–101 hpi on day 4 and 115–125 hpi on day 5) to 40.5 °C for 10 h were designated as Heat 1 and Heat 2 groups, respectively. The heat stress condition was designed to mimic the daily change in rectal temperatures in cows that are exposed to heat stress in the hot season [37]. The proportions of embryos that had developed to the blastocyst stage were compared among the Normal, Heat 1, and Heat 2 groups. The cultures were replicated four times, and the number of embryos allocated to each treatment group was approximately 18 per replicate. Blastocyst development was assessed on day 8.
Effects of Ax on the development of bovine embryos exposed to repeated heat stress
As described above, ≥8-cell embryos at 72 hpi were transferred to 500 μl of IVCM supplemented with 0.045 % (v/v) of AstaREAL® or the corresponding vehicle (control) and were subsequently cultured up to day 8 (192 hpi). The final concentration of Ax in the medium was 2.5 ppm. Embryos were cultured basically at 38.5 °C (Normal) or, in the heat-stressed group (Heat), were exposed twice to 40.5 °C for 10 h as in the Heat 2 treatment described above. Thus, the embryos were divided into four groups: (1) normal thermal conditions in the absence of Ax, (2) normal thermal conditions in the presence of Ax, (3) heat stress in the absence of Ax and (4) heat stress in the presence of Ax. The cultures were replicated five times, and the number of embryos allocated to each treatment group was approximately 18 per replicate. Blastocyst development was assessed on day 8.
Detection of Ax taken up by bovine preimplantation embryos
The ≥8-cell embryos were cultured in the presence or absence of Ax as described above, and cultures were extended up to day 10 to induce hatching from the zona pellucida. Pre-hatching, hatching, and completely hatched blastocysts were fixed and nuclear-stained with 10 % (v/v) formalin neutral buffer solution containing 10 μg/ml Hoechst 33342. Stained blastocysts were then mounted onto slides with a droplet of VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA) and flattened with a coverslip. The slides were examined under a fluorescence microscope (Olympus, Tokyo, Japan) in combination with observations using transmitted light.
In separate experiments, fixed day 5 morulae and day 10 blastocysts (pre-hatching to hatched stage) were mounted onto slides and covered with coverslips supported with 9:1 (v/v) Vaseline/paraffin spots at each corner. The slides were observed under a laser-scanning confocal microscope (Olympus). The fluorescence emission of Ax [19] excited at 488 nm by an argon laser was detected using BA565IF barrier filter.
To further confirm the cellular uptake of Ax in bovine preimplantation embryos, high-performance liquid chromatography (HPLC) was used. One-hundred blastocysts (pre-hatching to hatched stage) on day 10 that had been cultured with Ax were collected and extensively washed with PBS. Carotenoids were extracted from the blastocysts with 1 ml acetone at room temperature. The extract solution was evaporated to dryness. The residue was dissolved in 50 μl of acetone/hexane (2:8) solution and subjected to HPLC analysis. HPLC was performed on a Hitachi (Tokyo, Japan) L-6200 intelligent pump with a Hitachi L-4250 UV–VIS detector set at 470 nm absorbance. Ax was quantified by HPLC on a Cosmosil 5SL-II 250 × 4.6 mm i.d., column (Nacalai Tesque, Kyoto, Japan) with acetone/hexane as a solvent at a flow rate of 1.0 ml/min at room temperature. Standard Ax was provided by DSM Nutrition Japan (Tokyo, Japan).
Localization of Ax and mitochondria in bovine blastocysts
Hatched blastocysts cultured in the presence of Ax were obtained on day 10 and fixed with 10 % (v/v) formalin neutral buffer solution for 30 min. Then they were extensively washed with PBS containing 0.1 mg/ml polyvinylalcohol (PBS-PVA). Mitochondria were stained by incubating embryos in PBS-PVA containing 500 nM MitoTracker Green FM (Invitrogen, Carlsbad, CA) for 30 min at 37 °C in air with high humidity. After washing with PBS-PVA, embryos were mounted onto slides with Vaseline/paraffin spots as described above and observed under a laser-scanning confocal microscope. The MitoTracker (green) and Ax (red) fluorescence was excited at 488 nm by an argon laser and detected using both BA510IF and BA530RIF filters (channel 1) for green and BA610IF (channel 2) for red fluorescence, respectively. The filter for the detection of Ax emission (BA610IF) was different from the former experiment (BA565IF) to minimize the possible detection of MitoTracker-derived marginal red fluorescence, while the detection range was still within the range of Ax emission [19]. Moreover, the possibility of detecting this marginal fluorescence was excluded by the photomultiplier setting that had been validated through the staining of blastocysts cultured without Ax.
