Abstract
Deletions encompassing the X-linked STS gene (encoding steroid sulfatase) have been observed in subjects with neurodevelopmental disorders, including attention deficit hyperactivity disorder (ADHD). Recently, two single nucleotide polymorphisms (SNPs) within STS (rs12861247 and rs17268988) have been reported to be associated with ADHD risk and inattentive symptoms in ADHD, respectively. Using a UK sample of ADHD subjects (aged 5–18 years), we tested the hypothesis that rs12861247 is associated with ADHD risk using a case–control approach (comparing 327 ADHD cases with 358 male controls from the Wellcome Trust Case Control Consortium). Using a subset of males from the ADHD sample, we also examined whether variation within STS is associated with symptomatology/cognitive function in ADHD. We then tested whether SNPs associated with cognitive function in ADHD were also associated with cognitive function in healthy male subjects using a German sample (n = 143, aged 18–30 years), and whether STS was expressed in brain regions pertinent to ADHD pathology during development. We did not replicate the previously identified association with rs12861247. However, in ADHD males, variation at rs17268988 was associated with inattentive symptoms, while variation within STS was significantly associated with performance on three cognitive measures. Three SNPs associated with cognitive function in ADHD males were not associated with cognitive function in healthy males. STS was highly expressed in the developing cerebellar neuroepithelium, basal ganglia, thalamus, pituitary gland, hypothalamus and choroid plexus. These data suggest that genetic variants affecting STS expression and/or activity could influence the function of brain regions perturbed in ADHD.
Keywords: Association, attention, brain, cognition, dehydroepiandrosterone sulfate, neurosteroid, picture completion, testosterone, Wechsler Intelligence scales
Cytogenetic deletions on the short arm of the X chromosome encompassing the steroid sulfatase (STS) gene have been noted in cases of neurodevelopmental disorders associated with abnormal cognition, including attention deficit hyperactivity disorder (ADHD) (Doherty et al. 2003; Lonardo et al. 2007; Tobias et al. 2001), schizophrenia (Milunsky et al. 1999) and autism (Shinawi et al. 2009; Thomas et al. 1999; Vorstman et al. 2006). These findings suggest the possibility that a lack of the brain-expressed protein encoded by STS, the enzyme steroid sulfatase, might influence vulnerability to, or the presentation of, these particular disorders. The function of STS is to cleave sulfate groups from a variety of steroid hormones [e.g. dehydroepiandrosterone sulfate (DHEAS)], thereby modulating their neural function and activity (Reed et al. 2005). Dehydroepiandrosterone sulfate and its non-sulfated form DHEA are metabolized to a number of biologically significant molecules including testosterone (Reed et al. 2005), and influence key neurodevelopmental processes (Herbert 1998). Abnormal DHEA(S) levels have been implicated in the pathogenesis of a number of neuropsychiatric disorders, including schizophrenia (Maninger et al. 2009).
The link between STS dysfunction and ADHD (a disorder associated with attention deficits, impulsivity, hyperactivity, cognitive abnormalities and aggression in some subjects) has been strengthened by four observations: first, subjects with deletions of STS, or with inactivating mutations within the gene (and thus presenting with the dermatological condition X-linked ichthyosis, XLI), are at significantly increased risk of developing ADHD (Kent et al. 2008). Second, two single nucleotide polymorphisms (SNPs) within STS (rs2270112 and rs12861247) have been reported to be associated with ADHD, although only the latter survived correction for multiple testing; in the same study SNP rs17268988 was associated with inattentive symptoms (Brookes et al. 2008). Third, systemic levels of DHEA appear to correlate with ADHD symptomatology, while methylphenidate treatment may exert its therapeutic effect in ADHD through increasing DHEA levels (Golubchik et al. 2007). Finally, deletion of the STS gene in mice (or inhibition of the associated enzyme) is associated with attention deficits, with alterations in impulsivity, and with elevated aggression (Davies et al. 2009; Mortaud et al. 2010; Nicolas et al. 2001).
The aims of the present study were fourfold: (1) to try and replicate the most robust association finding previously identified with rs12861247 using a case–control protocol, (2) to investigate how variation across the STS gene might influence symptomatology and general cognitive function in ADHD subjects; with regard to effects on symptomatology, we focussed on possible association with inattentive, impulsive and aggressive symptoms given the previous findings in mouse and man, (3) to ascertain whether variants associated with symptomatology and/or cognitive function in an ADHD sample were also associated with effects on cognition in healthy volunteers and (4) to identify the pattern of expression of STS in the developing human brain with a view to understanding how STS activity might influence normal neurodevelopment, and thereby to understand whether there are plausible biological underpinnings to any observed genetic associations with cognitive endophenotypes.
Materials and methods
Case–control study: ADHD and control samples
A sample of 393 British Caucasian subjects in total (352 males, 41 females) between the ages of 5 and 18 years who had recently been diagnosed with ADHD was recruited from Child and Adolescent Psychiatrists and Paediatricians in the Greater Manchester, South Wales and Avon areas of the United Kingdom. The presence of symptoms and research diagnoses of Diagnostic and Statistical Manual of Mental Disorders, Version IV (DSM-IV ) ADHD and comorbid conditions were obtained from parent reports using the Child and Adolescent Psychiatric Assessment (CAPA) (Angold & Costello 2000), with pervasiveness of ADHD assessed using the Child ADHD Teacher Telephone Interview (ChATTI) (Holmes et al. 2004). All assessments were conducted by psychology graduates trained to high levels of inter-rater reliability. Individuals were excluded from the study if they had a full-scale IQ < 70, Tourette's syndrome, any neurological condition (including epilepsy) or a pervasive developmental disorder. To further assess this last criterion, participants were also assessed using the Autism Screening Questionnaire (Berument et al. 1999), and those scoring >14 were excluded from the study. All individuals met DSM-IV criteria for ADHD. The overall composition of our sample was 6% inattentive subtype, 81% combined subtype and 13% hyperactive-impulsive subtype, rates consistent with a previous study examining association in STS in UK and Irish ADHD samples (Brookes et al. 2008). Subjects with ADHD were genotyped at rs12861247 for the case–control analysis.
The control sample genotyped at rs12861247 for the case–control study (hereafter referred to as the control group) consisted of 360 males from the Wellcome Trust Case Control Consortium 1958 Birth Cohort sample, used in previous association studies in Cardiff (Wellcome Trust Case Control Consortium 2007). These control subjects, like the ADHD sample, comprise British Caucasians, and are thought to faithfully represent the genome of this population, with little evidence for population stratification (Wellcome Trust Case Control Consortium 2007). Sufficient control subjects were genotyped such that the combined sample size was calculated a priori to give a power of >80% to detect an odds ratio of >1.52, and a power of 100% to detect an odds ratio of >2.00, assuming a disorder prevalence of 6% (Thapar et al. 2005) (Brookes et al. 2008, association of rs12861247 with ADHD gave rise to an odds ratio of 2.05, with a lower 95% confidence interval of 1.88).
Symptomatology and cognitive analysis in ADHD subjects
Of the overall ADHD sample described above, a total of 266 male subjects were used to assay the effects of STS variation on symptomatology and cognition. Importantly, given the possible long- and short-term effects of medication on behavior and cognition, these subjects had been on medication for <1 year, and had been medication-free for at least 24 h prior to assessment. The mean age of this subset of subjects was 111.5 ± 1.5 months.
Using the CAPA, the number of parent-reported DSM-IV inattentive symptoms (maximum nine) and impulsive symptoms (maximum four) was ascertained (hyperactivity symptoms were also assessed, but were not analyzed here as there was no prior evidence for STS effects on this measure). The presence of DSM-IV aggressive conduct disorder symptoms, again ascertained using the CAPA, was also investigated. This subgroup of conduct disorder symptoms consists of seven measures: (1) often bullies, threatens or intimidates others, (2) often initiates physical fights, (3) has used a weapon that can cause serious physical harm to others, (4) has been physically cruel to people, (5) has been physically cruel to animals, (6) has stolen while confronting a victim and (7) has forced someone into sexual activity. The final measure was not assessed in this sample, resulting in a maximum of six possible aggressive symptoms being present.
Cognitive performance in this sample was tested using the Wechsler Intelligence Scales for Children Version III (WISC-III; Wechsler 1991). This assessment of cognitive ability consists of 13 subtests, 10 of which are used to calculate a standardized full-scale IQ score. Based on previous studies discriminating individuals with ADHD from control subjects using Wechsler scales (Antshel et al. 2007; Ek et al. 2007; Filippatou & Livaniou 2005; Mayes & Calhoun 2004; Pineda et al. 1999), we investigated performance on six measures by genotype: information, coding, block design, freedom from distractibility (derived from scores on the arithmetic and digit span subtests), picture completion and comprehension. We also examined performance on two general measures of cognition (performance IQ and verbal IQ) by genotype. Parents provided written informed consent, and children informed assent, prior to the assessments. The studies involving ADHD subjects were approved by North West and Wales Multicentre Research Ethics Committees and local National Health Service Research and Development offices.
Healthy volunteer samples and assessments
For this study, all available male participants aged 18–30 years (total n = 143, hereafter referred to as the healthy volunteer group) were drawn from a sample of 2337 healthy volunteers of German descent who were randomly selected from the general population of Munich, Germany, and contacted by mail. To exclude subjects with central neurological diseases and psychotic disorders or subjects who had first-degree relatives with psychotic disorders, several screenings were conducted before the volunteers were enrolled in our study. First, subjects who responded were initially screened by telephone for the absence of neuropsychiatric disorders. Second, detailed medical and psychiatric histories were assessed for both themselves and their first-degree relatives by using a semistructured interview. Third, if no exclusion criteria were fulfilled, the subjects were invited to a comprehensive interview including the Structured Clinical for DSM-IV Axis I Disorders – Patient Edition (First et al. 1995) and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (First et al. 1990) to validate the absence of any lifetime psychotic disorder. In addition, the Family History Assessment Module (Rice et al. 1995) was conducted to exclude psychotic disorders among first-degree relatives. A neurological examination was also conducted to exclude subjects with current central nervous system impairment. Subjects were administered the German version of the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Tewes 1991, the adult equivalent of the WISC-III) using all 11 subtests (vocabulary, comprehension, information, digit span, arithmetic, similarities, block design, picture completion, picture arrangement, object assembly and digit symbol coding). The recruitment, genotyping and behavioral testing of the healthy volunteer participants were approved by the Institutional Review Board of the Ludwig-Maximilians-University of Munich, Germany.
Genotyping in ADHD and control samples
Genomic DNA was extracted from saliva or whole blood samples according to routine procedures. Single-nucleotide polymorphism rs12861247 was genotyped in the ADHD sample and in the control group using TaqMan SNP genotyping assay C_321340_10 according to the manufacturer's instructions (Applied Biosystems, Warrington, UK).
Subjects for which symptomatology and cognitive data are presented were genotyped for eight Tag-SNPs covering the promoter and coding regions of the STS gene (Table 1). Single-nucleotide polymorphisms were chosen using Haploview Tagger (Barrett et al. 2005) at r2 = 0.8 in order to achieve widespread coverage of the gene locus (tagging ∼68% of the STS gene). Six of these SNPs (with the exceptions of rs12861247 and rs5978405) were genotyped in 233 ADHD males using a Sequenom MassARRAY system and Sequenom iPLEX GOLD chemistry according to the manufacturer's instructions. Assays were designed using Assay Design software version 3.1 (Sequenom Inc., San Diego, CA, USA) and genotyping was performed using Typer software version 4.0 (Sequenom Inc.). All thermocycling reactions were performed using an MJ Thermocycler. Specific details of reaction conditions and primers are available on request. Quality control assessment of genotyping assays after optimization involved analysis in the CEU individuals genotyped as part of the HapMap project prior to genotyping in the association sample (http://www.hapmap.org). Only assays with 100% concordance with HapMap genotypes were analyzed further. All association sample plates contained cases, controls, blanks and CEU samples. Genotypes were called in duplicate blind to sample identity and blind to the other rater. Single-nucleotide polymorphism rs12861247 was genotyped as described above. rs5978405 was genotyped using TaqMan SNP genotyping assay C_30005846_10 according to the manufacturer's instructions (Applied Biosystems). Linkage disequilibrium analysis was performed using Haploview (Barrett et al. 2005).
Table 1.
Allelic frequency at SNPs across the STS promoter and coding regions
| SNP assayed | Location of SNP within gene | Minor allele frequency from HapMap CEU sample (both sexes)/males only | Minor allele frequency from Brookes et al. (2008) Dublin ADHD sample | Minor allele frequency from Brookes et al. (2008) St Andrews ADHD sample | Minor allele frequency from present ADHD sample | Minor allele frequency from present healthy volunteer sample |
|---|---|---|---|---|---|---|
| rs5934671 | Putative promoter | 0.33/0.27 | N/A | N/A | 0.34 | N/A |
| rs2270112 | Intron 1 | 0.26/0.33 | 0.27 | 0.26 | 0.31 | N/A |
| rs12861247 | Intron 2 | 0.20/0.20 | 0.10 | 0.09 | 0.10 | N/A |
| rs5978405 | Intron 6 | 0.44/0.40 | N/A | N/A | 0.41 | 0.37 |
| rs17268974 | Intron 7 | 0.24/0.21 | N/A | N/A | 0.24 | N/A |
| rs4403552 | Intron 7 | 0.30/0.30 | 0.25 | 0.24 | 0.21 | N/A |
| rs17268988 | Intron 9 | 0.20/0.27 | 0.21 | 0.21 | 0.24 | 0.35 |
| rs5933863 | 3′-UTR | 0.22/0.20 | 0.16 | 0.16 | 0.17 | 0.11 |
3′-UTR, 3′-untranslated region.
HapMap minor allele frequencies are reported for combined male and female samples (n = 60) and for male founder samples alone (n = 30). Each of the eight SNPs assayed in our ADHD sample was genotyped in ≥179 males, and each of the three SNPs assayed in the healthy volunteer group was genotyped in ≥134 males.
Genotyping in healthy volunteer samples
Three SNPs, rs5978405, rs5933863 and rs17268988, which showed evidence for association (replicated in the case of rs17268988) with symptomatology/cognitive measures in the ADHD sample, were successfully genotyped in healthy volunteer subjects using the Sequenom MassARRAY system; rs12861247, which also showed strong evidence for association, could not be typed using this panel. Genomic DNA was extracted from whole blood samples according to routine procedures. One nanogram of DNA was assayed using the iPLEX assay on the MassARRAY MALDI-TOF mass spectrometer (SEQUENOM, Hamburg, Germany). DNA concentration was adjusted using the PicoGreen quantitation reagent (Invitrogen, Karlsruhe, Germany). Quality control assessment of genotyping assays involved analysis in the CEU individuals genotyped as part of the HapMap project (http://www.hapmap.org). Only assays with 100% concordance with HapMap genotypes were analyzed further.