Effects of Ax on mitochondrial membrane potential (ΔΨm) under heat-stressed conditions
The ≥8-cell embryos on day 3 were cultured with or without Ax basically at 38.5 °C and the heat-stressed group embryos were subjected to 40.5 °C/10 h/day on days 4 and 5 as described above. On day 6, embryos except for degenerated ones were incubated for 15 min with JC-1 (ImmunoChemistry Technologies, Bloomington, MN) in pre-equilibrated IVCM at 38.5 °C. After the incubation, embryos were washed with 50 μl of IVCM twice and then transferred to a 1-μl IVCM drop covered with mineral oil in a 4-well dish. The fluorescence of JC-1 J-aggregates (red) and monomers (green), which correspond to mitochondria with higher and lower membrane potentials, respectively, were captured using a fluorescence microscope (FSX100, Olympus) and measured using Image J software (National Institute of Health, Bethesda, MD). The ΔΨm of embryos was evaluated using the red/green fluorescence ratio. Data were obtained from 36 to 38 embryos per group in three replicates.
Statistical analysis
Blastocyst development data (Tables 1 and 2) were analyzed by the least squares analysis of variance (ANOVA) using the Mixed Models of SPSS (Chicago, IL). In all the models, replicates were included as a random variable. Fixed variables were treatment groups in the analysis for Table 1; heat stress, Ax, and their interaction for Table 2. When ANOVA showed a significant difference, comparisons among the treatments were performed by the least significant difference method. The data obtained in JC-1 staining were analyzed using non-parametric Kruskal–Wallis test followed by pairwise Mann–Whitney U-test. Significance was accepted at P < 0.05.
Table 1.
Effects of single or repeated heat stress on the blastocyst development of bovine embryos
| Treatment | No. of ≥8-cell embryos | No. of replicates | Blastocyst development %(least square means ± SEM) |
|---|---|---|---|
| Control | 73 | 4 | 40.6 ± 3.8a |
| Heat on day 4 | 72 | 4 | 31.8 ± 3.8ab |
| Heat on day 4 and 5 | 72 | 4 | 21.5 ± 3.8b |
a,bValues without common superscripts differ significantly (P = 0.015)
Table 2.
Effects of heat stress and astaxanthin (Ax) on the blastocyst development of bovine embryos
| Treatment | No. of ≥8-cell embryos | No. of replicates | Blastocyst development %(least square means ± SEM) | |
|---|---|---|---|---|
| Heat | Ax | |||
| − | − | 86 | 5 | 47.9 ± 5.2a |
| − | + | 94 | 5 | 44.2 ± 5.2a |
| + | − | 90 | 5 | 17.8 ± 5.2b |
| + | + | 91 | 5 | 38.5 ± 5.2a |
a,bValues without common superscripts differ significantly (P < 0.05)