Statistical analysis
The case–control data were analyzed using plink 1.06, which takes into account the location of the SNPs on the X chromosome (Purcell et al. 2007). Behavioral and cognitive data are presented as mean values ± standard error of the mean, and were analyzed using spss 16. As symptomatology data were not normally distributed, they were analyzed by Mann–Whitney U test or Kruskal–Wallis test to test for allelic association. Wechsler scale subtest scores were tested for statistical normality by Shapiro–Wilk's test, transformed if appropriate, and then subjected to two-tailed t-tests to compare allelic effects. Scores which were not normally distributed and which could not be transformed to approximate a normal distribution were compared using the Mann–Whitney U test. Nominal P values are reported. P values of <0.05 following extremely conservative Bonferroni correction for multiple testing (taking into account the number of SNPs genotyped and the number of phenotypes assayed) were regarded as significant (Goodarzi et al. 2007), i.e. P values <0.00078 prior to correction were regarded as significant in the ADHD sample analysis, while P values <0.0024 prior to correction were regarded as significant in the healthy volunteer sample.
In situ hybridization
A polymerase chain reaction (PCR) product corresponding to NM_000351.3 (bp 2570–3249) was subcloned into pGEM-T Easy vector (Promega, Southampton, UK). The vector was linearized using SalI or NcoI restriction enzymes (Promega), and transcribed using T7 or Sp6 polymerases to generate antisense or sense ribonucleotide probes, respectively. Digoxigenin-uridine triphosphate was incorporated into riboprobes during in vitro transcription using DIG RNA labeling mix (Roche Diagnostics, Burgess Hill, UK) according to the manufacturer's instructions. Labeled probes were then hybridized to human embryonic or fetal tissue obtained from the Human Developmental Biology Resource (University College London Institute of Child Health, London, UK) according to standard protocols (Wilkinson 1992).
Results
Case–control analysis
For our case–control study, a total of 327 ADHD cases (290 males, 37 females) and 358 male control subjects were successfully genotyped for rs12861247. The frequency of the minor allele (A) at this SNP did not differ significantly between the two groups: 0.107 in cases (0.097 in males and 0.149 in females) and 0.101 in control subjects, P = 0.762, odds ratio = 1.08, 95% confidence interval 0.667–1.737. When the analysis was limited to males only, again there was no significant difference between cases and controls (P = 0.888, odds ratio = 0.96, 95% confidence interval 0.570–1.614). Data from the females genotyped indicated that there was no significant deviation from Hardy–Weinberg equilibrium at this SNP (27 GG homozygotes, 9 GA heterozygotes, 1 AA homozygote, two-tailed Fisher's exact test, P = 1.0).
Genotyping and allelic frequency in the ADHD sample
Our genotyping success rate across all eight SNPs analyzed in the ADHD sample for which behavioral/cognitive data are presented was 90.5 ± 2.6%. The minor allele frequencies we observed were comparable with those seen previously in British and Irish ADHD samples (Brookes et al. 2008), and with those reported in residents of Utah with White European ancestry (the CEU population) in the HapMap database (Table 1). The Tag SNP markers that we analyzed were not in high-linkage disequilibrium with one another (Fig. 1).
Figure 1. The r2 linkage disequilibrium between typed markers across the STS locus in our ADHD sample.
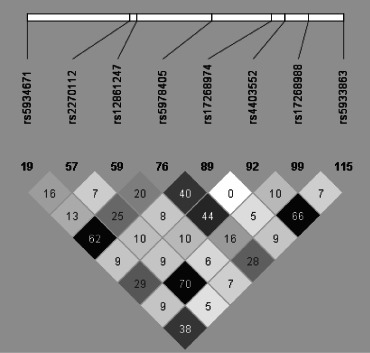
The Tag SNP markers analysed were not in high linkage disequilibrium with one another.
Genotyping and allelic frequency in the healthy volunteer sample
Our genotyping success rate across the three SNPs analyzed in the healthy volunteer sample was 96.3 ± 1.3%. Minor allele frequencies within the healthy volunteer sample are shown in Table 1.
Association between STS variants and symptomatology in male ADHD subjects
As the Y-linked homolog of STS is a non-expressed pseudogene (Yen et al. 1988), males will be hemizygous for STS alleles; hence, we initially looked for association in males, in that genotype–phenotype correlations should be easier to interpret in this sex. We found nominally significant evidence for allelic variation at one SNP (rs17268988) being associated with the number of DSM-IV inattentive symptoms, with males possessing a G allele (n = 51) showing more inattentive symptoms than those possessing the more common C allele (n = 163) (U = 4906, nominal P value = 0.026; Table 2). Importantly, the ages of the G- and C-allele groups were equivalent (Table S1). Although this result did not remain significant upon correcting for multiple comparisons, as it replicated a previous observation (Brookes et al. 2008), we decided to follow it up further. Specifically, given that ADHD is a developmental disorder characterized by symptom reduction with age (Langley et al. 2009), we tested whether the effect of the SNP on inattentive symptoms was age dependent, by subdividing our male sample in two around the median age of 109 months: subjects <109 months of age (C: n = 79, G: n = 24) and subjects ≥109 months of age (C: n = 84, G: n = 27); this resulted in two evenly sized groups (and hence maximal power to detect an age-dependent effect). We found no effect of allelic variation on inattentive symptoms in the younger subgroup (U = 915, P = 0.80), but a significant effect in the older subgroup, with males possessing a G allele showing, on average, 0.8 more inattentive symptoms than males possessing a C allele (U = 1544, P = 0.005) (Fig. 2). None of the other SNPs assayed were significantly associated with DSM-IV inattentive, impulsivity or aggressive symptoms (Table 2).
Table 2.
Relationship between STS genotype and DSM-IV symptoms in ADHD males
| SNP | Genotype | Inattentive symptoms | Nominal P value | Impulsivity symptoms | Nominal P value | Aggressive symptoms | Nominal P value |
|---|---|---|---|---|---|---|---|
| rs5934671 | C (n = 143) | 6.98 ± 0.14 | 0.85 | 3.26 ± 0.08 | 0.62 | 0.48 ± 0.07 | 0.73 |
| G (n = 74) | 6.96 ± 0.21 | 3.18 ± 0.11 | 0.42 ± 0.09 | ||||
| rs2270112 | C (n = 149) | 6.94 ± 0.14 | 0.62 | 3.22 ± 0.08 | 0.79 | 0.46 ± 0.07 | 0.82 |
| G (n = 68) | 7.05 ± 0.21 | 3.26 ± 0.11 | 0.47 ± 0.10 | ||||
| rs12861247 | G (n = 186) | 6.95 ± 0.13 | 0.37 | 3.24 ± 0.07 | 0.87 | 0.46 ± 0.06 | 0.98 |
| A (n = 17) | 7.41 ± 0.33 | 3.24 ± 0.25 | 0.47 ± 0.21 | ||||
| rs5978405 | T (n = 116) | 7.04 ± 0.16 | 0.34 | 3.33 ± 0.08 | 0.14 | 0.52 ± 0.08 | 0.48 |
| A (n = 85) | 6.79 ± 0.20 | 3.13 ± 0.11 | 0.40 ± 0.09 | ||||
| rs17268974 | T (n = 165) | 6.91 ± 0.14 | 0.51 | 3.26 ± 0.07 | 0.46 | 0.47 ± 0.06 | 0.87 |
| A (n = 53) | 7.17 ± 0.20 | 3.17 ± 0.13 | 0.43 ± 0.11 | ||||
| rs4403552 | G (n = 141) | 7.08 ± 0.14 | 0.08 | 3.22 ± 0.08 | 0.45 | 0.46 ± 0.07 | 0.62 |
| A (n = 38) | 6.47 ± 0.30 | 3.08 ± 0.17 | 0.50 ± 0.13 | ||||
| rs17268988 | C (n = 163) | 6.90 ± 0.13 | 0.03 | 3.25 ± 0.07 | 0.79 | 0.46 ± 0.06 | 0.95 |
| G (n = 51) | 7.31 ± 0.26 | 3.24 ± 0.12 | 0.47 ± 0.12 | ||||
| rs5933863 | G (n = 180) | 7.10 ± 0.12 | 0.13 | 3.23 ± 0.07 | 0.94 | 0.50 ± 0.07 | 0.59 |
| A (n = 36) | 6.56 ± 0.32 | 3.25 ± 0.16 | 0.33 ± 0.10 |
Nominal P values <0.05 are highlighted in bold.
Figure 2. Relationship between genotype at rs17268988, age and DSM-IV inattentive symptoms in ADHD males.
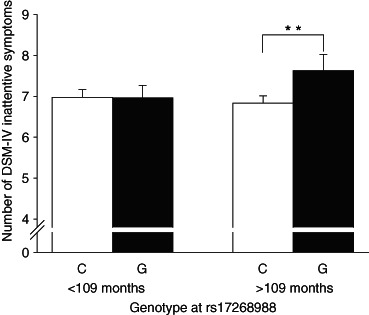
In ADHD males aged <109 months, genetic variation at rs17268988 appeared to have no effect on the number of DSM-IV inattentive symptoms (C: n = 79, G: n = 24). However, in subjects ≥109 months, the presence of a G allele at this SNP (n = 27) was associated with a significantly greater number of inattentive symptoms than the presence of a C allele (n = 84) (**P≤ 0.005).
Association between STS variants and cognitive function in male ADHD subjects
Wechsler Intelligence Scales for Children Version III data were not available for eight case individuals due to subject refusal or because they had previously been tested on an alternative IQ test. Of those subjects who were tested, rarely (<5% of the time) data could not be obtained for particular subtests. As with the symptomatology analysis, association with STS variants was initially tested in males only (Table 3). One SNP (rs5933863) was highly significantly associated with performance on the picture completion subtest (G: n = 171, A: n = 36) (U = 1909, P = 0.0003, P < 0.05 after correction for multiple testing), with a second SNP rs4403552 showing suggestive evidence for association with this subtest (G: n = 171, A: n = 36) (U = 3553, nominal P value = 0.0021). Significant associations were also noted between rs5978405 and verbal IQ (t[185] = 3.556, P = 0.000478) and between rs12861247 and comprehension subtest performance (U = 1970, P = 0.000052), indicated by corrected P values <0.05 in each case. These positive results cannot be attributed to age-dependent effects (Table S2).
Table 3.
Relationship between STS genotype and performance on WISC-III measures in ADHD males
| SNP | Genotype | PIQ | Nominal P value | VIQ | Nominal P value | I | Nominal P value | C | Nominal P value | BD | Nominal P value | FFD | Nominal P value | PC | Nominal P value | CP | Nominal P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs5934671 | C (n = 135) | 92.0 ± 1.2 | 0.28 | 91.4 ± 1.0 | 0.12 | 8.8 ± 0.3 | 0.035 | 8.4 ± 0.3 | 0.59 | 8.4 ± 0.3 | 0.93 | 89.8 ± 1.2 | 0.16 | 10.0 ± 0.2 | 0.16 | 8.2 ± 0.3 | 0.83 |
| G (n = 73) | 89.8 ± 1.4 | 88.6 ± 1.4 | 8.0 ± 0.3 | 8.4 ± 0.4 | 8.4 ± 0.4 | 87.9 ± 1.4 | 9.3 ± 0.3 | 8.2 ± 0.4 | |||||||||
| rs2270112 | C (n = 144) | 90.5 ± 1.1 | 0.30 | 89.8 ± 1.0 | 0.30 | 8.3 ± 0.2 | 0.083 | 8.4 ± 0.3 | 0.85 | 8.3 ± 0.3 | 0.57 | 89.1 ± 1.0 | 0.71 | 9.7 ± 0.2 | 0.66 | 8.3 ± 0.3 | 0.41 |
| G (n = 64) | 92.6 ± 1.7 | 91.7 ± 1.6 | 9.0 ± 0.4 | 8.5 ± 0.4 | 8.6 ± 0.4 | 89.3 ± 1.9 | 9.8 ± 0.3 | 7.9 ± 0.4 | |||||||||
| rs12861247 | G (n = 181) | 90.9 ± 1.0 | 0.77 | 90.3 ± 1.0 | 0.033 | 8.6 ± 0.2 | 0.28 | 8.3 ± 0.2 | 0.96 | 8.4 ± 0.2 | 0.62 | 89.5 ± 1.0 | 0.42 | 9.4 ± 0.2 | 0.043 | 7.9 ± 0.2 | 0.0001 |
| A (n = 15) | 92.3 ± 4.1 | 97.5 ± 2.6 | 9.4 ± 0.7 | 8.1 ± 0.7 | 8.6 ± 0.9 | 92.2 ± 3.2 | 10.9 ± 0.8 | 10.8 ± 0.5 | |||||||||
| rs5978405 | T (n = 105) | 92.5 ± 1.4 | 0.06 | 93.5 ± 1.3 | 0.000478 | 9.1 ± 0.3 | 0.0021 | 8.7 ± 0.3 | 0.16 | 8.4 ± 0.3 | 0.90 | 91.9 ± 1.4 | 0.016 | 9.8 ± 0.3 | 0.13 | 8.5 ± 0.3 | 0.018 |
| A (n = 82) | 89.0 ± 1.2 | 87.1 ± 1.2 | 7.9 ± 0.3 | 8.0 ± 0.3 | 8.3 ± 0.3 | 86.5 ± 1.2 | 9.1 ± 0.3 | 7.5 ± 0.3 | |||||||||
| rs17268974 | T (n = 157) | 91.0 ± 1.1 | 0.63 | 91.1 ± 1.0 | 0.19 | 8.8 ± 0.2 | 0.024 | 8.5 ± 0.3 | 0.52 | 8.4 ± 0.2 | 0.61 | 89.4 ± 1.1 | 0.70 | 9.7 ± 0.2 | 0.56 | 8.2 ± 0.2 | 0.78 |
| A (n = 52) | 91.9 ± 1.7 | 88.5 ± 1.6 | 7.8 ± 0.3 | 8.2 ± 0.5 | 8.5 ± 0.5 | 88.4 ± 1.5 | 9.9 ± 0.4 | 8.1 ± 0.4 | |||||||||
| rs4403552 | G (n = 133) | 92.0 ± 1.2 | 0.041 | 91.8 ± 1.1 | 0.020 | 8.7 ± 0.2 | 0.19 | 8.2 ± 0.3 | 0.95 | 8.5 ± 0.3 | 0.27 | 90.6 ± 1.1 | 0.072 | 10.1 ± 0.2 | 0.0021 | 8.3 ± 0.3 | 0.20 |
| A (n = 38) | 87.0 ± 1.8 | 86.5 ± 1.9 | 8.1 ± 0.4 | 8.2 ± 0.6 | 8.1 ± 0.4 | 86.5 ± 2.2 | 8.7 ± 0.4 | 7.6 ± 0.5 | |||||||||
| rs17268988 | C (n = 157) | 90.8 ± 1.0 | 0.36 | 89.8 ± 1.0 | 0.15 | 8.4 ± 0.2 | 0.11 | 8.4 ± 0.3 | 0.68 | 8.3 ± 0.2 | 0.27 | 89.2 ± 1.0 | 0.35 | 9.7 ± 0.2 | 0.49 | 8.2 ± 0.2 | 0.87 |
| G (n = 48) | 92.8 ± 2.0 | 92.8 ± 1.9 | 9.1 ± 0.4 | 8.3 ± 0.5 | 8.9 ± 0.5 | 89.7 ± 2.2 | 10.0 ± 0.4 | 8.2 ± 0.4 | |||||||||
| rs5933863 | G (n = 171) | 92.2 ± 1.0 | 0.056 | 91.5 ± 0.9 | 0.024 | 8.7 ± 0.2 | 0.15 | 8.4 ± 0.3 | 0.94 | 8.5 ± 0.2 | 0.37 | 89.9 ± 1.0 | 0.14 | 10.1 ± 0.2 | 0.0003 | 8.3 ± 0.2 | 0.22 |
| A (n = 36) | 87.6 ± 1.9 | 86.4 ± 2.0 | 7.9 ± 0.5 | 8.4 ± 0.5 | 8.2 ± 0.4 | 87.2 ± 2.2 | 8.4 ± 0.4 | 7.7 ± 0.5 |
BD, block design; C, coding; CP, comprehension; FFD, freedom from distractibility; I, information; PC, picture completion; PIQ, performance IQ; VIQ, verbal IQ.