Effects of Heat (P = 0.004), Ax (P = 0.131), and Heat x Ax (P = 0.036)
Results
We first investigated the effects of single or repeated exposure to heat stress during day 4 and 5 post-IVF on blastocyst development from ≥8-cell embryos (Table 1). Although single exposure to heat stress on day 4 did not significantly affect the rates of blastocyst formation, daily repeated exposure to heat stress on days 4 and 5 did significantly reduce blastocyst development as compared with the control (P < 0.05).
Next, the effects of Ax on impaired blastocyst development following repeated heat exposure were investigated (Table 2). As in the above experiment, daily repeated exposure to heat stress on days 4 and 5 decreased the rate of blastocyst development (P < 0.01). The addition of Ax into culture media ameliorated impaired blastocyst development following the heat stress. The addition of Ax to the normal thermal conditions did not affect blastocyst development. The effect of heat stress (P = 0.004) and the heat stress × Ax interaction (P = 0.036) were both significant.
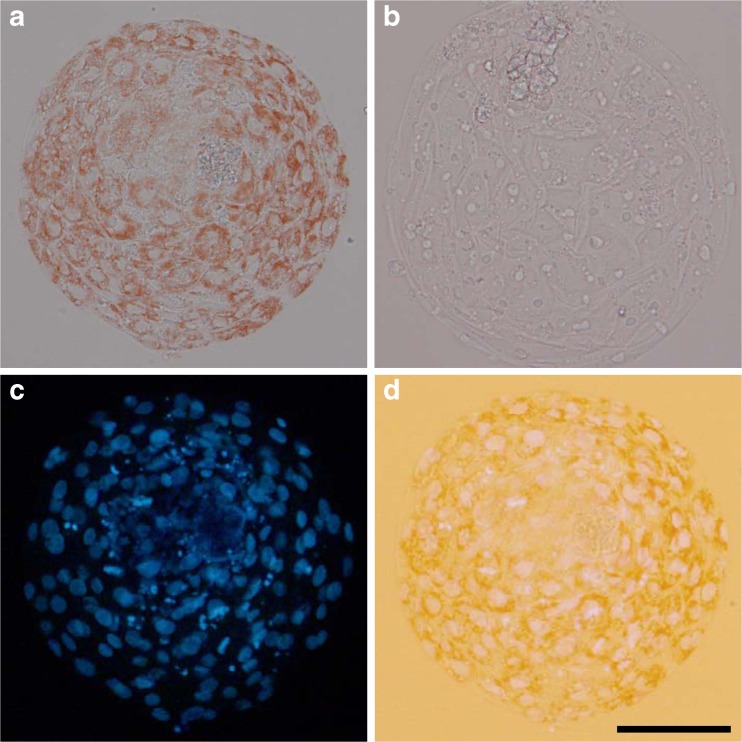
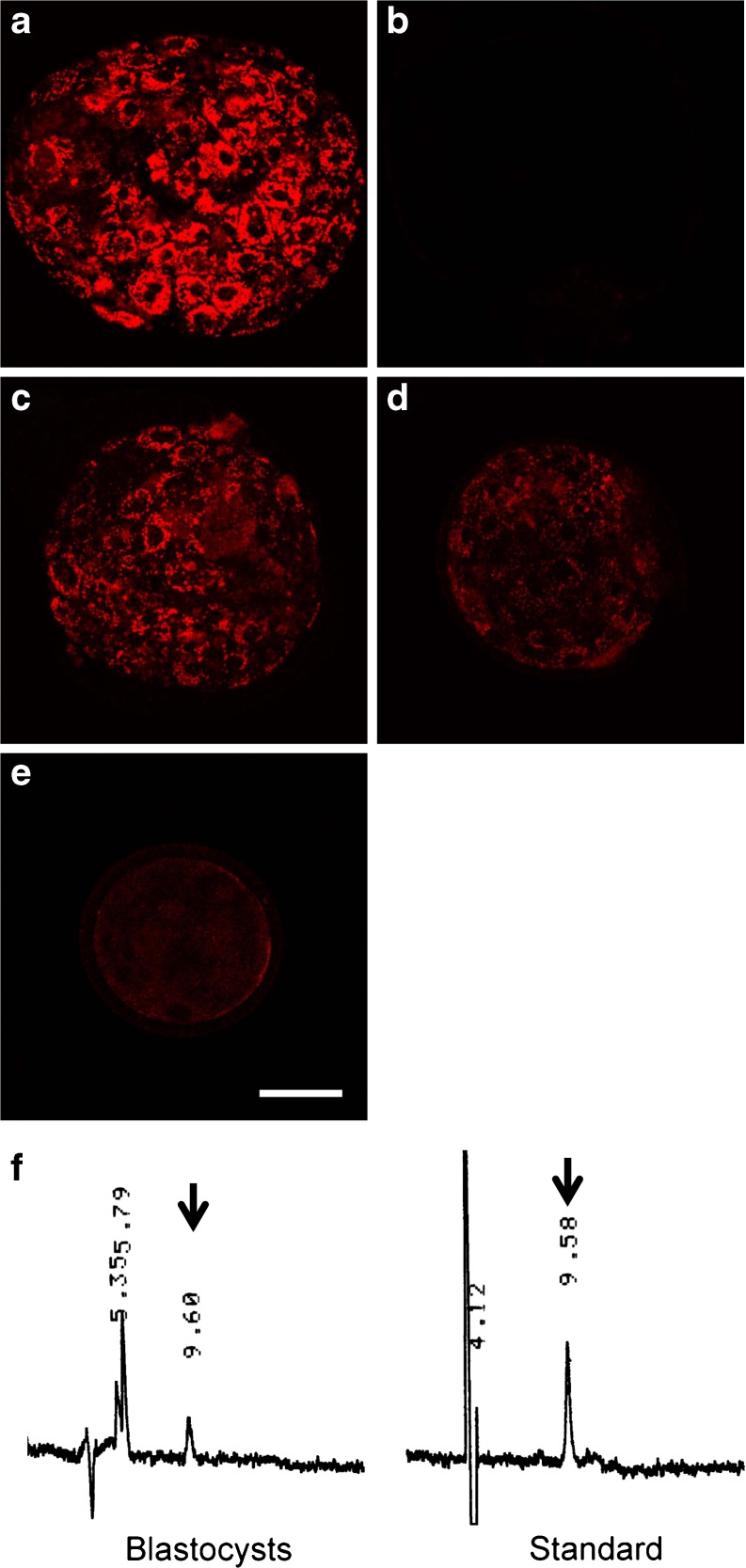
The cellular uptake of Ax in bovine preimplantation embryos was examined using three methodologies. First, blastocysts that were hatching or completely hatched from their zona pellucida were visibly red under bright-field microscopy in the culture with Ax (Fig. 1a), whereas their counterparts in the absence of Ax did not exhibit red coloring (Fig. 1b). Blastocysts that had not started hatching (pre-hatching blastocysts) did not exhibit visible red coloring even in the presence of Ax (data not shown). Furthermore, nuclear-staining with Hoechst 33342 showed that red pigments accumulated in the cytoplasm of the embryos (Fig. 1c, d). Second, based on the fluorescence emission of Ax [19], Ax was detected by laser-scanning confocal microscopy. Blastocysts that completely hatched exhibited relatively strong red fluorescence (Fig. 2a), whereas hatched blastocysts in the absence of Ax did not exhibit such fluorescence (Fig. 2b). Fluorescence was also seen in the hatching (Fig. 2c) and pre-hatching blastocysts (Fig. 2d), and day 5 morula stage embryos (Fig. 2e) to a lesser extent as compared with hatched blastocysts. In addition, Ax content in blastocysts was determined by HPLC. The extracts from blastocysts (pre-hatching to hatched stage) exhibited a chromatographic peak corresponding to Ax (Fig. 2f), and Ax content in the blastocysts was calculated as 0.02 ng/embryo.