Nominal P values <0.05 are highlighted in bold.
Association between STS variants and WAIS-R subtest scores in male healthy volunteer subjects
To simplify data interpretation and to provide maximum comparability with the ADHD data, association between cognitive measures and STS variants at SNPs rs5978405, rs5933863 and rs17268988 was initially tested in hemizygous young, male healthy volunteer subjects; cognitive data were obtained for all of these subjects. Data from measures analogous to those in the WISC-III were examined (performance and verbal IQs, verbal information, digit symbol coding, block design, picture completion and verbal comprehension). We detected no evidence for association between the SNPs tested and any of the cognitive measures examined within our healthy volunteer sample (Table 4).
Table 4.
Relationship between STS genotype and performance on WAIS-R subtests in healthy male volunteers ≤30 years of age
| SNP | Genotype | PIQ | Nominal P value | VIQ | Nominal P value | I | Nominal P value | C | Nominal P value | BD | Nominal P value | PC | Nominal P value | CP | Nominal P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs5978405 | T (n = 84) | 118.5 ± 1.4 | 0.07 | 119.2 ± 1.5 | 0.59 | 18.7 ± 0.4 | 0.42 | 63.3 ± 1.0 | 0.25 | 41.3 ± 0.6 | 0.097 | 14.9 ± 0.2 | 0.90 | 22.8 ± 0.3 | 0.24 |
| A (n = 50) | 114.3 ± 1.9 | 117.9 ± 1.8 | 18.2 ± 0.5 | 61.3 ± 1.4 | 38.9 ± 1.0 | 14.7 ± 0.3 | 22.4 ± 0.4 | ||||||||
| rs17268988 | C (n = 91) | 116.2 ±1.5 | 0.88 | 118.2 ± 1.5 | 0.84 | 18.5 ± 0.4 | 0.23 | 60.8 ± 1.0 | 0.089 | 40.5 ± 0.7 | 0.19 | 14.8 ± 0.2 | 0.90 | 22.6 ± 0.3 | 0.41 |
| G (n = 48) | 115.8 ± 1.6 | 117.7 ± 1.8 | 18.0 ± 0.5 | 63.8 ± 1.4 | 39.4 ± 0.8 | 14.8 ± 0.3 | 22.4 ± 0.4 | ||||||||
| rs5933863 | G (n = 124) | 116.6 ± 1.2 | 0.56 | 118.4 ± 1.2 | 0.52 | 18.5 ± 0.3 | 0.62 | 62.4 ± 0.9 | 0.13 | 40.2 ± 0.6 | 0.48 | 14.8 ± 0.2 | 0.76 | 22.6 ± 0.3 | 0.28 |
| A (n = 16) | 114.6 ± 2.7 | 116.4 ± 2.8 | 17.8 ± 1.1 | 58.5 ± 2.1 | 39.4 ± 1.5 | 15.1 ± 0.3 | 22.2 ± 0.6 |
BD, block design; C, coding; CP, comprehension; I, information; PC, picture completion; PIQ, performance IQ; VIQ, verbal IQ.
In situ hybridization
At Carnegie stage 18 (44 days of gestation), STS was expressed highly in various epithelial tissues, including cells of the olfactory epithelium, the subthalamic and thalamic neuroepithelia, and in the rhombencephalic choroid plexus (Fig. 3a–d). High levels of expression were also noted in the developing pituitary, particularly the ventral portion of the anterior lobe (Fig. 3e). The gene appeared to be expressed throughout much of the rest of the developing brain, but at much lower levels. Outside the brain, high levels of expression were seen in the developing tongue (Fig. S1). At Carnegie stage 23 (56–57 days of gestation), STS was expressed highly in the cortical plate, widely within the developing thalamus and at high levels throughout the choroid plexus (Fig. 3f). At 9 weeks of gestation, high expression levels were observed in the cerebellar neuroepithelium, widely within the thalamus, and also within the basal ganglia, hypothalamus and pituitary gland; lower expression levels were seen in the developing neocortex (Fig. 3g). High expression was also noted in the olfactory epithelium and in the thyroid gland. No hybridization signal was observed in tissue sections hybridized with the sense riboprobe (Fig. 3h).
Figure 3. Pattern of STS expression (blue staining) in embryonic and fetal human brain.
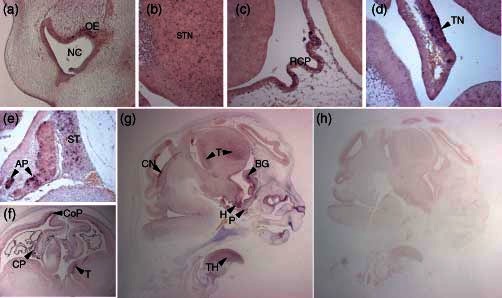
(a–e) Sagittal sections from CS18 (44 days of gestation) embryo. (a) OE, olfactory epithelium; NC, nasal cavity (×10 magnification). (b) STN, subthalamic neuroepithelium (×20 magnification). (c) RCP, rhombencephalic choroid plexus (×20 magnification). (d) TN, thalamic neuroepithelium (×20 magnification). (e) AP, anterior lobe of the pituitary gland; ST, sella turcica (×20 magnification). (f) Coronal brain section from CS23 (56–57 days of gestation) embryo. CoP, cortical plate; CP, choroid plexus; T, thalamus (×5 magnification). (g and h) Sagittal sections from subjects at 9 weeks of gestation. (g) T, thalamus; BG, basal ganglia; CN, cerebellar neuroepithelium; H, hypothalamus; P, pituitary gland; TH, thyroid gland (×1 magnification). (h) No blue staining was evident in tissue hybridized with sense riboprobes.
Discussion
The primary aim of this study was to examine whether genetic variants within the X-linked STS gene, encoding the enzyme steroid sulfatase, were associated with vulnerability to ADHD and with cognitive function in ADHD and healthy subjects. A secondary aim was to map the expression of STS during development, with a view to understanding how its altered expression/function might influence ADHD endophenotypes.