Fig. 1.
“Red blastocyst” observed in culture with Ax. Bright-field images of hatched blastocysts cultured in the presence a or absence b of Ax. c Nuclear-stained image of the blastocyst shown in a. d Transmitted light image of c. Bar represents 100 μm
Fig. 2.
Ax detection in bovine embryos. a-d Laser-scanning confocal observation of Ax in blastocysts cultured with or without Ax. a Hatched blastocyst in Ax treatment, b hatched blastocyst in the vehicle control, c hatching blastocyst, d pre-hatching blastocysts, and e morula in Ax treatment. Morulae and blastocysts were fixed on day 5 and 10, respectively. Bar represents 50 μm. f HPLC chromatograms of the extract from 100 blastocysts (pre-hatching to hatched stage) cultured in the presence of Ax and a standard Ax sample. Arrows indicate peaks corresponding to Ax
The relationship between the site of Ax accumulation and the location of mitochondria was investigated in hatched blastocysts which exhibited strong Ax-derived fluorescence. Ax was observed to coincide with or lined the mitochondria in blastocyst cells (Fig. 3). Because of the lesser Ax-derived fluorescence, it was difficult to assess the relationship between Ax and mitochondria localization in the earlier developmental stages.
Fig. 3.
Colocalization of Ax and mitochondria in blastocyst cells. a Confocal observation of Ax, b mitochondria (MitoTracker Green FM-staining), and c merged images of blastocyst cells in the Ax treatment. Ax coincided with (arrowheads) or lined (arrows) the mitochondria. Bar represents 10 μm
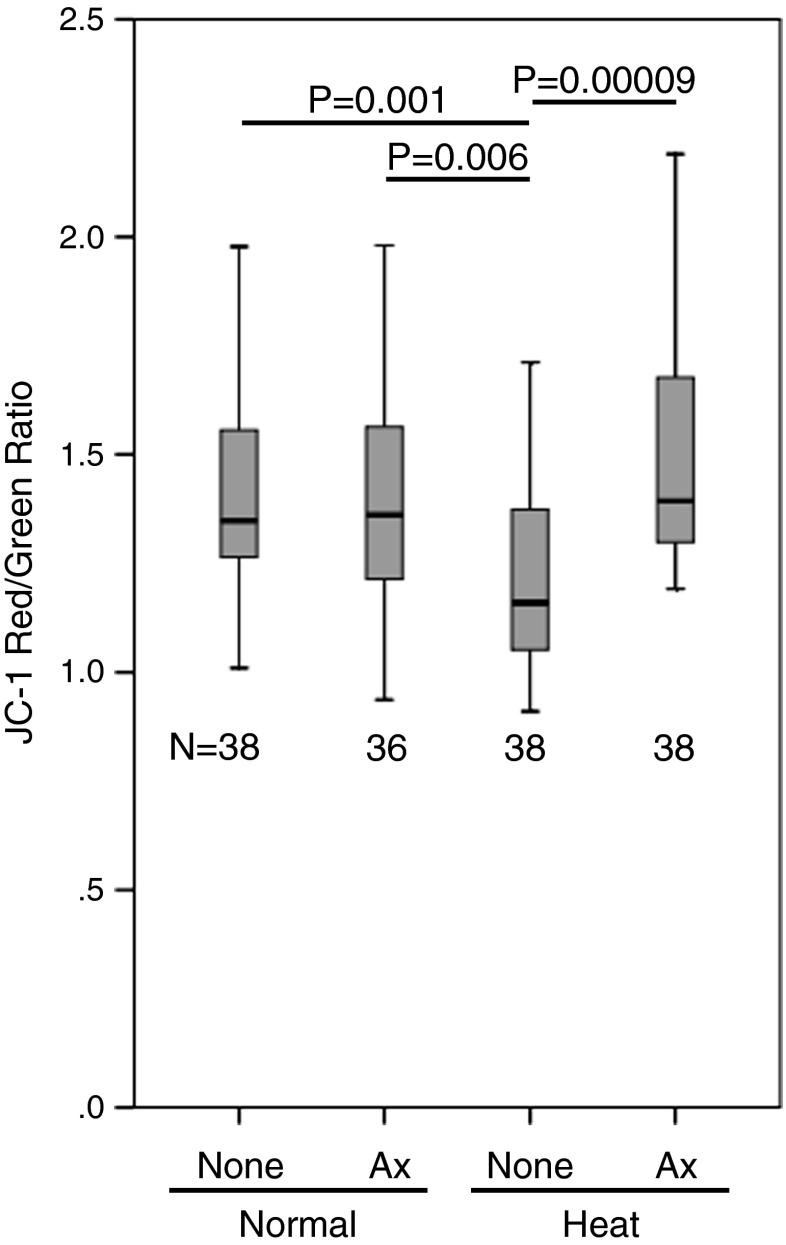
Because the colocalization of Ax with mitochondria was suggested, the effects of Ax on the mitochondrial membrane potential (ΔΨm) after heat stress were investigated using JC-1, a dual fluorescent dye (Fig. 4). JC-1 can selectively enter into mitochondria where it reversibly changes its fluorescence from green to red as ΔΨm increases [43]. Heat stress decreased (P < 0.01) the ΔΨm in day 6 embryos in the absence of Ax. However, Ax significantly (P < 0.0001) recovered the ΔΨm under the heat-stressed condition, which resulted in a level similar to the normal thermal condition (Fig. 4).