Using a case–control approach, we did not replicate the previously reported association between SNP rs12861247 and ADHD, despite having sufficient power to replicate the relatively large reported effect (for SNP association to a complex disorder) (Brookes et al. 2008). Both the family study by Brookes et al. (2008) and its recent extended follow-up (Brookes et al. 2010) were relatively small, and the previously reported association may therefore have been a false positive. However, it is possible that the original finding is a true positive, but that the true effect size is smaller than previously reported, and consequently our analysis was underpowered to detect it. To exclude that possibility very much larger sample sizes are needed. An alternative interpretation is that there are no common variants within STS that influence ADHD risk, and that only complete loss of function of the gene (as in the mutant mouse and XLI studies) results in ADHD endophenotypes. It is also important to be aware of two possible limitations of our control sample (from the Wellcome Trust Case Control Consortium): first, it was not specifically matched for IQ to our ADHD sample, although we did exclude ADHD subjects with evidence of learning disability (IQ < 70) from our study. Second, no formal assessment of childhood ADHD symptoms was made in this sample; it is plausible that the presence of subjects with undiagnosed ADHD in the control group may have slightly biased our study toward producing a type II error, although given the incidence of ADHD, this would be expected to have little impact on power (Moskvina et al. 2005).
It is also plausible that polymorphisms within STS may be associated with a particular ADHD subtype. Our data in mice suggest that a lack of Sts results in attentional deficits, but reduced impulsivity (Davies et al. 2009), while data from XLI subjects suggest that STS dysfunction predisposes mainly to inattentive subtype ADHD (Kent et al. 2008). It may therefore be worthwhile testing explicitly for association between STS polymorphisms and the inattentive ADHD subtype in a large multicenter sample.
Nevertheless, our data suggest that variation within this gene might influence the phenotypic presentation in ADHD subjects. We replicated the association previously observed between rs17268988 and inattentive symptoms (Brookes et al. 2008) in our sample. Specifically, we showed that possession of a G allele at this locus was associated with a greater number of inattentive symptoms, particularly in older boys. This age-dependent effect could be due to the cumulative deleterious effects of possessing this allele or, more speculatively, to some interaction between STS function and steroid hormone biosynthesis around puberty; interestingly, plasma DHEAS concentrations are higher in children older than 9 years of age than in younger children (Smith et al. 1989). We have preliminary evidence that variation at rs17268988 is also associated with aspects of cognition (block design) in schizophrenia (data not shown). Variation within STS was associated with performance on the picture completion subtest in ADHD males. In this test, subjects must identify a missing item from a series of pieces of artwork within a limited time frame. The task taxes three major psychological processes: distinguishing relevant from irrelevant information, visual alertness and long-term visual memory (Groth-Marnat 1999). Data from mice indicate that visual alertness (‘visuospatial attention’) may be influenced by STS function (Davies et al. 2009), while picture completion performance has been suggested as a reasonable predictor of attentional function in children and adults (Fuentes et al. 2003; Groth-Marnat & Baker 2003). Moreover, STS inhibition in rats has been shown to elicit effects on spatial memory (Johnson et al. 2000). Hence, it is plausible that the observed associations with picture completion performance are consequent upon primary associations with attentional function and/or memory. We also found significant association between STS variants and performance on the verbal IQ and comprehension subtests; it is noteworthy that in the latter case, association was seen with rs12861247, the SNP identified as being associated with ADHD and altered frontal cortex gene expression by Brookes et al. [specifically, inferior performance was seen in subjects possessing the presumed ‘risk’ allele (G)] (Brookes et al. 2008, 2010).
An important potential limitation with studies such as this is the possibility of type I errors. The fact that the results above remain significant upon correction for stringent multiple testing, that the association between rs5933863 and picture completion is replicated in a small sample of ADHD females whereby homozygotes for the G allele significantly outperform heterozygotes and homozygotes for the A allele (data not shown), and that the only subject from previous publications (of whom we are aware) with STS deficiency and ADHD tested using the WISC (family 2, case C) has been reported to show an uneven cognitive profile, with relative strength in picture completion and weakness in comprehension (Kent et al. 2008), argues against the reported findings being spurious and suggests that STS may truly influence performance in these areas in ADHD subjects.
We could find no evidence that the three SNPs genotyped in healthy male volunteers were associated with cognitive function. This may be because the associations are only manifest in the context of an abnormally developing brain, because the associations vary with age, because of subtle methodological differences between the WISC-III and the WAIS-R, because any effects within this homogeneous population would be too small to detect with our limited sample size or because the findings from the ADHD sample were indeed false positives. However, as we have argued above, we believe that the latter possibility is unlikely.
STS was found to be most highly expressed in various epithelia during development, including the olfactory, thalamic and cerebellar neuroepithelia. High STS expression was also observed in the choroid plexus, the hypothalamus and pituitary gland, the thalamus and the basal ganglia. Expression was noted throughout much of the rest of the brain at lower levels, including the developing neocortex. These findings are broadly consistent with previous limited data examining the localization of STS protein/enzyme activity in specific adult brain regions in man (Kriz et al. 2008; Steckelbroeck et al. 2004), monkeys (Kriz et al. 2005) and bovines (Park et al. 1997); they are also consistent with data from studies examining the expression of STS in the developing brains of rodents (Compagnone et al. 1997) and frogs (Cevasco et al. 2009). It is likely that STS activity in the aforementioned brain regions affects their development, and possibly their ongoing function in adulthood. By extension, altered STS expression is likely to adversely affect the development of these structures. Hence, high levels of STS expression in the thalamus, basal ganglia and cerebellar neuroepithelium (which gives rise to GABAergic and glutamatergic neurons of the cerebellum; Hoshino et al. 2005) were of particular interest, given prior data suggesting aberrant development of these regions in both ADHD (Dickstein et al. 2006; Perlov et al. 2009; Silk et al. 2009; Valera et al. 2007) and schizophrenia (Ellison-Wright & Bullmore 2010; Maloku et al. 2010). Similarly, altered STS function in the hypothalamus/pituitary gland, olfactory epithelium and tongue could feasibly play a role in the altered hypothalamic-pituitary-adrenal axis responsivity (van West et al. 2009), odor detection abnormalities (Karsz et al. 2008; Romanos et al. 2008) and tongue characteristics (Atmetlla et al. 2006) previously described in ADHD.
Recent data from monkeys have shown that the thalamus is a key mediator of visual attention (Rees 2009). Although little is known about the neurobiological correlates of subtest performance on the Wechsler scales, there is some evidence that picture completion performance is relatively specifically affected in subjects with cerebellar lesions (Molinari et al. 2004; Ruessmann et al. 1989; Schmahmann & Sherman 1998), and is correlated with midline cerebellar volume in adolescents born preterm (Allin et al. 2005), implying a role for the cerebellum in performing this cognitive task. Furthermore, picture completion is the most highly correlated WISC-III subtest with basal ganglia volume in children born preterm (Peterson et al. 2000), implicating this second brain structure in picture completion performance. In contrast, block design performance does not appear to be markedly affected by cerebellar lesions (Molinari et al. 2004), but may be specifically affected by thalamic lesion (Carlesimo et al. 2007). Hence, the expression patterns of STS are consistent with the associated protein having discrete effects on attention and other aspects of cognition.
Several of the analyses presented herein are exploratory and, as such, should be confirmed in alternative samples. However, when taken together, these data suggest the possibility that variation within STS might be associated with altered brain development and affect the severity of inattention symptoms in ADHD. It is possible that there is an association with cognitive function in ADHD, but some caution is required due to testing of multiple phenotypes.