Fig. 4.
The effects of Ax in combination with heat stress on the mitochondrial membrane potential (ΔΨm). The fluorescence ratios of JC-1 staining on day 6 are shown. The top, middle line, and bottom of the box represent the 75th percentile, median, and 25th percentile, respectively. The whiskers represent the highest and lowest values that are not outliers or extreme values. N denotes the number of embryos per each group. P value for Kruskal–Wallis test is 0.0003. P values shown in the figure are from pairwise Mann–Whitney U-test
Discussion
Compared with the oocyte stage and the early cleavage stage (1- to 8-cell) of mammalian preimplantation development, later-stage embryos (≥8-cell) are more resistant to heat stress [4, 10, 40]. However, the results of our first experiment indicate that later-stage embryos, when exposed to repeated, not single as previous reports [4, 10, 40], heat stress on two consecutive days can show impaired blastocyst development. Using this developmental inhibition model, we examined whether Ax treatment in culture can ameliorate the detrimental effects of heat stress. We found that Ax increased blastocyst development under the heat-stressed condition. We previously reported the heat stress-ameliorating effects of Ax on early cleavage stage (1- to 8-cell) embryos [30]. The present result therefore further demonstrates the favorable effects of Ax on the later stages of preimplantation development.
It is, however, unknown how Ax restores proper embryonic development following heat stress. Over the course of our culture experiments, we observed “red blastocysts” in Ax-treated cultures and the red color was observed in the cytoplasm of the embryonic cells (Fig. 1). The visibly red blastocysts were restricted to embryos that were hatching or completely hatched from the zona pellucida. However, laser excitation at 488 nm resulted in fluorescence emission even from pre-hatching blastocysts and also from day 5 embryos that were not visibly red (Fig. 2d, e). Considering the excitation spectra of Ax [19], the red fluorescence from Ax-treated blastocysts is considered to be due to Ax that has been incorporated into the cells. Furthermore, Ax was detected by HPLC analysis of blastocyst extracts (Fig. 2f). From these results, it can be concluded that Ax was incorporated into the cytoplasm of bovine preimplantation embryos. This is the first report that demonstrates the uptake and accumulation of carotenoids in mammalian preimplantation embryos. The lower intensities of visible and fluorescent red coloring in pre-hatching blastocysts as compared with post-hatching blastocysts suggest that the zona pellucida affects Ax uptake.
The confocal observations of Ax and mitochondria demonstrated that Ax effectively colocalized with mitochondria. Two patterns of colocalization were observed, i.e., Ax coincided with the mitochondria or it lined the mitochondria (Fig. 3). The accumulation of Ax in mitochondria has been reported in other cell and tissue types, including leukocytes [33], mesangial cells [25], liver, and muscles [45]. The result of our present study suggests the direct action of Ax on mitochondria in bovine preimplantation embryos.
Mitochondria are responsible for the vital regulation of cellular homeostasis through the metabolism of respiratory substrate, OXPHOS, ion homeostasis, ROS production, and apoptosis [13, 35]. Several lines of investigation have suggested the possible associations of high-potential mitochondria with the ability of matured oocytes to be fertilized and the developmental competence of postfertilization embryos [9, 49, 51]. Furthermore, the influence of environmental insults, including heat stress, on mitochondrial function in mammalian oocytes has been documented previously [29, 44]. Therefore, we further examined the effects of Ax in combination with heat stress on the membrane potential of mitochondria (ΔΨm) of day 6 embryos (exclusively morula stage). As a result, the heat treatment on day 4 and 5 resulted in decreased ΔΨm on day 6 (Fig. 4). Interestingly, Ax treatment effectively (P < 0.0001) recovered the impaired ΔΨm in the heat-stressed condition. Although the ΔΨm-promoting effect of Ax was previously shown in cultured HeLa cells [54], the present results show for the first time that the effect has an interaction with the heat-stress condition in preimplantation embryos. In contrast to the case of oocytes [29, 44] and preimplantation embryos (present results), heat stress enhanced the ΔΨm in some cases, including peripheral blood mononuclear cells [6] and skeletal muscles [3, 28]. In these cases, heat-induced ΔΨm elevation acts in a cell-protective manner against cell perturbation. Therefore, it is plausible that higher levels of ΔΨm in embryos are beneficial for cellular survival, especially under heat-stressed conditions.
Bovine oocytes and preimplantation embryos represent a popular animal model for studying the development of human counterparts because of the similarity and/or proximity in the mono-ovulatory manner, cell size, timing of development and embryonic genome activation, and the mode of cell-lineage determination [2, 22, 27, 32]. Regarding mitochondria, oocytes in both species contain varied number of mitochondria, i.e., 20,000 to over 800,000 in human [48] and 2,000 to 1,200,000 in bovine [47] on mitochondrial DNA (mtDNA) basis. In human IVF, higher numbers of mtDNA are correlated with higher fertilizability or developmental competence of resulting eggs after fertilization [36, 42]. Along with the number of mitochondria, the higher ΔΨm has been proposed as a relevant factor to developmental competence also in human oocytes and preimplantation embryos [1, 20, 50, 52]. Sufficient number and activity of mitochondria are considered to be crucial for generating energy through the formation of ATP, which is required for proper embryonic development [49, 51]. As in the present study, physiological, environmental, or clinical factors can decrease ΔΨm in human oocytes and early embryos, which is one of proposed causes of their compromised quality and IVF outcome. These factors include advanced age of women [34, 52], clinical hyperstimulation protocol [8], cryopreservation [20], and the condition of in vitro culture [53]. Therefore, ΔΨm-promoting effects of Ax in challenged conditions shown in the present study may be applicable for ameliorating the effects of deleterious factors involved in human IVF. On the other hand, it is to be noted that human oocytes and preimplantation embryos are anecdotally known to contain much less intracellular lipid compared with bovine [24, 26]. Taking the lipophilic property of Ax into account, there may be differences in the level of Ax accumulation between human and bovine cases; consequently, Ax may give rise to different actions on these cells reflecting its different interactions with cellular lipid components depending on the species. Therefore, further studies are needed in order to elucidate the effects of Ax in different species including human.
In summary, the present study demonstrated that the blastocyst development of ≥8-cell stage bovine embryos was inhibited by exposure to repeated heat stress and that the addition of Ax ameliorated heat stress-induced developmental inhibition. Furthermore, Ax was incorporated into the cytoplasm of embryos, preferentially localized with mitochondria, and recovered the heat stress-induced reduction of ΔΨm. These results suggest that the direct action of Ax on mitochondria which enhances ΔΨm under the heat-stressed condition is a mechanism of the ameliorating effects of Ax on heat stress-induced developmental defects.
Acknowledgements
The authors thank the staff at the second wholesale market of Kyoto City for providing bovine ovaries. We appreciate the donation of bull semen from the Laboratory of Reproductive Biology, Kyoto University. This work was supported in part by ASKA Pharmaceutical Co., Ltd. and JSPS KAKENHI Grant Number 24658233.
Footnotes
Capsule
Astaxanthin, mitochondria and heat-stressed embryo.
References
- 1.Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod. 2004;10(1):23–32. doi: 10.1093/molehr/gah004. [DOI] [PubMed] [Google Scholar]
- 2.Adjaye J, Herwig R, Brink TC, Herrmann D, Greber B, Sudheer S, Groth D, Carnwath JW, Lehrach H, Niemann H. Conserved molecular portraits of bovine and human blastocysts as a consequence of the transition from maternal to embryonic control of gene expression. Physiol Genomics. 2007;31(2):315–27. doi: 10.1152/physiolgenomics.00041.2007. [DOI] [PubMed] [Google Scholar]
- 3.Azad MA, Kikusato M, Sudo S, Amo T, Toyomizu M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp Biochem Physiol A Mol Integr Physiol. 2010;157(3):266–71. doi: 10.1016/j.cbpa.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Bonilla AQ, Oliveira LJ, Ozawa M, Newsom EM, Lucy MC, Hansen PJ. Developmental changes in thermoprotective actions of insulin-like growth factor-1 on the preimplantation bovine embryo. Mol Cell Endocrinol. 2011;332(1–2):170–9. doi: 10.1016/j.mce.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Caserta D, Mantovani A, Marci R, Fazi A, Ciardo F, La Rocca C, Maranghi F, Moscarini M. Environment and women’s reproductive health. Hum Reprod Update. 2011;17(3):418–33. doi: 10.1093/humupd/dmq061. [DOI] [PubMed] [Google Scholar]
- 6.Chiu HY, Tsao LY, Yang RC. Heat-shock response protects peripheral blood mononuclear cells (PBMCs) from hydrogen peroxide-induced mitochondrial disturbance. Cell Stress Chaperones. 2009;14(2):207–17. doi: 10.1007/s12192-008-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cow–a review. Theriogenology. 2003;60(6):1139–51. doi: 10.1016/S0093-691X(03)00126-2. [DOI] [PubMed] [Google Scholar]