Acknowledgments
The authors' work is supported by the Medical Research Council (MRC) UK, The Wellcome Trust, Action Medical Research, the German Research Council (DFG), Cardiff University and a Research Councils UK Fellowship to W.D. The authors would like to thank the ADHD and healthy volunteer subjects and their families for their participation in this research, the field teams for their help with the interviews and Professor Stephanie van Goozen for critical reading of the manuscript. Human embryonic material was provided by the Medical Research Council/Wellcome Trust Human Developmental Biology Resource.
The authors declare that they have no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1: STS expression was detectable (bluestaining) in the mid and surface layers of the tongue at CS18 (44days of gestation) (sagittal section, ×5 magnification).
Table S1: The ages of male ADHD subjects with major and minor alleles at rs17268988 at the time of assessment of DSM-IV ADHD symptoms did not differ.
Table S2: The ages of male ADHD subjects with major and minor alleles at SNPs rs12861247, rs5978405 and rs5933863 at the time of assessment of WISC-III scores did not differ.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Allin MP, Salaria S, Nosarti C, Wyatt J, Rifkin L, Murray RM. Vermis and lateral lobes of the cerebellum in adolescents born very preterm. Neuroreport. 2005;16:1821–1824. doi: 10.1097/01.wnr.0000185014.36939.84. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) J Am Acad Child Adolesc Psychiatry. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Faraone SV, Stallone K, Nave A, Kaufmann FA, Doyle A, Fried R, Seidman L, Biederman J. Is attention deficit hyperactivity disorder a valid diagnosis in the presence of high IQ? Results from the MGH longitudinal family studies of ADHD. J Child Psychol Psychiatry. 2007;48:687–694. doi: 10.1111/j.1469-7610.2007.01735.x. [DOI] [PubMed] [Google Scholar]
- Atmetlla G, Burgos V, Carrillo A, Chaskel R. Behavior and orofacial characteristics of children with attention-deficit hyperactivity disorder during a dental visit. J Clin Pediatr Dent. 2006;30:183–190. doi: 10.17796/jcpd.30.3.g66h2750h11242p6. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Hawi Z, Kirley A, Barry E, Gill M, Kent L. Association of the steroid sulfatase (STS) gene with attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1531–1535. doi: 10.1002/ajmg.b.30873. [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Hawi Z, Park J, Scott S, Gill M, Kent L. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1417–1424. doi: 10.1002/ajmg.b.31120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesimo GA, Serra L, Fadda L, Cherubini A, Bozzali M, Caltagirone C. Bilateral damage to the mammillo-thalamic tract impairs recollection but not familiarity in the recognition process: a single case investigation. Neuropsychologia. 2007;45:2467–2479. doi: 10.1016/j.neuropsychologia.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Cevasco A, Pittaluga A, Do Rego JL, Luu-The V, Pelletier G, Vaudry H, Mandich A. Immunohistochemical localization of hydroxysteroid sulfotransferase and sulfatase in the brain of Rana esculenta tadpoles. Ann N Y Acad Sci. 2009;1163:365–368. doi: 10.1111/j.1749-6632.2009.04436.x. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Salido E, Shapiro LJ, Mellon SH. Expression of steroid sulfatase during embryogenesis. Endocrinology. 1997;138:4768–4773. doi: 10.1210/endo.138.11.5504. [DOI] [PubMed] [Google Scholar]
- Davies W, Humby T, Kong W, Otter T, Burgoyne PS, Wilkinson LS. Converging pharmacological and genetic evidence indicates a role for steroid sulfatase in attention. Biol Psychiatry. 2009;66:360–367. doi: 10.1016/j.biopsych.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Doherty MJ, Glass IA, Bennett CL, Cotter PD, Watson NF, Mitchell AL, Bird TD, Farrell DF. An Xp; Yq translocation causing a novel contiguous gene syndrome in brothers with generalized epilepsy, ichthyosis, and attention deficits. Epilepsia. 2003;44:1529–1535. doi: 10.1111/j.0013-9580.2003.61702.x. [DOI] [PubMed] [Google Scholar]
- Ek U, Fernell E, Westerlund J, Holmberg K, Olsson PO, Gillberg C. Cognitive strengths and deficits in schoolchildren with ADHD. Acta Paediatr. 2007;96:756–761. doi: 10.1111/j.1651-2227.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Filippatou DN, Livaniou EA. Comorbidity and WISC-III profiles of Greek children with attention deficit hyperactivity disorder, learning disabilities, and language disorders. Psychol Rep. 2005;97:485–504. doi: 10.2466/pr0.97.2.485-504. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams BW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1990. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Fuentes LJ, Gonzalez C, Estevez AF, Carranza JA, Daza M, Galian MD, Alvarez D. Sensitivity of certain standardised tests to executive attention functioning in seven-year-old children. Electron J Res Educ Psychol. 2003;1:23–36. [Google Scholar]
- Golubchik P, Lewis M, Maayan R, Sever J, Strous R, Weizman A. Neurosteroids in child and adolescent psychopathology. Eur Neuropsychopharmacol. 2007;17:157–164. doi: 10.1016/j.euroneuro.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Antoine HJ, Azziz R. Genes for enzymes regulating dehydroepiandrosterone sulfonation are associated with levels of dehydroepiandrosterone sulfate in polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2659–2664. doi: 10.1210/jc.2006-2600. [DOI] [PubMed] [Google Scholar]
- Groth-Marnat G. Handbook of Psychological Assessment. 3rd edn revised. New York: Wiley; 1999. [Google Scholar]
- Groth-Marnat G, Baker S. Digit Span as a measure of everyday attention: a study of ecological validity. Percept Mot Skills. 2003;97:1209–1218. doi: 10.2466/pms.2003.97.3f.1209. [DOI] [PubMed] [Google Scholar]
- Herbert J. Neurosteroids, brain damage, and mental illness. Exp Gerontol. 1998;33:713–727. doi: 10.1016/s0531-5565(98)00039-4. [DOI] [PubMed] [Google Scholar]
- Holmes J, Lawson D, Langley K, Fitzpatrick H, Trumper A, Pay H, Harrington R, Thapar A. The Child Attention-Deficit Hyperactivity Disorder Teacher Telephone Interview (CHATTI): reliability and validity. Br J Psychiatry. 2004;184:74–78. doi: 10.1192/bjp.184.1.74. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, Watanabe M, Bito H, Terashima T, Wright CV, Kawaguchi Y, Nakao K, Nabeshima Y. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Wu T, Li P, Maher TJ. The effect of steroid sulfatase inhibition on learning and spatial memory. Brain Res. 2000;865:286–290. doi: 10.1016/s0006-8993(00)02372-6. [DOI] [PubMed] [Google Scholar]
- Karsz FR, Vance A, Anderson VA, Brann PG, Wood SJ, Pantelis C, Brewer WJ. Olfactory impairments in child attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69:1462–1468. doi: 10.4088/jcp.v69n0914. [DOI] [PubMed] [Google Scholar]
- Kent L, Emerton J, Bhadravathi V, Weisblatt E, Pasco G, Willatt LR, McMahon R, Yates JR. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519–524. doi: 10.1136/jmg.2008.057729. [DOI] [PubMed] [Google Scholar]