- 8.Dell’Aquila ME, Ambruosi B, De Santis T, Cho YS. Mitochondrial distribution and activity in human mature oocytes: gonadotropin-releasing hormone agonist versus antagonist for pituitary down-regulation. Fertil Steril. 2009;91(1):249–55. doi: 10.1016/j.fertnstert.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20(3):346–53. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Edwards JL, Hansen PJ. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol Reprod Dev. 1997;46(2):138–45. doi: 10.1002/(SICI)1098-2795(199702)46:2<138::AID-MRD4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Gomez E, Caamano JN, Rodriguez A, De Frutos C, Facal N, Diez C. Bovine early embryonic development and vitamin A. Reprod Domest Anim. 2006;41(Suppl 2):63–71. doi: 10.1111/j.1439-0531.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 12.Hansen PJ. To be or not to be–determinants of embryonic survival following heat shock. Theriogenology. 2007;68(Suppl 1):S40–8. doi: 10.1016/j.theriogenology.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Harvey A, Gibson T, Lonergan T, Brenner C. Dynamic regulation of mitochondrial function in preimplantation embryos and embryonic stem cells. Mitochondrion. 2011;11(5):829–38. doi: 10.1016/j.mito.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46(2):185–96. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 15.Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod. 2006;69(3):443–9. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- 16.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5(4):e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda S, Kitagawa M, Imai H, Yamada M. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. J Reprod Dev. 2005;51(1):23–35. doi: 10.1262/jrd.51.23. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda S, Sugimoto M, Kume S. Importance of methionine metabolism in morula-to-blastocyst transition in bovine preimplantation embryos. J Reprod Dev. 2012;58(1):91–7. doi: 10.1262/jrd.11-096H. [DOI] [PubMed] [Google Scholar]
- 19.Jørgensen K, Stapelfeldt H, Skibsted LH. Fluorescence of carotenoids. Effect of oxygenation and cis/trans isomerization. Chem Phys Lett. 1992;190(5):514–9. doi: 10.1016/0009-2614(92)85183-B. [DOI] [Google Scholar]
- 20.Jones A, Van Blerkom J, Davis P, Toledo AA. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential: implications for developmental competence. Hum Reprod. 2004;19(8):1861–6. doi: 10.1093/humrep/deh313. [DOI] [PubMed] [Google Scholar]
- 21.Koyama H, Ikeda S, Sugimoto M, Kume S. Effects of folic acid on the development and oxidative stress of mouse embryos exposed to heat stress. Reprod Domest Anim. 2012;47(6):921–7. [DOI] [PubMed]
- 22.Leidenfrost S, Boelhauve M, Reichenbach M, Gungor T, Reichenbach HD, Sinowatz F, Wolf E, Habermann FA. Cell arrest and cell death in mammalian preimplantation development: lessons from the bovine model. PLoS One. 2011;6(7):e22121. doi: 10.1371/journal.pone.0022121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Gatius F. Factors of a noninfectious nature affecting fertility after artificial insemination in lactating dairy cows. A review. Theriogenology. 2012;77(6):1029–41. doi: 10.1016/j.theriogenology.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Yang X, Liang X. Obesity does not aggravate vitrification injury in mouse embryos: a prospective study. Reprod Biol Endocrinol. 2012;10:68. doi: 10.1186/1477-7827-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manabe E, Handa O, Naito Y, Mizushima K, Akagiri S, Adachi S, Takagi T, Kokura S, Maoka T, Yoshikawa T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J Cell Biochem. 2008;103(6):1925–37. doi: 10.1002/jcb.21583. [DOI] [PubMed] [Google Scholar]
- 26.McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil. 2000;118(1):163–70. doi: 10.1530/reprod/118.1.163. [DOI] [PubMed] [Google Scholar]
- 27.Menezo YJ, Herubel F. Mouse and bovine models for human IVF. Reprod Biomed Online. 2002;4(2):170–5. doi: 10.1016/S1472-6483(10)61936-0. [DOI] [PubMed] [Google Scholar]
- 28.Mujahid A, Akiba Y, Toyomizu M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R690–8. doi: 10.1152/ajpregu.90974.2008. [DOI] [PubMed] [Google Scholar]
- 29.Nabenishi H, Takagi S, Kamata H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. The role of mitochondrial transition pores on bovine oocyte competence after heat stress, as determined by effects of cyclosporin A. Mol Reprod Dev. 2012;79(1):31–40. doi: 10.1002/mrd.21401. [DOI] [PubMed] [Google Scholar]
- 30.Namekawa T, Ikeda S, Sugimoto M, Kume S. Effects of astaxanthin-containing oil on development and stress-related gene expression of bovine embryos exposed to heat stress. Reprod Domest Anim. 2010;45(6):e387–91. doi: 10.1111/j.1439-0531.2010.01584.x. [DOI] [PubMed] [Google Scholar]
- 31.Negro-Vilar A. Stress and other environmental factors affecting fertility in men and women: overview. Environ Health Perspect. 1993;101(Suppl 2):59–64. doi: 10.1289/ehp.93101s259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niakan KK, Eggan K. Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev Biol. 2013;375(1):54–64. doi: 10.1016/j.ydbio.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Park JS, Kim HW, Mathison BD, Hayek MG, Massimino S, Reinhart GA, Chew BP. Astaxanthin uptake in domestic dogs and cats. Nutr Metab (Lond) 2010;7:52 [DOI] [PMC free article] [PubMed]
- 34.Picton HM, Elder K, Houghton FD, Hawkhead JA, Rutherford AJ, Hogg JE, Leese HJ, Harris SE. Association between amino acid turnover and chromosome aneuploidy during human preimplantation embryo development in vitro. Mol Hum Reprod. 2010;16(8):557–69. doi: 10.1093/molehr/gaq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update. 2009;15(5):553–72. doi: 10.1093/humupd/dmp016. [DOI] [PubMed] [Google Scholar]
- 36.Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F, Barriere P, Malthiery Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7(5):425–9. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 37.Rivera RM, Hansen PJ. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction. 2001;121(1):107–15. doi: 10.1530/rep.0.1210107. [DOI] [PubMed] [Google Scholar]
- 38.Roth Z, Aroyo A, Yavin S, Arav A. The antioxidant epigallocatechin gallate (EGCG) moderates the deleterious effects of maternal hyperthermia on follicle-enclosed oocytes in mice. Theriogenology. 2008;70(6):887–97. doi: 10.1016/j.theriogenology.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 39.Saeki K, Hoshi M, Leibfried-Rutledge ML, First NL. In vitro fertilization and development of bovine oocytes matured with commercially available follicle stimulating hormone. Theriogenology. 1990;34:1035–9. doi: 10.1016/S0093-691X(05)80002-0. [DOI] [Google Scholar]
- 40.Sakatani M, Kobayashi S, Takahashi M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol Reprod Dev. 2004;67(1):77–82. doi: 10.1002/mrd.20014. [DOI] [PubMed] [Google Scholar]
- 41.Sakatani M, Suda I, Oki T, Kobayashi S, Takahashi M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J Reprod Dev. 2007;53(3):605–14. doi: 10.1262/jrd.18124. [DOI] [PubMed] [Google Scholar]
- 42.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85(3):584–91. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991;88(9):3671–5. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soto P, Smith LC. BH4 peptide derived from Bcl-xL and Bax-inhibitor peptide suppresses apoptotic mitochondrial changes in heat stressed bovine oocytes. Mol Reprod Dev. 2009;76(7):637–46. doi: 10.1002/mrd.20986. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi K, Watanabe M, Takimoto T, Akiba Y. Uptake and distribution of astaxanthin in several tissues and plasma lipoproteins in male broiler chickens fed a yeast (Phaffia rhodozyma) with a high concentration of astaxanthin. Br Poult Sci. 2004;45(1):133–8. doi: 10.1080/00071660410001668950a. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi Y, First NL. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology. 1992;37(5):963–78. doi: 10.1016/0093-691X(92)90096-A. [DOI] [PubMed] [Google Scholar]
- 47.Tamassia M, Nuttinck F, May-Panloup P, Reynier P, Heyman Y, Charpigny G, Stojkovic M, Hiendleder S, Renard JP, Chastant-Maillard S. In vitro embryo production efficiency in cattle and its association with oocyte adenosine triphosphate content, quantity of mitochondrial DNA, and mitochondrial DNA haplogroup. Biol Reprod. 2004;71(2):697–704. doi: 10.1095/biolreprod.103.026104. [DOI] [PubMed] [Google Scholar]
- 48.Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128(3):269–80. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 49.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17(2):393–406. doi: 10.1093/humrep/17.2.393. [DOI] [PubMed] [Google Scholar]
- 51.Wilding M, Coppola G, Dale B, Di Matteo L. Mitochondria and human preimplantation embryo development. Reproduction. 2009;137(4):619–24. doi: 10.1530/REP-08-0444. [DOI] [PubMed] [Google Scholar]
- 52.Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16(5):909–17. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 53.Wilding M, Fiorentino A, De Simone ML, Infante V, De Matteo L, Marino M, Dale B. Energy substrates, mitochondrial membrane potential and human preimplantation embryo division. Reprod Biomed Online. 2002;5(1):39–42. doi: 10.1016/S1472-6483(10)61595-7. [DOI] [PubMed] [Google Scholar]
- 54.Wolf AM, Asoh S, Hiranuma H, Ohsawa I, Iio K, Satou A, Ishikura M, Ohta S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J Nutr Biochem. 2010;21(5):381–9. doi: 10.1016/j.jnutbio.2009.01.011. [DOI] [PubMed] [Google Scholar]