- Kriz L, Bicikova M, Hill M, Hampl R. Steroid sulfatase and sulfuryl transferase activity in monkey brain tissue. Steroids. 2005;70:960–969. doi: 10.1016/j.steroids.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kriz L, Bicikova M, Mohapl M, Hill M, Cerny I, Hampl R. Steroid sulfatase and sulfuryl transferase activities in human brain tumors. J Steroid Biochem Mol Biol. 2008;109:31–39. doi: 10.1016/j.jsbmb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Langley K, Fowler TA, Grady DL, Moyzis RK, Holmans PA, van den Bree MB, Owen MJ, O’Donovan MC, Thapar A. Molecular genetic contribution to the developmental course of attention-deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18:26–32. doi: 10.1007/s00787-008-0698-4. [DOI] [PubMed] [Google Scholar]
- Lonardo F, Parenti G, Luquetti DV, Annunziata I, Della Monica M, Perone L, De Gregori M, Zuffardi O, Brunetti-Pierri N, Andria G, Scarano G. Contiguous gene syndrome due to an interstitial deletion in Xp22.3 in a boy with ichthyosis, chondrodysplasia punctata, mental retardation and ADHD. Eur J Med Genet. 2007;50:301–308. doi: 10.1016/j.ejmg.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Maloku E, Covelo IR, Hanbauer I, Guidotti A, Kadriu B, Hu Q, Davis JM, Costa E. Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci U S A. 2010;107:4407–4411. doi: 10.1073/pnas.0914483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Similarities and differences in Wechsler Intelligence Scale for Children–Third Edition (WISC-III) profiles: support for subtest analysis in clinical referrals. Clin Neuropsychol. 2004;18:559–572. doi: 10.1080/13854040490888530. [DOI] [PubMed] [Google Scholar]
- Milunsky J, Huang XL, Wyandt HE, Milunsky A. Schizophrenia susceptibility gene locus at Xp22.3. Clin Genet. 1999;55:455–460. doi: 10.1034/j.1399-0004.1999.550610.x. [DOI] [PubMed] [Google Scholar]
- Molinari M, Petrosini L, Misciagna S, Leggio MG. Visuospatial abilities in cerebellar disorders. J Neurol Neurosurg Psychiatry. 2004;75:235–240. [PMC free article] [PubMed] [Google Scholar]
- Mortaud S, Nicolas L, Pinoteau W, Tordjman S, Carlier M, Roubertoux PL. Brain pathways mediating the pro-aggressive effect of the steroid sulfatase (sts) gene. Behav Genet. 2010;40:211–219. doi: 10.1007/s10519-010-9340-6. [DOI] [PubMed] [Google Scholar]
- Moskvina V, Holmans P, Schmidt KM, Craddock N. Design of case-controls studies with unscreened controls. Ann Hum Genet. 2005;69:566–576. doi: 10.1111/j.1529-8817.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- Nicolas LB, Pinoteau W, Papot S, Routier S, Guillaumet G, Mortaud S. Aggressive behavior induced by the steroid sulfatase inhibitor COUMATE and by DHEAS in CBA/H mice. Brain Res. 2001;922:216–222. doi: 10.1016/s0006-8993(01)03171-7. [DOI] [PubMed] [Google Scholar]
- Park IH, Han BK, Jo DH. Distribution and characterization of neurosteroid sulfatase from the bovine brain. J Steroid Biochem Mol Biol. 1997;62:315–320. doi: 10.1016/s0960-0760(97)00042-3. [DOI] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Matthies S, Drieling T, Maier S, Bubl E, Hesslinger B, Buechert M, Henning J, Ebert D, Tebartz Van Elst L. Spectroscopic findings in attention-deficit/hyperactivity disorder: review and meta-analysis. World J Biol Psychiatry. 2009;10:355–365. doi: 10.1080/15622970802176032. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Pineda D, Ardila A, Rosselli M. Neuropsychological and behavioral assessment of ADHD in seven- to twelve-year-old children: a discriminant analysis. J Learn Disabil. 1999;32:159–173. doi: 10.1177/002221949903200206. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev. 2005;26:171–202. doi: 10.1210/er.2004-0003. [DOI] [PubMed] [Google Scholar]
- Rees G. Visual attention: the thalamus at the centre? Curr Biol. 2009;19:R213–R214. doi: 10.1016/j.cub.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Romanos M, Renner TJ, Schecklmann M, Hummel B, Roos M, von Mering C, Pauli P, Reichmann H, Warnke A, Gerlach M. Improved odor sensitivity in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64:938–940. doi: 10.1016/j.biopsych.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Ruessmann K, Sondag HD, Beneicke U. Visuospatial disorders and related lesions of the brain. Int J Neurosci. 1989;46:123–126. doi: 10.3109/00207458908986248. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121((Pt. 4)):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Shinawi M, Patel A, Panichkul P, Zascavage R, Peters SU, Scaglia F. The Xp contiguous deletion syndrome and autism. Am J Med Genet A. 2009;149A:1138–1148. doi: 10.1002/ajmg.a.32833. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. Structural development of the basal ganglia in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Psychiatry Res. 2009;172:220–225. doi: 10.1016/j.pscychresns.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Smith CP, Dunger DB, Williams AJ, Taylor AM, Perry LA, Gale EA, Preece MA, Savage MO. Relationship between insulin, insulin-like growth factor I, and dehydroepiandrosterone sulfate concentrations during childhood, puberty, and adult life. J Clin Endocrinol Metab. 1989;68:932–937. doi: 10.1210/jcem-68-5-932. [DOI] [PubMed] [Google Scholar]
- Steckelbroeck S, Nassen A, Ugele B, Ludwig M, Watzka M, Reissinger A, Clusmann H, Lutjohann D, Siekmann L, Klingmuller D, Hans VH. Steroid sulfatase (STS) expression in the human temporal lobe: enzyme activity, mRNA expression and immunohistochemistry study. J Neurochem. 2004;89:403–417. doi: 10.1046/j.1471-4159.2004.02336.x. [DOI] [PubMed] [Google Scholar]
- Tewes U. Hamburg-Wechsler Intelligenztest fur Erwachsene (HAWIE-R) Germany: Hogrefe, Gottingen; 1991. [Google Scholar]
- Thapar A, O’Donovan M, Owen MJ. The genetics of attention deficit hyperactivity disorder. Hum Mol Genet. 2005;14:R275–R282. doi: 10.1093/hmg/ddi263. (Spec No. 2) [DOI] [PubMed] [Google Scholar]
- Thomas NS, Sharp AJ, Browne CE, Skuse D, Hardie C, Dennis NR. Xp deletions associated with autism in three females. Hum Genet. 1999;104:43–48. doi: 10.1007/s004390050908. [DOI] [PubMed] [Google Scholar]
- Tobias ES, Bryce G, Farmer G, Barton J, Colgan J, Morrison N, Cooke A, Tolmie JL. Absence of learning difficulties in a hyperactive boy with a terminal Xp deletion encompassing the MRX49 locus. J Med Genet. 2001;38:466–470. doi: 10.1136/jmg.38.7.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Staal WG, van Daalen E, van Engeland H, Hochstenbach PF, Franke L. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry. 2006;11:18–28. doi: 10.1038/sj.mp.4001781. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scales for Children Version III. San Antonio, Texas: The Psychological Corporation; 1991. [Google Scholar]
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. In Situ Hybridisation: A Practical Approach. New York: Oxford University Press; 1992. [Google Scholar]
- van West D, Claes S, Deboutte D. Differences in hypothalamic-pituitary-adrenal axis functioning among children with ADHD predominantly inattentive and combined types. Eur Child Adolesc Psychiatry. 2009;18:543–553. doi: 10.1007/s00787-009-0011-1. [DOI] [PubMed] [Google Scholar]
- Yen PH, Marsh B, Allen E, Tsai SP, Ellison J, Connolly L, Neiswanger K, Shapiro LJ. The human X-linked steroid sulfatase gene and a Y-encoded pseudogene: evidence for an inversion of the Y chromosome during primate evolution. Cell. 1988;55:1123–1135. doi: 10.1016/0092-8674(88)90257-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


